Introduction
Primary diffuse large B-cell lymphoma (Bcl) of the
central nervous system (CNS) is a rare tumor of the brain and
spinal cord, which has been estimated to account for ≤1% of all
lymphomas, 4–6% of all extranodal lymphomas and ~1–3% of primary
CNS tumors (1–3). Immunocompromised individuals are
considered most at risk of the disease, however, the incidence of
primary diffuse large Bcl of the CNS (PCNSL) is increasing in
immunocompetent populations (4).
Due to the absence of a typical clinical
presentation, multiple neuroimaging appearances, heterogeneity of
the pathological morphology and no specific laboratory examination,
the immunohistochemistry and molecular biology are of vital
importance in accurate diagnosis of PCNSL (5). At present, the methods of treatment
include surgery, chemotherapy and radiotherapy (5). Although the introduction of systemic
chemotherapy and radiotherapy has consistently improved survival,
the prognosis of PCNSL remains poor (5), with a median survival time of 17–45
months following symptomatic treatment (6). The current study reports the case of a
patient with PCNSL and reviews the literature regarding the
presentation, diagnosis, treatment and prognosis of the disease.
The present study was approved by the Ethics Committee of The First
Hospital Of Jilin University (Changchun, China) and written
informed consent was obtained from the patient.
Case report
A 58-year-old male presented to The First Hospital
Of Jilin University (Changchun, China) with slurred speech and
facial paralysis. A physical examination revealed that the left
nasolabial fold was shallow. The patient denied a family history of
genetic or immunodeficiency disorders. The cerebrospinal fluid
(CSF) appeared to be normal and free from tumor cells. The
ultrasound examination results and the peripheral blood tests,
including for the human immunodeficiency virus and Epstein-Barr
virus, were either normal or negative. The brain magnetic resonance
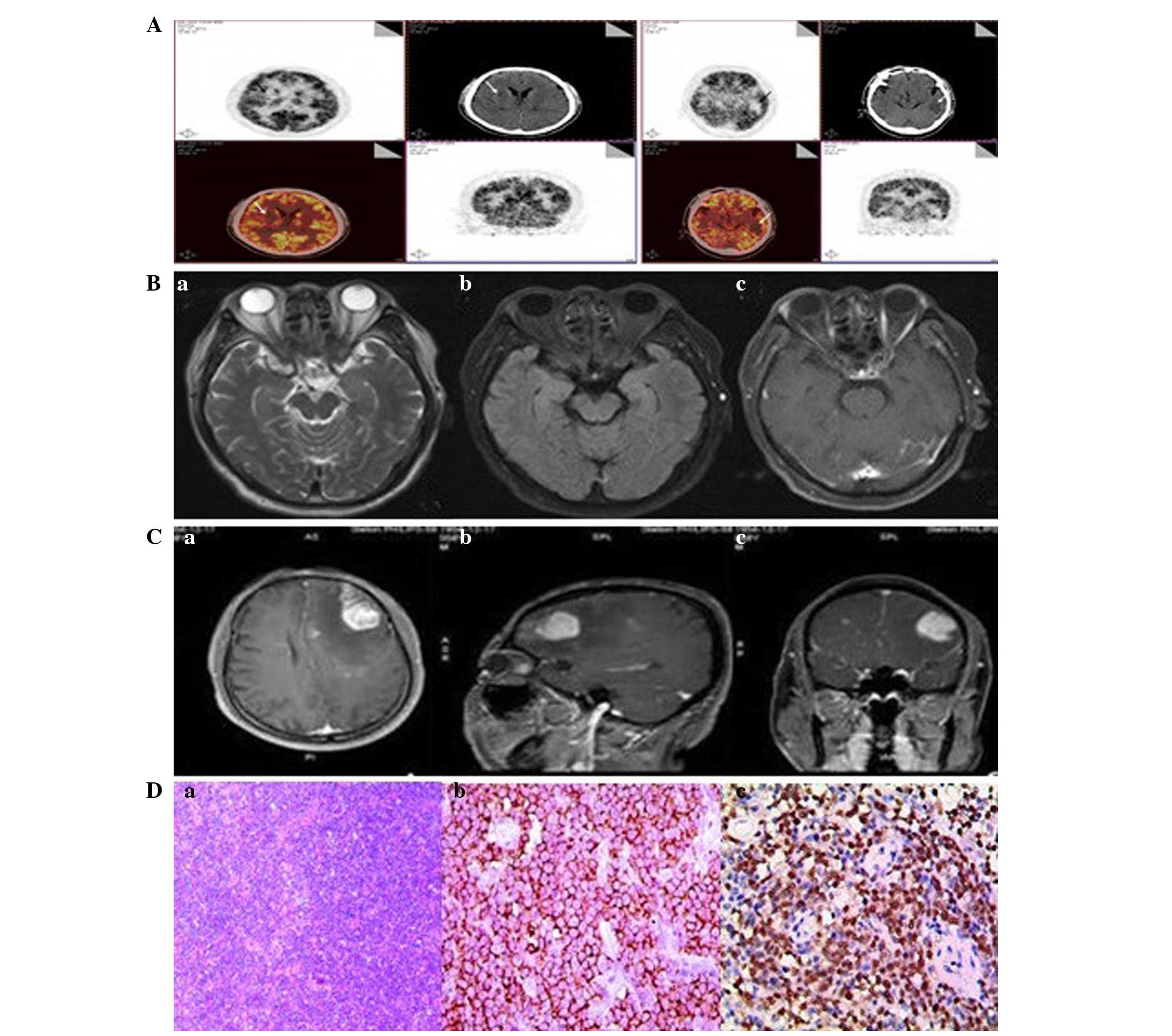
