Introduction
A schwannoma is a benign nerve sheath tumor derived
from Schwann cells, the sheath cells that cover myelinated nerve
fibers (1). These tumors are
typically located in the skin and subcutaneous tissue of the head
and neck, or along the flexor surfaces of the extremities (1). Intraosseous schwannomas are rare benign
tumors that account for <1% of primary bone tumors (2). Fewer than 200 cases have been previously
reported; of these cases, the most common sites of the involved
bones were the mandible and sacrum (2). Although a number of cases of
intraosseous schwannoma involving the long bones have been
previously reported (3–8), this type of disease is relatively rare,
and the typical location at which an intraosseous schwannoma of the
long bones may arise is uncertain. Intraosseous schwannoma presents
as a slowly enlarging and painless mass (8); however, if the bone becomes affected and
a microfracture is caused, the mass becomes painful. Since
intraosseous schwannoma is a benign bone tumor, it has good
prognosis following surgery with curettage and bone grafting
(8).
The radiological findings of intraosseous schwannoma
appear as features of benign bone tumors: i) A well-defined lytic
lesion with a thin sclerotic margin; ii) trabeculation or
multiloculation; iii) cortical expansion; and iv) no internal
calcification (3,4). Although it has been reported that
pathological fractures are rare in intraosseous schwannoma
(4), pathological fractures may occur
in slender bones, including the ulna (6). Radiographically, it may be difficult to
distinguish intraosseous schwannomas from other benign bone tumors,
including solitary bone cyst, aneurysmal bone cyst, giant cell
tumor, benign chondroblastoma or fibrous dysplasia (4). On magnetic resonance imaging (MRI), soft
tissue schwannomas typically appear isointense to muscle on
T1-weighted images and homogeneously or heterogeneously
hyperintense to fat on T2-weighted images (9), but not specifically. The tumors may only
be diagnosed by microscopic findings, which is difficult without
biopsy or surgery (1,6).
Microscopically, the histological characteristics of
intraosseous schwannomas are similar to schwannomas in soft tissue
(1,2).
The tumor is composed of two types of cell arrangement: Antoni A
and Antoni B (1,2). The Antoni A area is composed of
compactly arranged spindle-shaped and ovoid tumor cells with
palisading nuclei known as Verocay bodies, in a fibrous background.
The Antoni B area consists of loose cellularity with a myxoid
matrix and frequent cystic degeneration and hemorrhage. It is
clinically difficult to truly distinguish between soft tissue
schwannomas involving the bone and intraosseous schwannomas
involving the surrounding soft tissues (4).
Treatment of intraosseous schwannoma is generally
performed by curettage or en bloc resection followed by bone
grafting. Recurrence is rare, but may be associated with incomplete
resection (10).
Therefore, in the current case report a case of
intraosseous schwannoma arising in the ulna is presented. A benign
bone tumor was suspected from plain radiographs and MRI, and
intraosseous schwannoma was diagnosed based on the pathological
findings of the surgically resected tumor. In addition, relevant
previously reported cases of intraosseous schwannoma involving the
long bones in the upper and lower extremities with clear
radiographic figures are reviewed. Based on the findings of the
present case report and the review of the existing literature, it
is possible that the location of intraosseous schwannoma involving
the long bones may be determined by the position of the
intraosseous artery.
Case report
In March 2014, an 87-year-old woman presented to
Toyama University Hospital (Toyama, Japan) with a 1-month history
of a rapidly-growing mass with tenderness on the medial side of her
left elbow. On physical examination, there was no limitation of
range of motion of the elbow joint and no Tinel's sign detected in
the swollen portion. The patient did not demonstrate muscle
weakness or sensory disturbance.
