Introduction
α-fetoprotein (AFP) is an oncofetal glycoprotein
that is mainly produced by the yolk sac and liver during fetal
development, and to a lesser extent, in the fetal gastrointestinal
tract (1,2). The greatest serum AFP levels are
observed between 12–15 weeks after birth, which decrease to normal
adult levels subsequent to approximately one year (3,4). AFP is
detected in the serum of adults with hepatocellular carcinoma, yolk
sac tumors and noncancerous liver disease (2–6). Rising
AFP levels may also be detected in the malignant tumors of various
organs, including the stomach, lung, pancreas, colon, bladder and
ovary (7–13). The stomach is one of the most common
tumor locations (10). AFP-producing
gastric cancer (AFP-GC) was first reported in 1970 by Bourreille
(14). The incidence of AFP-GC in all
gastric carcinoma ranges between 1.3–15.0% (15–19). Nagai
et al proposed that the defining diagnosis of AFP-GC may be
based on the recognition of characteristic histological features
(20). AFP-GC may be divided into two
subtypes, consisting of AFP-producing non-hepatoid adenocarcinoma
and hepatoid adenocarcinoma. AFP-GC is usually diagnosed in the
advanced stages of disease and demonstrates a poor prognosis
(20–22). In patients exhibiting serum AFP levels
of >300 ng/ml, the 1-, 3-, and 5-year survival rates are 15.4,
7.7%, and 0%, respectively. By contrast, in patients exhibiting
serum AFP levels of 20–300 ng/ml, the 1-, 3-, 5- and 10-year
survival rates are 46.7, 28.9, 17.8 and 13.3%, respectively
(23). Liver metastasis is considered
the major independent prognostic factor for AFP-GC patients
(24). As AFP-GCs are aggressive
tumors, radical resection is the standard treatment, whereas
palliative resection is reserved for patients exhibiting distant
metastasis. Furthermore, adjuvant chemotherapy may be administered
to improve the surgical outcomes (25). The present study describes the rare
case of a AFP-producing non-hepatoid adenocarcinoma of the stomach,
with a review of the literature. Written informed consent was
obtained from the patient.
Case report
A 66-year-old man presented to a local doctor with a
20-day history of retrosternal pain. A radiographic examination of
the upper gastrointestinal tract revealed a gastric mass, which was
suspected to be gastric cancer. The patient was referred to the
Department of Gastrointestinal Surgery, Qianfoshan Hospital,
Shandong University (Jinan, China) for additional examination and
treatment. A laboratory investigation revealed that the serum AFP
levels of the patient were elevated to 46.49 ng/ml (normal range,
<12.00 ng/ml), and the serum carcinoembryonic antigen (CEA)
levels were 382.22 ng/ml (normal range, <5.00 ng/ml). The
results of other laboratory tests, including liver function tests,
were all within normal limits. A computed tomography (CT) scan
revealed a thickening of the wall of the cardia and massive lymph
node swelling in the lesser curvature of the stomach and head of
the pancreas (Fig. 1A). However, no
metastasis was observed. Endoscopy revealed a tumor that
demonstrated necrosis and hemorrhage in the lesser curvature of the
stomach, which extended between the antrum and body of the stomach.
Biopsies of the region identified a poorly differentiated
adenocarcinoma. AFP-GC was diagnosed. Laparotomy was performed,
which indicated that the carcinoma had extended into the lumen of
small lymphatic and venular vessels.
