Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a
worldwide healthcare dilemma (1). Due
to the improved quality of imaging modalities and other diagnostic
procedures, the annual incidence of PDAC has increased in various
countries, being the fourth leading cause of cancer-associated
mortality in the USA, with an overall 5-year survival rate of ~10%
(2). One of the standard treatments
for PDAC is gemcitabine chemotherapy, as ~80% of patients present
with unresectable or metastatic disease (3). Clinical trials have demonstrated that
several new chemotherapy regimens have superior anti-tumor efficacy
compared with gemcitabine (4,5). However, gemcitabine remains a key
chemotherapeutic agent for palliative chemotherapy in patients with
PDAC due to its moderate anti-tumor effect and limited adverse
events. Numerous studies aiming to stratify patients using
molecular markers, including human equilibrative nucleoside
transporter 1 and ribonucleotide reductase regulatory subunit M1,
have been conducted to determine candidates for whom gemcitabine
treatment would be optimal (6–8). These
molecular markers are reliable predictive markers for gemcitabine
response in patients with PDAC, but typically require specialized
equipment that is difficult to utilize in routine clinical
practice.
Previous studies have revealed that tumor
inflammation is important in carcinogenesis, cancer progression and
chemotherapy resistance (9–11). Among various immunological processes,
neutrophils and lymphocytes are reported to have a vital role in
tumor inflammation and immunology. It has been demonstrated that
the peripheral blood neutrophil-to-lymphocyte ratio (NLR), or more
recently the derived NLR (dNLR), may be a reliable prognostic
marker in various types of cancer, including PDAC (12–18),
However, the use of the dNLR in predicting susceptibility to
chemotherapy has not been evaluated. Therefore, the present study
aimed to evaluate whether the dNLR is a reliable predictive marker
for gemcitabine response in patients with unresectable PDAC. In
addition, the efficacy of the dNLR was compared with other
clinicopathological markers, including serum carbohydrate antigen
(CA) 19–9 level. We consider it clinically important to identify
patients that will not have a robust response to gemcitabine and to
identify those that should instead be treated with highly intensive
chemotherapy regimens, in order to maximize the therapeutic
window.
Patients and methods
The present retrospective cohort study reviewed data
from patients diagnosed with unresectable PDAC, including locally
advanced and metastatic disease, at Fukushima Medical University
(Fukushima, Japan) between September 2006 and January 2014.
Patients with histopathologically confirmed PDAC were included,
whereas those who were assumed to have PDAC based on imaging
findings or serum tumor marker levels were excluded from the study.
Additionally, patients with rare primary pancreatic neoplasms,
including acinar cell carcinoma or neuroendocrine carcinoma, were
excluded. All patients were chemotherapy-naïve prior to undergoing
>2 cycles of gemcitabine treatment at 1,000 mg/m2 on
days 1, 8 and 15 of a 28-day cycle. Adverse events were graded
according to the National Cancer Institute Common Toxicity Criteria
version 4.0, within the Cancer Therapy Evaluation Program
(http://dctd.cancer.gov/ProgramPages/CTEP/, accessed on
May 6, 2013). If severe hematological toxicity (>grade 3) was
observed, the dose was reduced to 800 mg/m2; if patients
were unable to tolerate this reduced dose, the dose was
administered biweekly. Patients were treated with gemcitabine alone
until disease progression or unacceptable toxicity (grade >3
adverse events in biweekly treatment protocol) was observed. When
gemcitabine treatment failed, additional treatment was administered
based on the physicians' decision. All clinicopathological data
utilized in the present study were measured immediately prior to
the initial chemotherapy session. The dNLR was calculated using the
following formula: Neutrophil count / (white blood cell count -
neutrophil count) (19). The present
study was approved by the institutional review board (IRB) of
Fukushima Medical University (Fukushima, Japan) as a retrospective
cohort study (protocol number, 2286). The IRB of Fukushima Medical
University waived the need for written informed patient consent due
to the retrospective non-interventional nature of the study.