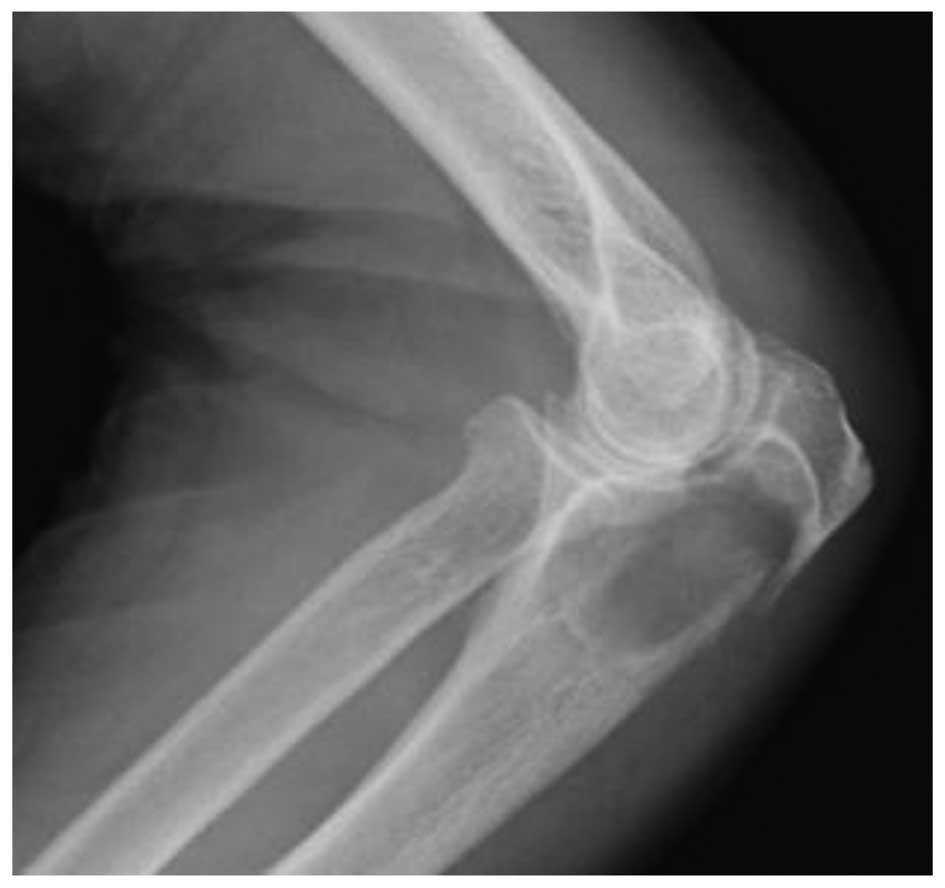
Plain radiographs demonstrated a well-defined lytic
lesion, with a pathological fracture and no intralesional
calcification, in the proximal metaphysis of the left ulna
(Fig. 1). The lesion was accompanied
by thinning of cortical bone. Computed tomography (CT;
SOMATOM® Definition AS; Siemens Healthcare, Erlangen,
Germany) confirmed the lesion with cortical expansion and thinning
in the proximal metaphysis of the ulna. There was no calcification.