imaging (MRI) scan revealed an occupied lesion of 1.8 cm at the
right basal ganglia region and corona radiata (Fig. 1A). The patient refused a stereotactic
biopsy; however, the patient's symptoms improved with symptomatic
treatment. In particular, the patient's speech regained fluency,
seven days subsequent to the administration of mannitol (50 g,
three times daily) and dexamethasone (10 mg, twice daily) for three
days.
Two weeks subsequent to the identification of the
lesion, the patient received local γ knife treatment at the Central
Hospital of Changchun (Changchun, China) on September 23, 2012, and
was re-admitted to the hospital on October 3, 2012, with a headache
and numbness on the left side of the body that had lasted for 5
days. Another brain MRI scan revealed high and low signal in the
right basal ganglia and radial area. The size of the lesion was
1.3×1.2×1.6 cm. Compared with the previous MRI, the size of the
lesion was significantly decreased (Fig.
1B). In addition, a single-voxel magnetic resonance
spectroscopy (MRS) scan that used a point resolved spectroscopy
protocol with a short (35 msec) echo time revealed a notable
elevation of the choline peak, a stable creatine peak and a
decreasing N-acetylaspartate (NAA) peak. The MRS scan also revealed
a lactate peak and elevation of the glutamate and glutamine peaks.
These data indicated that the patient possessed a malignant tumor
(Fig. 1C). One week later, 3 cycles
of temozolomide therapy (first dose, 150 mg/m2;
subsequent doses, 200 mg/m2) were administered. Another
brain MRI scan revealed that the size of the lesion was
significantly decreased (Fig. 1D);
however, the positron emission tomography-computed tomography
(PET/CT) scan reported highly metabolic nodules in the right basal
ganglia and left temporal lobe (Fig.
2A). Four months later, a follow-up MRI indicated that the left
temporal lobe lesion was resolved (Fig.
2B). Six months subsequent to the temozolomide treatment, the
tumor appeared to be in full remission.