Statistical analysis
The progression-free survival (PFS) and overall
survival (OS) time were calculated from the date of histological
diagnosis to the date of disease progression and mortality,
respectively, since no complete response was observed in any of the
patients of the present cohort. Receiver operating curve analysis
was conducted to determine ideal cut-off values of poor prognosis
for the following continuous variables: Tumor diameter, serum CA
19–9 level and the dNLR. The association of each
clinicopathological parameter [age, gender, tumor location, maximum
tumor diameter, disease stage (locally advanced unresectable PDAC
or PDAC with metastatic lesions), serum CA 19–9 level and dNLR]
with PFS and OS were investigated. Survival analysis was performed
using the Kaplan-Meier method with log-rank test in univariate
analysis. Multivariate Cox regression analysis using forward
stepwise selection was performed to determine the effect of
clinicopathological variables on survival time. Statistical
analysis was performed using SPSS (version 21; IBM SPSS, Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Between September 2006 and January 2014, 243
patients were diagnosed with PDAC. Among them, 31 patients who met
the inclusion criteria were included in the present retrospective
analysis. The majority of patients were excluded because of loss to
follow-up. Clinical patient characteristics are indicated in
Table I. Briefly, the cohort included
17 male and 14 female patients with a median age of 61 years
(range, 49–75 years). The median PFS and OS times were 96 days
(range, 20–700 days) and 251 days (range, 71–781 days),
respectively.
 | Table I.Univariate analysis of
clinicopathological variables according to PFS and OS time. |
Table I.
Univariate analysis of
clinicopathological variables according to PFS and OS time.
|
|
| PFS time | OS time |
|---|
|
|
|
|
|
|---|
| Variable | Total patients, n
(%) | Median survival,
days | P-value | Median survival,
days | P-value |
|---|
| Age, years |
|
| 0.874 |
| 0.458 |
|
<65 | 18 (58.1) | 145.1 |
| 291.0 |
|
| ≥65 | 13 (41.9) | 114.5 |
| 254.6 |
|
| Gender |
|
| 0.338 |
| 0.320 |
| Male | 17 (54.8) | 108.3 |
| 253.5 |
|
|
Female | 14 (45.2) | 161.6 |
| 302.7 |
|
| Location |
|
| 0.356 |
| 0.140 |
| Head | 14 (45.1) | 104.1 |
| 223.7 |
|
|
Body/tail | 17 (54.9) | 155.7 |
| 318.5 |
|
| Diameter, mm |
|
| 0.053 |
| 0.210 |
|
<23 | 4
(12.9) | 214.7 |
| 403.5 |
|
| ≥23 | 27 (87.1) | 120.2 |
| 256.8 |
|
| Stage |
|
| 0.021 |
| 0.006 |
| Locally
advanced | 8
(25.8) | 239.5 |
| 422.3 |
|
|
Metastatic | 23 (71.2) | 95.1 |
| 224.7 |
|
| CA 19–9, U/ml |
|
| 0.121 |
| 0.134 |
|
<3,800 | 21 (67.7) | 150.4 |
| 298.1 |
|
|
≥3,800 | 10 (32.3) | 94.5 |
| 228.8 |
|
| dNLR |
|
| 0.002 |
| 0.006 |
|
<2.5 | 15 (48.4) | 187.3 |
| 358.7 |
|
| ≥2.5 | 16 (51.6) | 80.9 |
| 197.9 |
|
The ideal cut-off values to predict the clinical
outcome of continuous variables were tumor diameter of 23 mm, serum
CA 19–9 level of 3,800 U/ml and dNLR of 2.5.
Univariate analysis revealed that there were no
significant differences in PFS and OS time as a function of age
(<65 vs. ≥65 years), gender (male vs. female), tumor location
(head of the pancreas vs. body and tail of the pancreas), tumor
diameter (<23 vs. ≥23 mm) or serum CA 19–9 level (<3,800 vs.