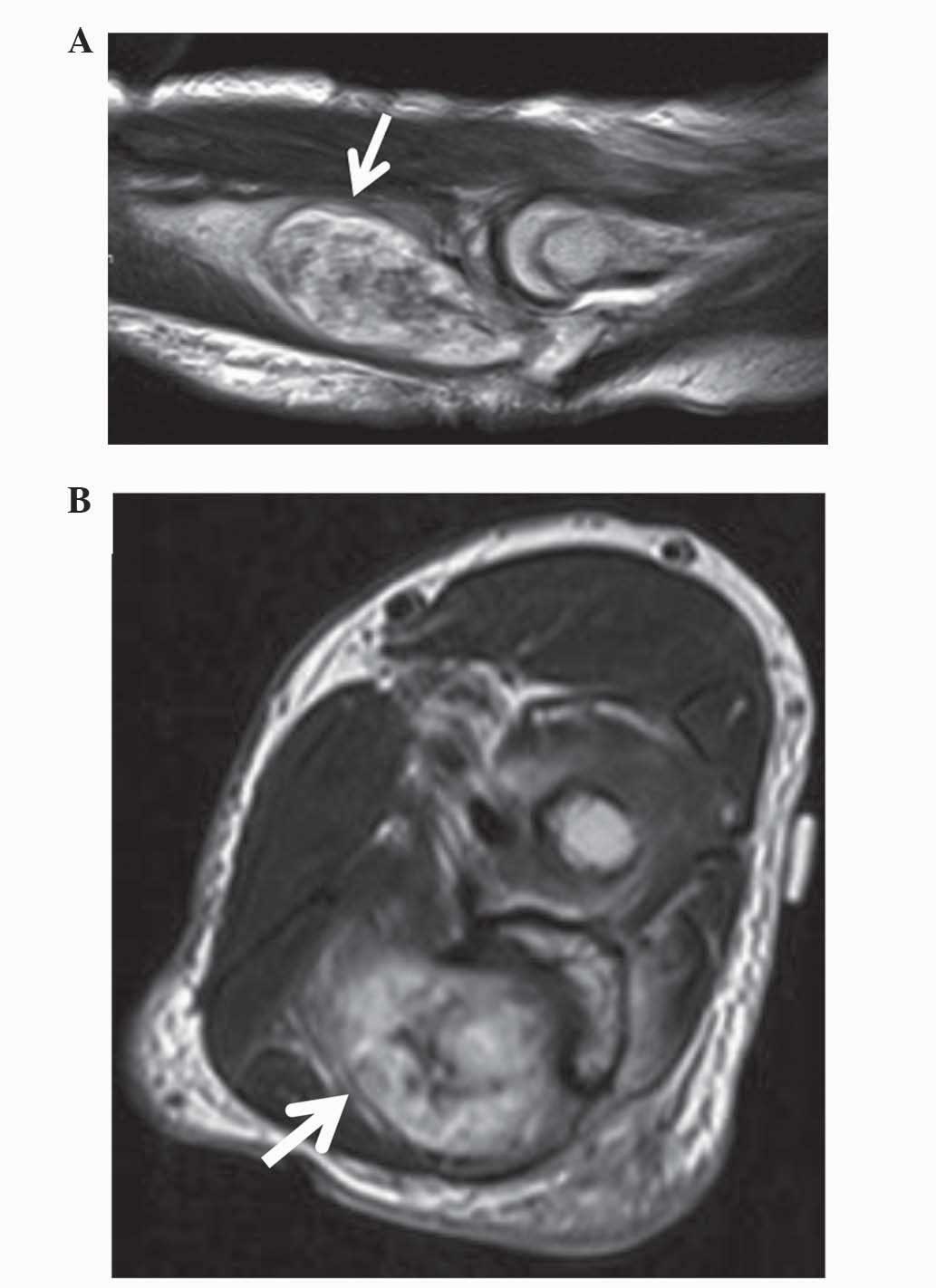
MRI (MAGNETOM Avanto; Siemens Healthcare) of the proximal ulna
revealed that the intraosseous mass was spreading out from the
cortical defect. The lesion appeared isointense to skeletal muscle
on T1-weighted images and hyperintense or heterogeneous on
T2-weighted images (Fig. 2A and
B).
Histological examination of the needle biopsy
revealed a benign spindle cell tumor suggestive of a schwannoma.