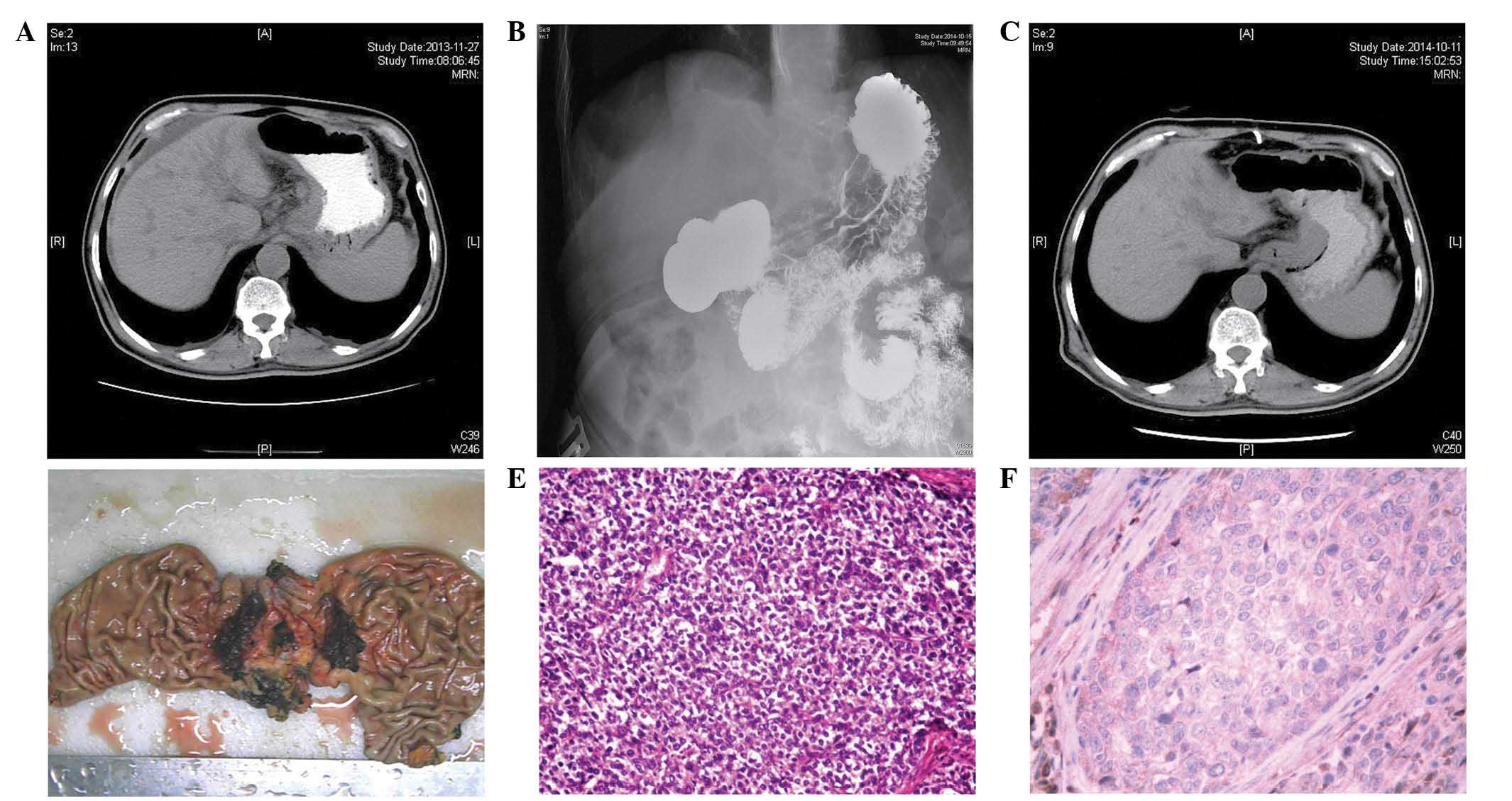 | Figure 1.(A) A CT scan revealed a thickening of
the cardia wall and massive lymph node swelling in the lesser
curvature of the stomach and the head of the pancreas. (B) Upper
gastrointestinal radiological studies revealed an irregular
structure, a filling defect and wall stiffness at the cardia. (C) A
CT scan revealed that the enlarged lymph nodes had decreased in
size. (D) The surgically resected specimen revealed an elevated
tumor (maximal diameter, 4.0 cm) at the cardia, which possessed
surface erosion, located between the lesser curvature and the
posterior-wall. (E) Microscopically, the tumor was a poorly
differentiated adenocarcinoma, and possessed no hepatoid features
(H&E stain; magnification, ×100). (F) Immunohistochemistry
revealed that the tumor was diffusely negative for α-fetoprotein
expression (H&E stain; magnification, ×200). CT, computed
tomography; H&E, hematoxylin and eosin. |
The tumor was surgically unresectable, so the
patient received systemic adjuvant chemotherapy, consisting of 1
cycle of oxaliplatin (150 mg; day 1)-fluorouracil (1.0 g; days
2–6)-calcium folinate (0.3 g; days 2–6), 4 cycles of paclitaxel (80
mg; day 1 and 8, repeated day 21) and capecitabine (1,000
g/m2, twice daily; days 1–14, repeated day 21), and 2
cycles of oxaliplatin (130 mg/m2; day 1, repeated day
21) and S-1 (100 mg/d; day 1- day 14; repeated day 21). These
treatments resulted in partial remission; the serum AFP levels
remained between 44.5 and 32.7 ng/ml, and the serum CEA decreased
to normal levels (normal range, <5.0 ng/ml). Upper
gastrointestinal radiological studies revealed an irregular
structure, a filling defect and wall stiffness at the cardia
(Fig. 1B). The CT scan showed that
the enlarged lymph nodes had decreased in size (Fig. 1C), and there was no evidence of
ascites or liver metastasis. A radical gastrectomy was performed on
20 October 2014. The surgically resected specimen revealed an
elevated tumor (diameter, 4.0 cm) with surface erosion at the
cardia (Fig. 1D) that was located
between the lesser curvature and the posterior-wall.
Microscopically, the tumor was a poorly
differentiated adenocarcinoma (Fig.
1E) that invaded the serous layer region, with local and