≥3,800 U/ml). By contrast, disease stage (locally advanced vs.
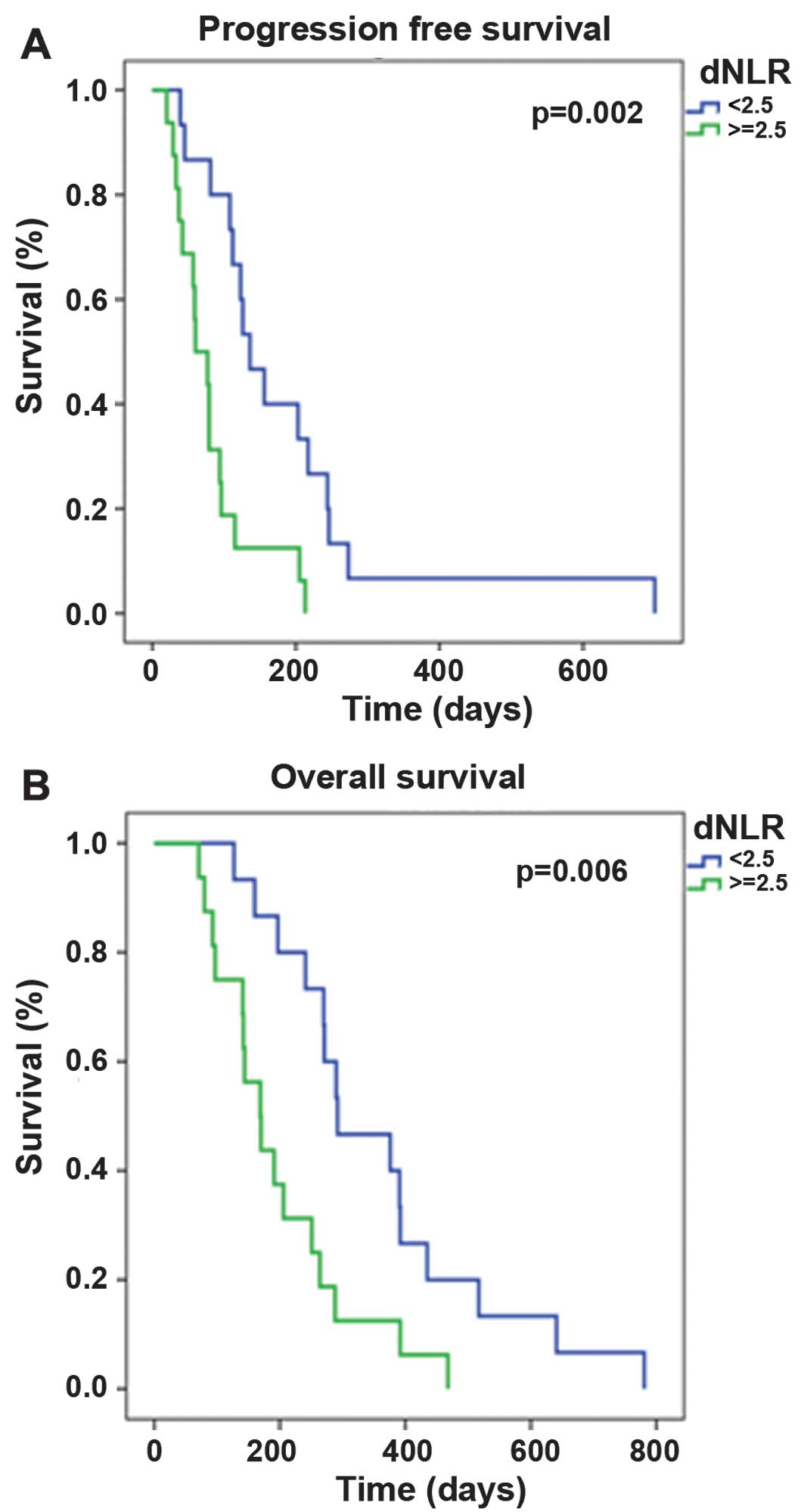
metastatic) and the dNLR (<2.5 vs. ≥2.5) significantly affected
the PFS (P=0.02 and P=0.002, respectively) and OS (P=0.006 and
P=0.006, respectively; Table I). The
dNLR was an independent prognostic factor for PFS and OS time
according to multivariate analysis (P=0.003 and P=0.026,
respectively; Table II) and
Kaplan-Meier survival analysis (P=0.002 and P=0.006, respectively;
Fig. 1).