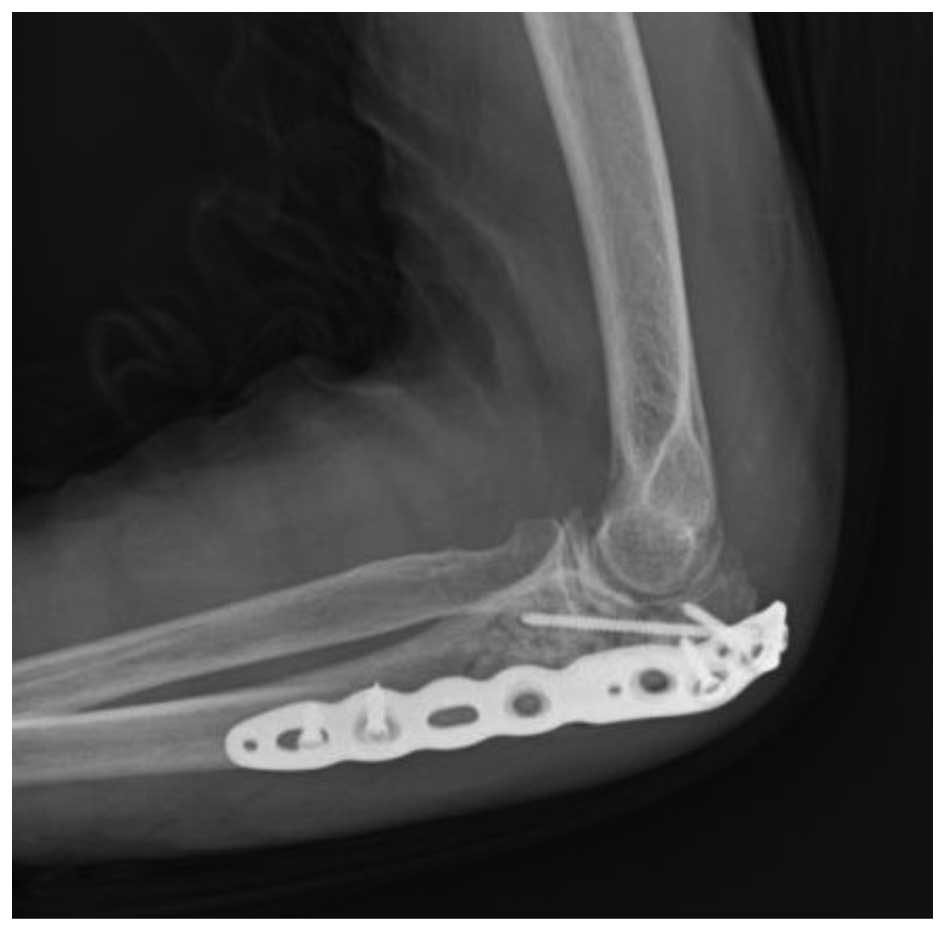
Therefore, marginal resection and curettage, followed by
hydroxyapatite granule (REGENOS; Kuraray Co., Ltd., Kurashiki,
Japan) packing and plate (VariAx Olecranon Locking Plate; Stryker
Japan, Tokyo, Japan) fixation (Fig.
3), was performed. Macroscopically, the encapsulated tumor in
the bone was ovoid and soft, measuring 5×2×1.5 cm. The cut surface
of the resected tumor was gray-yellowish and slightly myxomatous.
The resected tumor tissue was fixed in formalin, embedded in
paraffin, cut into 4-µm sections and stained with hematoxylin and
eosin (H&E; Wako Pure Chemical Industries, Ltd., Osaka, Japan).