In July 2013, the patient was re-admitted to the
hospital with weakness, slurred speech and memory loss that had
lasted for 3 days. A neurological examination revealed receptive
aphasia, weakness of the right side and shallowness of the right
nasolabial fold. A brain MRI scan revealed a hyperdense lesion with
a diameter of 5.5 cm in the left frontal lobe. Signs of edema and a
rightward shift of the midline structure were also present
(Fig. 2C). Additionally, there was an
abnormal enhancement in the bilateral frontal lobe and right basal
ganglia. A left frontal lobe tumor resection was performed.
Intraoperatively, the tumor was gray red, soft and 4.8 cm in
diameter. The tumor did not possess an envelope but had a rich
blood supply. Postoperatively, the patient demonstrated notable
improvements in speech and function, and exhibited no signs of
complication.
A pathological examination of the resected tumor
confirmed the diagnosis of PCNSL. The immunohistochemical analysis
revealed that the tumor did not express the glial gibrillary acidic
protein, cluster of differentiation (CD)3, CD43, cyclin D1, CD10,
CD20, CD21, CD30, cancer antigen of Ki-67, anaplastic lymphoma
kinase or myeloperoxidase proteins (Fig.
2D). The tumor did express the paired box protein-5, Bcl-2,
Bcl-6, B-cell related and forkhead box protein. In situ
hybridization of the tumor identified Epstein-Barr virus-encoded
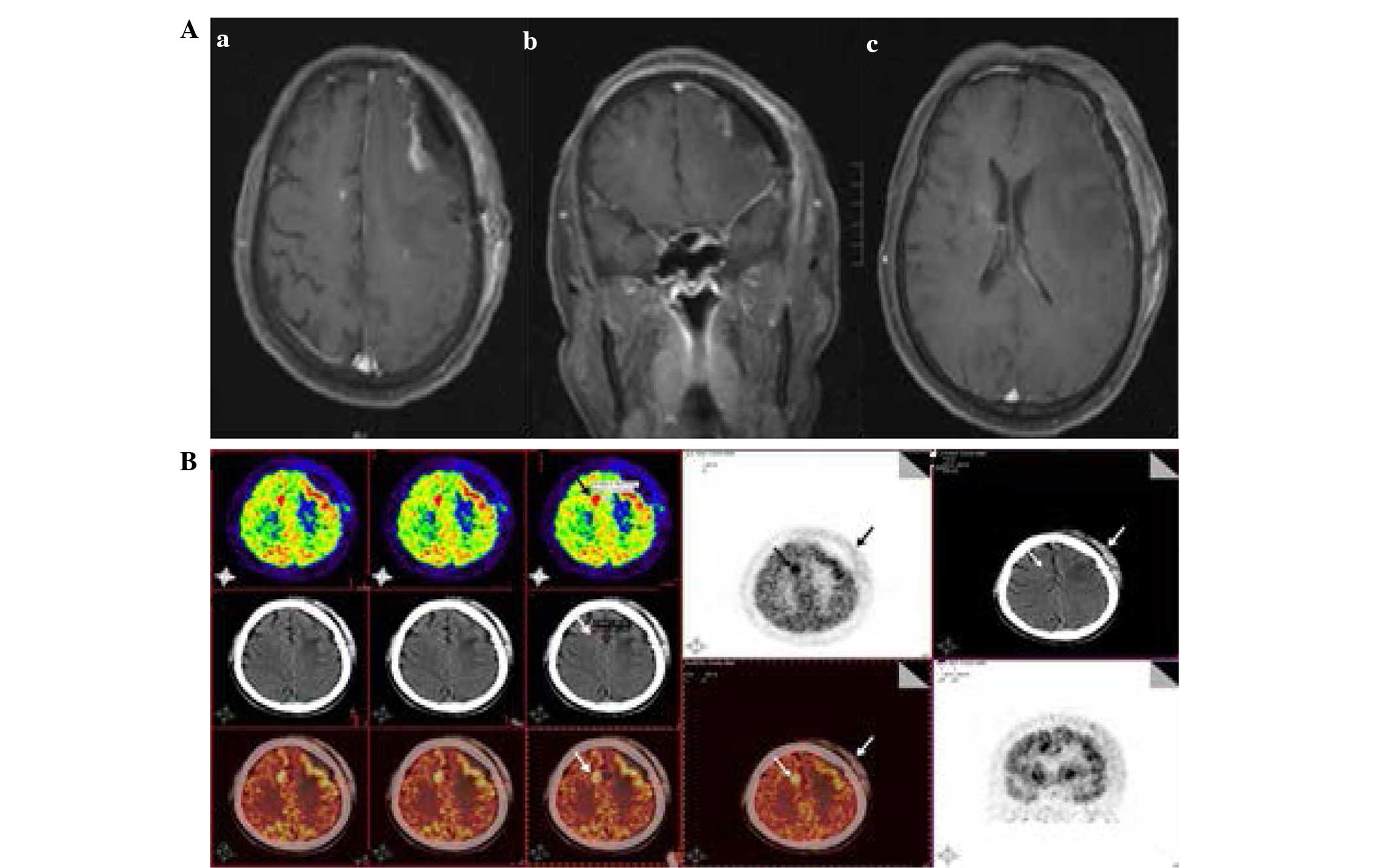
RNA (Fig. 2D). The post-surgical MRI
revealed the postoperative changes to the left frontal lobes
without residual tumor (Fig. 3A).
Another PET/CT scan revealed high metabolic nodules in the right
frontal lobe next to the falx cerebri that demonstrated a maximum
standardized uptake value (SUV) of 25 (Fig. 3B). In addition, the lactate
dehydrogenase and macroglobulin levels and the sedimentation rate
were all normal. The patient subsequently received six three-week
cycles of high-dose methotrexate (3.5 g/m2 daily; days
1, 8 and 14) treatment, followed by whole-brain radiation therapy
[40 Gy/25 fractions (f); once a week] for six weeks, and an
additional dose of 9 Gy/5 f two weeks later. The patient has
demonstrated no signs of relapse and has remained stable during the
20-month follow-up.
Discussion
PCNSL is a B-cell non-Hodgkin's lymphoma that