neuroendocrine differentiation and no hepatoid features.
Immunohistochemistry revealed that the tumor did not demonstrate
the diffusion of AFP (Fig. 1F).
Specific staining for CEA was focally demonstrated in the poorly
differentiated adenocarcinomatous region. Three lymph nodes on the
lesser curvature and one lymph node on the greater curvature
demonstrated massive swelling. The prominent intravascular
proliferation of non-hepatoid tumor cells, which occasionally form
tumor thrombi, was frequently observed. These immunohistochemical
findings combined with the previously described features supported
the diagnosis of AFP-producing non-hepatoid adenocarcinoma of the
stomach. The tumor was diagnosed as stage IIIB (T3N2M0), according
to the American Joint Committee on Cancer guidelines (26).
Postoperatively, the serum AFP levels decreased to
18.58 ng/ml within 2 weeks and the serum AFP levels reached 3.90
ng/ml 1 month subsequent to the surgery. The patient was treated
with systemic immunity-enhancing therapy and has been free of
recurrence for 2 months.
Discussion
AFP is an oncofetal glycoprotein mainly produced by
the yolk sac and liver during fetal development and, to a lesser
extent, in the fetal gastrointestinal tract (1,2). The
highest serum levels appear between 12–15 weeks of gestation, and
decrease to normal adult levels approximately one year later
(3,4).
AFP is frequently detected in the serum of adults that have
hepatocellular carcinoma, yolk sac tumors and noncancerous liver
disease (2–6). Elevated levels of AFP have been detected
in the malignant tumors of various organs, including the stomach,
lung, pancreas, colon, bladder and ovary (7–13). The
stomach is one of the most common locations of these tumors
(10). AFP-GC was first reported in
1970 by Bourreille (14). The
incidence of AFP-GC in all gastric carcinoma ranges between
1.3–15.0% (15–19).
Numerous studies have described the histological
features of AFP-GC (20,27–29). In
1981, Kodama et al described two histological types of
AFP-GC based on immunohistochemical staining, consisting of a
medullary type characterized by polygonal cells arranged in solid
nests or sheets, and a well-differentiated papillary or tubular
type, with clear cytoplasm (27).