The representative tumor section was immunohistochemically stained
with a specific antibody against S-100 protein (polyclonal rabbit;
dilution, 1:500; catalog no. Z0311; Dako Japan Co., Tokyo, Japan)
using the streptavidin-biotin peroxidase complex method. The
H&E stained section and immunohistochemical staining section
were evaluated by two pathologists (Department of Diagnostic
Pathology, Faculty of Medicine, University of Toyama, Toyama,
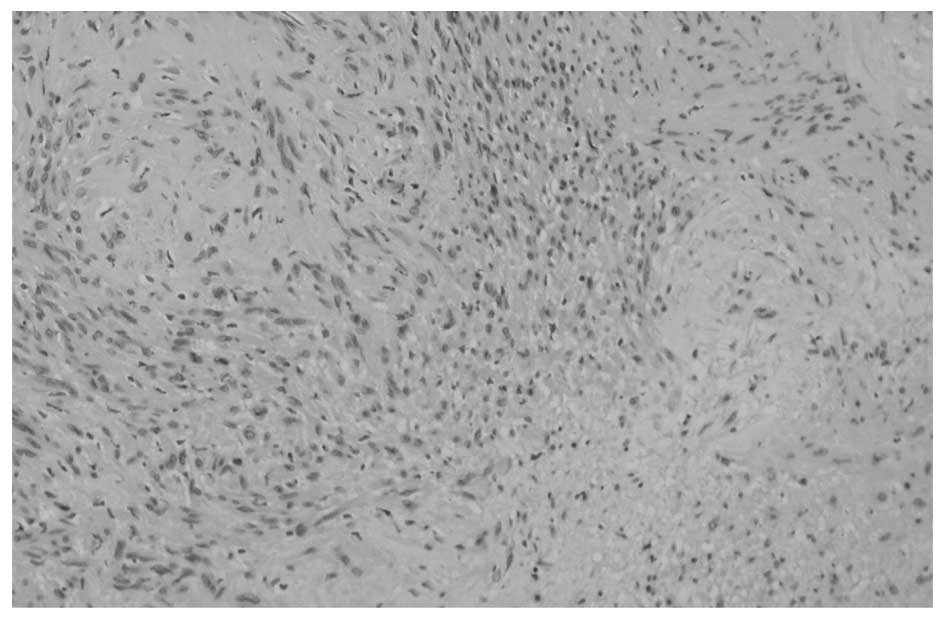
Japan). Microscopic examination (Olympus BX61; Olympus Corporation,
Tokyo, Japan) confirmed that the tumor was comprised of Antoni A
and B areas, with a myxoid stroma (Fig.
4). The tumor cells were positive for S-100 protein on
immunohistochemical staining, with only a few cells positive for
Mindbomb E3 Ubiquitin Protein Ligase 1. The resected tumor was
diagnosed as an intraosseous schwannoma based on these pathological
findings. A total of one year subsequent to surgery, the patient
exhibits no symptoms, and there is no evidence of disease
recurrence.
The present study was conducted following a clinical
research review by the ethics committee of the Toyama University
Hospital (Toyama, Japan). Written informed consent was obtained
from the patient, who was advised that the data from their case
would be submitted for publication in the present case report.
Discussion
Schwannomas typically arise from a peripheral nerve
in the skin or the subcutaneous tissue of the head and neck, or
along the flexor surfaces of the extremities (1). Intraosseous schwannomas are extremely
rare, accounting for <1% of total benign bone tumors (2). There is no sex predilection, and ~1/2 of
patients who develop intraosseous schwannoma have been reported to
be in their second or third decade of life (2). The mandible, maxilla and sacrum are the
most common sites of intraosseous schwannoma involvement (2,11). Of a
total of 165 cases in five previous review studies, 81 cases (49%)
affected the mandible, 12 (7%) the maxilla, 10 (6%) the sacrum, 40
(24%) the long bones, 6 (4%) the vertebra, 5 (3%) the rib, 4 (2%)
the patella, 3 (2%) the pelvis, 2 (1%) the scapula and 2 (1%)
affected other bones (3,4,10–12).