originates from, and localizes to, the cerebellum, spinal cord, pia
mater, retina or optic nerve (1,2). According
to the updated WHO classification of tumors of haematopoietic and
lymphoid tissues, the disease is recognized as a discrete entity
(3). PCNSL has been
characteristically associated with immunodeficient patients.
However, the exact mechanism has not yet been identified (7). In immunocompetent populations, the
median age of PCNSL occurrence is 53–57 years old, with a
male:female ratio of 1.2:1. In immunocompromised populations, the
median age of occurrence is 31–35 years old, with a male:female
ratio of 7.38:1. However, the incidence of PCNSL in immunocompetent
populations is increasing (8,9).
PCNSL is characterized by a supratentorial
localization. The clinical manifestations of PCNSL are similar to
those of other intracranial tumors, including the high intracranial
pressure and focal neurological deficit. The patient in the present
study showed no evidence of immunodeficiency. The patient
originally presented with dysarthria and received γ knife and
temozolomide treatment. The patient experienced 2 events of ectopic
intracranial spreading.
The typical imaging characteristics of lymphoma may
be used to differentiate between diagnoses. However, determining a
diagnosis may be challenging; in particular, when attempting to
rule out CNS metastasis, glioblastoma, inflammation, multiple
sclerosis or other CNS demyelination. The most common imaging
characteristics of PCNSL are hypointense T1 and hyperintense T2
signals, homogeneous or heterogeneous diffusion weighted imaging
(DWI) and high circular signals. In addition, the gadopentetic acid
enhancement may show a mass reinforcement, and the proton magnetic
resonance spectroscopy may exhibit an elevation of choline and
lactate peak. The fluorodeoxyglucose PET/CT scan may show single or
multiple high metabolic signals that exhibit SUV elevation
(10–18). In the present study, the MRI results
differed from a typical PCNSL case: The lesion size was smaller and
exhibited prominent surrounding edema, lateral ventricle
compression and an evident occupied effect. Future diagnoses may be
improved through the combined use of MRI scans with enhancement,
DWI, 1-h MRS and PET/CT scans. However, pathological analysis
remains to be the gold standard for the diagnosis of PCNSL.