Ishikura et al proposed the term ‘hepatoid adenocarcinoma of
the stomach’ for primary gastric carcinomas that were characterized
by hepatoid differentiation and the production of large amounts of
AFP (28,29). To discriminate between non-hepatoid
adenocarcinoma and hepatoid adenocarcinoma of the stomach, the
measurement of AFP isoforms is also useful, and hepatoid
adenocarcinoma of the stomach demonstrates a liver-type binding
pattern with the lectin concanavalin A (29). Nagai et al proposed a novel
definition, that the diagnosis of AFP-GC may be based on
characteristic histological features (20).
AFP-GC may be divided into two subtypes,
AFP-producing non-hepatoid adenocarcinoma and hepatoid
adenocarcinoma. In the present study, the tumor differed from a
hepatocellular carcinoma in histological and morphological
characteristics, which led to the diagnosis of an AFP-producing
non-hepatoid adenocarcinoma of the stomach. The number of studies
reporting this type of tumor at present is fairly small. AFP-GC is
usually diagnosed in the advanced stages of disease (20–22). The
prognosis of AFP-GC is closely associated with the tumor stage
(20,30). Other factors have also been reported
as important for predicting the prognosis of patients with AFP-GC
(31), including the depth of
invasion, degree of lymph node metastasis and AFP expression, and
the expression of p21 (32).
Previously, factors including increased mitosis, cell movement,
proliferative activity, tumor progression, hepatocyte and receptor
growth factor, c-Met, vascular endothelial growth factor (VEGF) and
the isoform VEGF-C expression were identified as being associated
with AFP-GC, and as poor prognostic factors of cancer (33–35).
Previously, Dhar et al proposed a novel theory that the poor
prognosis of AFP-GC may be associated with the presence of
P-glycoprotein (36). Chang et
al reported that, even if no metastasis is present
preoperatively, liver metastasis may occur within a year following
surgery, and that the close observation and long-term follow-up of
patients is required (27). In
addition, AFP-producing tumors were indicated to demonstrate an
increased likelihood of expressing chemoresistance-associated
proteins. Hirashima et al (37) proposed that AFP-GC is sensitive to
cisplatin, but resistant to fluoropyrimidine prodrugs such as
capecitabine, which require conversion into 5-fluorouracil by
thymidine phosphorylase (38). Due to
the poor prognosis of AFP-GC, accurate diagnosis is important.
Clinicians may be required to consider more aggressive treatment
options for patients with a good performance status, and recommend
resections and additional chemotherapy.
References
|
1
|
Gitlin D, Perricelli A and Gitlin GM:
Synthesis of α-fetoprotein by liver, yolk sac, and gastrointestinal
tract of the human conceptus. Cancer Res. 32:979–982.
1972.PubMed/NCBI
|
|
2
|
Babalı A, Cakal E, Purnak T, Bıyıkoğlu I,
Cakal B, Yüksel O and Köklü S: Serum α-fetoprotein levels in liver
steatosis. Hepatol Int. 3:551–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizejewski GJ: Levels of alpha-fetoprotein
during pregnancy and early infancy in normal and disease states.
Obstet Gynecol Surv. 58:804–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breborowicz J, Mackiewicz A and
Breborowicz D: Microheterogeneity of alpha-fetoprotein in patient
serum as demonstrated by lectin affino-electrophoresis. Scand J
Immunol. 14:15–20. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ezaki T, Yukaya H, Ogawa Y, Chang YC and
Nagasue N: Elevation of alpha-fetoprotein level without evidence of
recurrence after hepatectomy for hepatocellular carcinoma. Cancer.