 | Table II.Multivariate analysis of
clinicopathological variables according to PFS and OS time. |
Table II.
Multivariate analysis of
clinicopathological variables according to PFS and OS time.
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| PFS |
|
|
Location |
| 0.026 |
|
Head | 1 (reference) |
|
|
Body and tail | 2.50 (1.16–5.5) |
|
| dNLR |
| 0.003 |
|
<2.5 | 1 (reference) |
|
|
≥2.5 | 0.28 (0.13–0.65) |
|
| OS |
|
|
Location |
| <0.001 |
|
Head | 1 (reference) |
|
|
Body and tail | 3.68
(1.49–9.05) |
|
|
Stage |
| 0.022 |
|
Locally
advanced | 1 (reference) |
|
|
Metastatic | 0.27
(0.09–8.31) |
|
|
dNLR |
| 0.026 |
|
<2.5 | 1 (reference) |
|
|
≥2.5 | 0.41
(0.19–0.90) |
|
Discussion
To the best of our knowledge, there have been no
studies investigating the association between the dNLR and tumor
response to gemcitabine in patients with unresectable PDAC. The
present study aimed to clarify the role of dNLR and other
clinicopathological factors to predict PFS and OS time in patients
with locally advanced unresectable or metastatic disease treated
with gemcitabine.
With regard to gemcitabine, several molecular
markers have been observed to predict response and prognosis. Human
equilibrative nucleoside transporter 1, which regulates the
intracellular uptake of gemcitabine into cancer cells, is a
well-established molecular marker that is able to predict
susceptibility to gemcitabine or prognosis in patients with
pancreatic cancer following treatment (6,20,21). Other molecular markers, including
serum tumor markers carcinoembryonic antigen and CA 19–9, tumor
tissue Notch 3 mRNA expression levels, and serum interleukin (IL)-6
and IL-1β levels, have been proposed as good markers to predict
response to gemcitabinen in PDAC patients (22–24).
However, various of the above markers of gemcitabine response have
not been routinely utilized in clinical practice due to excessive
costs and technically challenging factors.
Proctor et al developed the dNLR by utilizing
the readily accessible clinical variables of white blood cell and
neutrophil counts (19). Although
dNLR was revealed to be as useful as NLR in predicting the
prognosis of patients with breast and colorectal cancer, the
usefulness of dNLR as a marker for predicting the prognosis of
patients with PDAC could not be verified in that study, due to the
limited number of PDAC patients included in the heterogeneous
population analyzed by the authors (19). Subsequently, Absenger et al
performed an external validation study of the dNLR on a large
cohort of patients with PDAC and confirmed that the pre-treatment
dNLR was an independent prognostic factor for the clinical outcome
of patients with PDAC (16). Based on
the aforementioned results, the present study further focused on
the role of the pre-treatment dNLR to predict response to
gemcitabine in patients with unresectable PDAC. A dNLR of >2.5
was an independent predictive marker of poor PFS and OS time,
whereas the traditional predictive marker CA 19–9 did not exhibit
any significance in predicting response or survival.