Although the use of surgery for PCNSL patients has
been debated (19,20), the present study supports that
surgical resections are critical for the management of PCNSL,
particularly when used in combination with chemotherapy and
radiotherapy regimens. Postoperatively, the symptoms of the patient
in the present study have significantly improved without
complication. The pathological analysis confirmed the diagnosis of
PCNSL, and provided useful information for the additional
management of the disease. Therefore, resection surgery is
recommended to be the first treatment for patients with a single
occupied lesion and increased intracranial pressure. However, MRI
stereotactic biopsies may be used for patients with multiple
sporadic lesions that are located deeply in the brain or any
important functional area. Ideally, steroid therapy may not be
commenced for 1–2 weeks following a biopsy (21). Lesions in multiple locations may be
biopsied to avoid misdiagnosis.
Radiation and chemotherapy may markedly improve the
survival rates of PCNSL patients, particularly when chemotherapy
agents are administered prior to radiation therapy. Recently,
high-dose methotrexate (HD-MTX) has been widely used for the
treatment of PCNSL, of which intrathecal MTX delivers a systemic
high dose of MTX (8 g/m2) (22). Osmotic pressure drugs may also be
applied to the open blood-brain barrier to facilitate drug delivery
to the lesions (23).
A previous study demonstrated that five cycles of
HD-MTX (3.5 g/m2) and procarbazine (100
mg/m2/day) is beneficial for patients with a 90%
objective response rate and median survival of 60 months (20). Other combination treatments included
high-dose MTX chemotherapy, temozolomide and rituximab (24,25).
Temozolomide is a chemotherapy agent with a high
oral bioavailability that may cross the blood-brain barrier and may
be effective in patients with glioma, leukemia, melanoma and
lymphoma. The National Comprehensive Cancer Network has recommended
that temozolomide may be used for refractory PCNSL (26,27). In
regards to radiation therapy, whole brain irradiation of 40–50 Gy
followed by localized irradiation of 60 Gy on regions of edema is
recommended as the best regimen (22).
The patient initially developed a tumor in the left
temporal lobe, which demonstrated ectopic dissemination following
three courses of temozolomide treatment. The lesion was
undetectable 6 months subsequent to the temozolomide therapy. This
result indicates that temozolomide combined with radiotherapy may
be highly effective for PCNSL patients. In addition, the patient
received 6 cycles of high dose chemotherapy (3.5 g/m2
MTX), whole brain radiation therapy (40 Gy/25 f) and additional
radiotherapy of 9 Gy/5 f, subsequent to pathological
confirmation.
Previous studies have indicated that PCNSL patients
only survive for 3–6 months without treatment (12,28).
Although comprehensive therapy improves progression-free and
overall survival (OS) rates compared with untreated patients, the
5-year survival rate of patients that receive treatment remains
20–25% (29). In addition, 35–60% of
patients experience ectopic recurrence within two years of
diagnosis. Patients that experience the recurrence of PCNSL
demonstrate an OS of 8–18 months. At present, there is no standard
treatment for recurrent PCNSL (30).
Subsequent to 20 months of follow-up, the patient in the present
study had a normal life and experienced no additional
recurrence.