61:1880–1883. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ganjei P, Nadji M, Albores-Saavedra J and
Morales AR: Histologic markers in primary and metastatic tumors of
the liver. Cancer. 62:1994–1998. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamagata T, Yamagata Y, Nakanishi M,
Matsunaga K, Minakata Y and Ichinose M: A case of primary lung
cancer producing alpha-fetoprotein. Can Respir J. 11:504–506. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamanaka W, Yoneda S, Shirakusa T, et al:
Alpha-fetoprotein (AFP)-producing adrenocortical carcinoma-long
survival with various therapeutic strategies including a lung
resection: Report of a case. Surg Today. 38:275–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsueda K, Yamamoto H, Yoshida Y and
Notohara K: Hepatoid carcinoma of the pancreas producing protein
induced by vitamin K absence or antagonist II (PIVKA-II) and
alpha-fetoprotein (AFP). J Gastroenterol. 41:1011–1019. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinjo T, Taniguchi H, Kushima R, Sekine S,
Oda I, Saka M, Gotoda T, Kinjo F, Fujita J and Shimoda T:
Histologic and immunohistochemical analyses of
α-fetoprotein-producing cancer of the stomach. Am J Surg Pathol.
36:56–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cappetta A, Bergamo F, Mescoli C, Lonardi
S, Rugge M and Zagonel V: Hepatoid adenocarcinoma of the colon:
What should we target? Pathol Oncol Res. 18:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawamura N, Hatano K, Kakuta Y, Takada T,
Hara T and Yamaguchi S: A case of hepatoid adenocarcinoma of the
urinary bladder. Hinyokika Kiyo. 55:619–622. 2009.(In Japanese).
PubMed/NCBI
|
|
13
|
Isonishi S, Ogura A, Kiyokawa T, Suzuki M,
Kunito S, Hirama M, Tachibana T, Ochiai K and Tanaka T:
Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly
woman. Int J Clin Oncol. 14:70–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha feto protein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.(In French). PubMed/NCBI
|
|
15
|
Mehlman DJ, Bulkley BH and Wiernik PH:
Serum alpha-1-fetoglobulin with gastric and prostatic carcinomas. N
Engl J Med. 285:1060–1061. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akai S and Kato K: Serum
α-fetoprotein-positive stomach cancer. Gann Monogr Cancer Res.
14:149–154. 1973.
|
|
17
|
Takahashi Y, Mai M, Ogino T, Ueda H,
Sawaguchi K and Ueno M: Clinicopathological study of AFP producing
gastric cancer - significance of AFP in gastric cancer. Nihon Geka
Gakkai Zasshi. 88:696–700. 1987.(In Japanese). PubMed/NCBI
|
|
18
|
Yonemura Y, Hashimoto T, Sawa T, Shima Y,
Kamata T, Nishimura G, Sugiyama K, Koyasaki N, Fujimura T, Tani T,
et al: The significance of measurement of serum CEA, AFP and hCG in
gastric cancer patients. J Jpn Soc Clin Surg. 48:174–179. 1987.(In
Japanese).
|
|
19
|
Nishio Y, Urakawa T, Nakamoto M, Yamaguchi
T, Tanaka H, Idei H, Noshi T, Iso A, Uematsu K, Iyoroi A and Setoh
K: Study of nine cases of α-fetoprotein (AFP) producing gastric
cancer. J Jpn Soc Clin Surg. 50:1176–80. 1989.(In Japanese).
|
|
20
|
Nagai E, Ueyama T, Yao T and Tsuneyoshi M:
Hepatoid adenocarcinoma of the stomach. A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kono K, Amemiya H, Sekikawa T, Iizuka H,
Takahashi A, Fujii H and Matsumoto Y: Clinicopathologic features of
gastric cancers producing alpha-fetoprotein. Dig Surg. 19:359–365.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin HJ, Hsieh YH, Fang WL, Huang KH and Li
AF: Clinical manifestations in patients with
alpha-fetoprotein-producing gastric cancer. Curr Oncol.