Schwannomas primarily originate from sensory nerves
(13). The underlying mechanism of
the development of schwannoma involving bone are as follows: i) An
extraosseous tumor causes secondary erosion of bone; ii) a tumor
arises within the nutrient canal and grows in a dumbbell-shaped
configuration, producing enlargement of the canal; and iii) a tumor
arises within the bone (3). The
reason that the majority of cases involve the mandible may be
attributed to the long intraosseous canal of the mandibular nerve,
a predominantly sensory nerve (3),
and intraosseous schwannomas of the sacrum are associated with the
numerous sensory nerve roots that run through the sacral foramina
(13). It is often difficult to
determine whether the tumor truly originates from bone or arises
from the nerve roots and bone is secondarily involved (14). Intraosseous nerves are typically
associated with arterial vessels in the nutrient canal and
participate in vasomotor functions, and the majority appear to be
non-myelinated (15). Therefore, an
intraosseous schwannoma involving a long bone may be associated
with the second mechanism, the tumor arises and develops within the
nutrient canal, and related to the site of the nutrient artery;
however, the most common location of intraosseous schwannomas in
long bones remains to be elucidated. A total of 4 cases of
intraosseous schwannoma of the fibula were previously reported with
clear radiographic figures (4–6), and 3 of
these cases occurred in the diaphysis of the fibula. As free
vascularized fibular grafts have been widely used to cover skeletal
bone defects larger than 6 cm (16),
it has been reported that the nutrient canal into the fibula is
positioned between 12 and 18 cm from the tip of the fibula, in the
diaphysis (17). Therefore,
intraosseous schwannomas of the fibula may be more likely to occur
in the diaphysis. The extra- and intraosseous blood supply of the
distal radius was investigated by Sheetz et al (18). In that study, the authors observed
that certain nutrient arteries were associated with distal radial
bone, and they also identified the location of nutrient arteries
penetrating cancerous bone. The nutrient arteries in the distal
radius usually penetrated cancerous bone at the proximal area to
the radiocarpal joint with a distance of 4–21 mm, in the area of
epiphysis and metaphysis (18).
Therefore, a case of intraosseous schwannoma involving the radius
was reported to occur in the distal metaphysis (17). Kimball et al (19) investigated the intraosseous blood
supply to the distal humerus. The large nutrient vessels entered
the anterior medial diaphysis, an average of 11.4 cm (8 specimens;
range, 9.5–14.0 cm) proximal to the medial epicondyle (19). The lateral column metaphysis was
predominately supplied by posterior segmental vessels, whereas
anterior and posterior vessels passed into the medial column
metaphysis (19). The trochlea,
olecranon fossa and coronoid fossa were watershed areas (19). A total of 2 cases of intraosseous
schwannoma of the humerus were reported to arise in the distal
metaphysis. One case was in the medial epicondyle and one was in
the lateral epicondyle (8,10). Although 8 cases of intraosseous
schwannoma involving the ulna have been observed in a large series
of reviews (4,6,7), their
detailed locations in the ulnar bone were unclear. In the present
case, the intraosseous schwannoma involving the ulna was located in
the proximal metaphysis. This area was supplied by an intraosseous
artery, which was a proximal branch from the large nutrient vessel
entering the anterior cortex of the ulnar metaphysis in a
relatively consistent site at the level of the biceps tuberosity,
and was 7.5 cm distal to the tip of the olecranon (20). The midsubstance of the olecranon
appeared to be a relative watershed area for the intraosseous
circulation. The tumor in the present case was located in the
distal area from the watershed area of the proximal ulna, at a site
9–42 mm distal to the tip of the olecranon. Therefore, the most
common location of intraosseous schwannomas involving long bones
may be determined by the position of the intraosseous nutrient
artery, particularly by the second mechanism of intraosseous
schwannoma; the tumor arises and develops within the nutrient
canal.
In conclusion, the present study reported a case of
intraosseous schwannoma of the ulnar bone, and the association
between the location of intraosseous schwannoma involving long
bones and the sites of nutrient vessels in the distal radius,
distal humerus, proximal ulna and fibula was considered.
Intraosseous schwannoma is able to occur in all long bones, and the
most common site of occurrence may be determined by the position of
the intraosseous nutrient artery. As it is difficult to totally
distinguish intraosseous schwannomas from other benign bone tumors
using imaging including plain radiographs and MRI, histological
diagnosis by biopsy is necessary.
Acknowledgements
The present study was supported in part by the
Japanese Government Grant-in-Aid for Scientific Research (KAKENHI;
grant no. 24592227).