Overall, clinicians are recommended to consider the
following issues. The clinical and imaging findings of PCNSL are
complicated and varied, particularly in the early phase of
remission stages, due to unremarkable symptoms, non-typical imaging
findings and the misdiagnosis as inflammation. Usually, the
specificity and sensitivity of the detection rate of tumors in the
CSF by cytological findings is low. Single-photon emission computed
tomography and molecular biology technology have not been widely
applied. A stereotactic biopsy may be a better method for the
diagnosis of PCNSL. Steroid treatment may improve the clinical
symptoms, but recurrence is common. For patients that are
misdiagnosed with multiple sclerosis or sarcoma, glucocorticoid
treatment may dissolve lymphoid cells and damage normal morphology.
Therefore, an accurate diagnosis of PCNSL is critical to avoid
complications from inappropriate treatment.
References
|
1
|
Gerstner ER and Batchelor TT: primary
central nervous system lymphoma. Arch Neurol. 67:291–297. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Senocak E, Oguz KK, Ozgen B, et al:
Parenchymal lymphoma of the brain on initial MR imaging: A
comparative study between primary and secondary brain lymphoma. Eur
J Radiol. 79:288–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kluin PM, Deckert M and Ferry JA: Primary
diffuse large B-cell lymphoma of the CNS. WHO Classification of
Tumours of Haematopietic and Lymphoid Tissues. Swerdlow SH, Campo
E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J and Vardiman
JW: 2:(4th). IARC Press. (Lyon). 240–241. 2008.
|
|
4
|
del Rio Sierra M, Rousseau A, Soussain C,
Ricard D and Hoang-Xuan K: Primary CNS lymphoma in immunocompetent
patients. Oncologist. 14:526–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu F, Liu Y and Zhang J: The
diagnosis and treatment of primary central nervous system lymphoma.
Zhonghua Xue Ye Xue Za Zhi. 35:771–773. 2014.(In Chinese).
PubMed/NCBI
|
|
6
|
Aki H, Uzunaslan D, Saygin C, Batur S,
Tuzuner N, Kafadar A, Ongoren S and Oz B: Primary central nervous
system lymphoma in immunocompetent individuals: A single center
experience. Int J Clin Exp Pathol. 6:1068–1075. 2013.PubMed/NCBI
|
|
7
|
Ferreri AJ and Marturano E: primary CNS
lymphoma. Best Pract Res Clin Haematol. 25:119–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soussain C and Hoang-Xuan K: primary
central nervous system lymphoma: An update. Curr Opin Oncol.
21:550–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mrugala MM, Rubenstein JL, Ponzoni M and
Batchelor TT: Insights into the biology of primary central nervous
system lymphoma. Curr Oncol Rep. 11:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haque S, Law M, Abrey LE and Young RJ:
Imaging of lymphoma of the central nervous system, spine, and
orbit. Radiol Clin North Am. 46:339–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Server A, Josefsen R, Kulle B, Maehlen J,
Schellhorn T, Gadmar Ø, Kumar T, Haakonsen M, Langberg CW and
Nakstad PH: Proton magnetic resonance spectroscopy in the
distinction of high-grade cerebral gliomas from single metastatic
brain tumors. Acta Radiol. 51:316–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barajas RF Jr, Rubenstein JL, Chang JS,
Hwang J and Cha S: Diffusion-weighted MR imaging derived apparent
diffusion coefficient is predictive of clinical outcome in primary
central nervous system lymphoma. AJNR Am J Neuroradiol. 31:60–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohile NA, Deangelis LM and Abrey LE: The
utility of body FDG PET in staging primary central nervous system
lymphoma. Neuro Oncol. 10:223–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karantanis D, O'eill BP, Subramaniam RM,
Witte RJ, Mullan BP, Nathan MA, Lowe VJ, Peller PJ and Wiseman GA:
18F-FDG PET/CT in primary central nervous system lymphoma in
HIV-negative patients. Nucl Med Commun. 28:834–841. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawai N, Okubo S, Miyake K, Maeda Y,
Yamamoto Y, Nishiyama Y and Tamiya T: Use of PET in the diagnosis
of primary CNS lymphoma in patients with atypical MR findings. Ann
Nucl Med. 24:335–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raoux D, Duband S, Forest F, Trombert B,
Chambonnière ML, Dumollard JM, Khaddage A, Gentil-Perret A and
Péoc'h M: primary central nervous system lymphoma:
Immunohistochemical profile and prognostic significance.