21:e394–e399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirajima S, Komatsu S, Ichikawa D, Kubota
T, Okamoto K, Shiozaki A, Fujiwara H, Konishi H, Ikoma H and Otsuji
E: Liver metastasis is the only independent prognostic factor in
AFP-producing gastric cancer. World J Gastroenterol. 19:6055–6061.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JF, Shi SS, Shao YF and Zhang HZ:
Clinicopathological and prognostic features of hepatoid
adenocarcinoma of the stomach. Chin Med J. 124:1470–1476.
2011.PubMed/NCBI
|
|
26
|
Sobin LH, Gospodarowicz M and Wittekind C:
Stomach. In: UICC International Union Against Cancer. TNM
Classification of Malignant Tumours (7th). Wiley-Blackwell.
(Oxford). 73–77. 2009.
|
|
27
|
Kodama T, Kameya T, Hirota T, Shimosato Y,
Ohkura H, Mukojima T and Kitaoka H: Production of α-fetoprotein,
normal serum proteins, and human chorionic gonadotropin in stomach
cancer: Histologic and immunohistochemical analyses of 35 cases.
Cancer. 48:1647–1655. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishikura H, Fukasawa Y, Ogasawara K,
Natori T, Tsukada Y and Aizawa M: An AFP-producing gastric
carcinoma with features of hepatic differentiation: A case report.
Cancer. 56:840–848. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishikura H, Kirimoto K, Shamoto M,
Miyamoto Y, Yamagiwa H, Itoh T and Aizawa M: Hepatoid
adenocarcinomas of the stomach: An analysis of seven cases. Cancer.
58:119–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adachi Y, Tsuchihashi J, Shiraishi N,
Yasuda K, Etoh T and Kitano S: AFP-producing gastric carcinoma:
Multivariate analysis of prognostic factors in 270 patients.
Oncology. 65:95–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chun H and Kwon SJ: Clinicopathological
characteristics of alpha-fetoprotein-producing gastric cancer. J
Gastric Cancer. 11:23–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Yu H, Cai H and Wang Y: Expression
of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric
cancer: Correlation with clinicopathologic characteristics and
survival. J Surg Oncol. 109:859–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koide N, Nishio A, Igarashi J, Kajikawa S,
Adachi W and Amano J: Alpha-fetoprotein-producing gastric cancer:
Histochemical analysis of cell proliferation, apoptosis, and
angiogenesis. Am J Gastroenterol. 94:1658–1663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amemiya H, Kono K, Mori Y, Takahashi A,
Ichihara F, Iizuka H, Sekikawa T and Matsumoto Y: High frequency of
c-Met expression in gastric cancers producing alpha-fetoprotein.
Oncology. 59:145–151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kamei S, Kono K, Amemiya H, Takahashi A,
Sugai H, Ichihara F, Fujii H and Matsumoto Y: Evaluation of VEGF
and VEGF-C expression in gastric cancer cells producing
alpha-fetoprotein. J Gastroenterol. 38:540–547. 2003.PubMed/NCBI
|
|
36
|
Dhar DK, Nagasue N, Yoshimura H, Tachibana
M, Tahara H, Matsuura H, Abe S, Chang YC and Nakamura T:
Overexpression of P-glycoprotein in untreated AFP-producing gastric
carcinoma. J Surg Oncol. 60:50–54. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirashima Y, Kitajima K, Sugi S, Kagawa K,
Kumamoto T, Murakami K, Fujioka T and Noguchi T: Successful
bi-weekly paclitaxel treatment of an AFP-producing gastric cancer
patient with peritoneal dissemination and multiple liver
metastasis. Gan To Kagaku Ryoho. 33:517–519. 2006.PubMed/NCBI
|
|
38
|
Kamoshida S, Suzuki M, Sakurai Y, Ochiai
M, Kimura F, Kuwao S, Sakamoto K, Sugimoto Y, Fukushima M and
Tsutsumi Y: Expression of chemoresistance-related proteins in
alpha-fetoprotein-producing adenocarcinoma of the digestive organs.
Oncol Rep. 16:721–727. 2006.PubMed/NCBI
|