References
|
1
|
Antonescu CR, Perry A and Woodruff JM:
Schwannoma (including variants). WHO Classification of Tumours of
Soft Tissue and Bone. Fletcher CDM, Bridge JA, Hogendoorn P and
Mertens F: 5:(4th). IARC Press. (Lyon). 170–172. 2013.
|
|
2
|
Unni KK and Inwards CY: Miscellaneous
unusual tumors of bone. Dahlin's Bone Tumors: General Aspects and
Data on 10,165 Cases (6th). Lippincott. (Williams & Wilkins,
Philadelphia, PA). 295–298. 2010.
|
|
3
|
de la Monte SM, Dorfman HD, Chandra R and
Malawer M: Intraosseous schwannoma: Histologic features,
ultrastructure, and review of the literature. Hum Pathol.
15:551–558. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ida CM, Scheithauer BW, Yapicier O, Carney
JA, Wenger DE, Inwards CY, Bertoni F, Spinner RJ and Unni KK:
Primary schwannoma of the bone: A clinicopathologic and radiologic
study of 17 cases. Am J Surg Pathol. 35:989–997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoki J, Tanikawa H, Fujioka F, Ishii K,
Seo GS, Karakida O and Sone S: Intraosseous neurilemmoma of the
fibula. Skeletal Radiol. 26:60–63. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palocaren T, Walter NM, Madhuri V and
Gibikote S: Schwannoma of the fibula. J Bone Joint Surg Br.
90:803–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giné J, Calmet J, Sirvent JJ and Domènech
S: Intraosseous neurilemmoma of the radius: A case report. J Hand
Surg Am. 25:365–369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mutema GK and Sorger J: Intraosseous
schwannoma of the humerus. Skeletal Radiol. 31:419–421. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stull MA, Moser RP Jr, Kransdorf MJ,
Bogumill GP and Nelson MC: Magnetic resonance appearance of
peripheral nerve sheath tumors. Skeletal Radiol. 20:9–14. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wirth WA and Bray CB Jr: Intra-osseous
neurilemmoma. Case report and review of thirty-one cases from the
literature. J Bone Joint Surg Am. 59:252–255. 1977.PubMed/NCBI
|
|
11
|
Gordon EJ: Solitary intraosseous
neurilemmoma of the tibia: Review of intraosseous neurilemmoma and
neurofibroma. Clin Orthop Relat Res. 117:271–282. 1976.PubMed/NCBI
|
|
12
|
Zhang L, Xia BQ, Sun H, Wang LZ, Zhao ZL,
Li B and Wang XD: Intraosseous schwannomas of the jaws: 2 case
reports and review of the literature. Oral Surg Oral Med Oral
Pathol Oral Radiol. 114:e13–e17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turk PS, Peters N, Libbey NP and Wanebo
HJ: Diagnosis and management of giant intrasacral schwannoma.
Cancer. 70:2650–2657. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Unni KK, Inwards CY, Bridge JA, Kindblom
LG and Wold LE: Miscellaneous tumors. AFIP Atals of Tumor Pathology
Series IV: Tumors of the Bones and Joints (1st). 2:ARP Press.
(Washington DC). 309–319. 2005.
|
|
15
|
Sherman MS: The nerves of bone. J Bone
Joint Surg Am. 45:522–528. 1963.
|
|
16
|
Beris AE, Lykissas MG, Korompilias AV,
Vekris MD, Mitsionis GI, Malizos KN and Soucacos PN: Vascularized
fibula transfer for lower limb reconstruction. Microsurgery.
31:205–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuura M, Ohno K, Michi K, Egawa K and
Takiguchi R: Clinicoanatomic examination of the fibula: Anatomic
basis for dental implant placement. Int J Oral Maxillofac Implants.
14:879–884. 1999.PubMed/NCBI
|
|
18
|
Sheetz KK, Bishop AT and Berger RA: The
arterial blood supply of the distal radius and ulna and its
potential use in vascularized pedicled bone grafts. J Hand Surg Am.
20:902–914. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimball JP, Glowczewskie F and Wright TW:
Intraosseous blood supply to the distal humerus. J Hand Surg Am.
32:642–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardy BT, Glowczewskie F Jr and Wright TW:
Vascular anatomy of the proximal ulna. J Hand Surg Am. 36:808–810.
2011. View Article : Google Scholar : PubMed/NCBI
|