Neuropathology. 30:232–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinoshita M, Hashimoto N, Izumoto S, Okita
Y, Kagawa N, Maruno M, Ohnishi T, Arita N and Yoshimine T:
Immunohistological profiling by B-cell differentiation status of
primary central nervous system lymphoma treated by high-dose
methotrexate chemotherapy. J Neurooncol. 99:95–101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleihues P and Cavenee WK: Pathology and
Genetics of Tumours of the Nervous System. IARC Press. Lyon:
198–203. 2000.
|
|
19
|
Angelov L, Doolittle ND, Kraemer DF,
Siegal T, Barnett GH, Peereboom DM, Stevens G, McGregor J, Jahnke
K, Lacy CA, et al: Blood-brain barrier disruption and
intra-arterial methotrexate-based therapy for newly diagnosed
primary CNS lymphoma: A multi-institutional experience. J Clin
Oncol. 27:3503–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Hu LB, Henning TD, Ravarani EM,
Zou LG, Feng XY, Wang WX and Wen L: MRI findings of primary CNS
lymphoma in 26 immunocompetent patients. Korean J Radiol.
11:269–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basso U and Brandes AA: Diagnostic
advances and new trends for the treatment of primary central
nervous system lymphoma. Eur J Cancer. 38:1298–1312. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, He J, Dang Y, Xia X, Dai Y and Xu
R: One case report and literature review of primary central nervous
system non Hodgkin's lymphoma. Lin Chuang Shen Jing Wai Ke Za Zhi.
5:373–375. 2014.(In Chinese).
|
|
23
|
Abrey LE, Yahalom J and DeAngelis LM:
Treatment for primary CNS lymphoma: The next step. J Clin Oncol.
18:3144–3150. 2000.PubMed/NCBI
|
|
24
|
Glantz MJ, Cole BF, Recht L, Akerley W,
Mills P, Saris S, Hochberg F, Calabresi P and Egorin MJ: High-dose
intravenous methotrexate for patients with nonleukemic
leptomeningeal cancer: Is intrathecal chemotherapy necessary? J
Clin Oncol. 16:1561–1567. 1998.PubMed/NCBI
|
|
25
|
Guha-Thakurta N, Damek D, Pollack C and
Hochberg FH: Intravenous methotrexate as initial treatment for
primary central nervous system lymphoma: Response to therapy and
quality of life of patients. J Neurooncol. 43:259–268. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murakami M, Fujimaki T, Asano S, Nakaguchi
H, Yamada SM, Hoya K, Yamazaki K, Ishida Y and Matsuno A:
Combination therapy with rituximab and temozolomide for recurrent
and refractory primary central nervous system lymphoma. Yonsei Med
J. 52:1031–1034. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Makino K, Nakamura H, Hide T and Kuratsu
J: Salvage treatment with temozolomide in refractory or relapsed
primary central nervous system lymphoma and assessment of the MGMT
status. J Neurooncol. 106:155–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamanaka R, Morii K, Shinbo Y, Homma J,
Sano M, Tsuchiya N, Yajima N, Tamura T, Hondoh H, Takahashi H, et
al: Results of treatment of 112 cases of primary CNS lymphoma. Jpn
J Clin Oncol. 38:373–380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weaver JD, Vinters HV, Koretz B, Xiong Z,
Mischel P and Kado D: Lymphomatosis cerebri presenting as rapidly
progressive dementia. Neurologist. 13:150–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suh C, Kim JE and Yoon DH: Relapse pattern
and prognostic factors for patients with primary CNS lymphoma.
Korean J Hematol. 47:155–156. 2012. View Article : Google Scholar : PubMed/NCBI
|