Newer chemotherapy regimens, such as FOLFIRINOX or
gemcitabine plus nab-paclitaxel, demonstrate superior anti-tumor
efficacy compared with gemcitabine (4,5), although
not all patients can tolerate these regimens. In a previous study,
the incidence of severe neutropenia and thrombocytopenia was
significantly higher in patients treated with FOLFIRINOX compared
with those administered gemcitabine alone (45.7 vs. 21.0% and 9.1
vs. 3.6%, respectively) (4).
Similarly, grade 3 or 4 neutropenia and febrile neutropenia were
more frequent when a combination of gemcitabine plus nab-paclitaxel
was administered compared with gemcitabine alone (38 vs. 27% and
3.0 vs. 1.0%, respectively) (5).
However, a post-hoc analysis of the metastatic adenocarcinoma of
the pancreas study revealed that the combination of gemcitabine
plus nab-paclitaxel may contribute to longer OS compared with
gemcitabine monotherapy, even in patients with high inflammation
marker levels (for example, high NLR) (25). Taken together, the findings of
previous studies and the present study suggest that patients with a
low dNLR can be treated with gemcitabine monotherapy, whereas those
with a high dNLR may require highly intensive regimens for disease
management.
A limitation of the current study was that it was a
single-center study with a limited number of patients; therefore,
the results should be validated in a larger population across
multiple clinical sites.
In conclusion, the pre-treatment dNLR appears to be
an independent prognostic factor for predicting the OS and PFS time
of patients with unresectable PDAC. In addition, the results
indicate the potential role of the dNLR to stratify patients who
should be treated with highly intensive regimens rather than
gemcitabine alone.
Acknowledgements
The present study thanks Elsevier B.V.
(Philadelphia, PA, USA) for their writing assistance.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surveillance, Epidemiology, and End
Results (SEER): Program Research Data (1973–2007). National Cancer
Institute, DCCPS, Surveillance Research Program, Surveillance
Systems Branch. 2010.
|
|
3
|
Sener SF, Fremgen A, Menck HR and
Winchester DP: Pancreatic cancer: A report of treatment and
survival trends for 100,313 patients diagnosed from 1985–1995,
using the national cancer database. J Am Coll Surg. 189:1–7. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardiére C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenhalf W, Ghaneh P, Neoptolemos JP,
Palmer DH, Cox TF, Lamb RF, Garner E, Campbell F, Mackey JR,
Costello E, et al: Pancreatic cancer hENT1 expression and survival
from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer
Inst. 106:djt3472014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakagawa N, Murakami Y, Uemura K, Sudo T,
Hashimoto Y, Kondo N and Sueda T: Combined analysis of intratumoral
human equilibrative nucleoside transporter 1 (hENT1) and
ribonucleotide reductase regulatory subunit M1 (RRM1) expression is
a powerful predictor of survival in patients with pancreatic
carcinoma treated with adjuvant gemcitabine-based chemotherapy
after operative resection. Surgery. 153:565–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashida R, Nakata B, Shigekawa M, Mizuno N,
Sawaki A, Hirakawa K, Arakawa T and Yamao K: Gemcitabine
sensitivity-related mRNA expression in endoscopic ultrasound-guided
fine-needle aspiration biopsy of unresectable pancreatic cancer. J
Exp Clin Cancer Res. 28:832009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farrow B, Sugiyama Y, Chen A, Uffort E,
Nealon W and Mark Evers B: Inflammatory mechanisms contributing to
pancreatic cancer development. Ann Surg. 239:763–771. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lesina M, Kurkowski MU, Ludes K, Rose-John
S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S,
et al: Stat3/Socs3 activation by IL-6 trans-signaling promotes
progression of pancreatic intraepithelial neoplasia and development
of pancreatic cancer. Cancer Cell. 19:456–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Logsdon CD, Simeone DM, Binkley C,
Arumugam T, Greenson JK, Giordano TJ, Misek DE, Kuick R and Hanash
S: Molecular profiling of pancreatic adenocarcinoma and chronic
pancreatitis identifies multiple genes differentially regulated in
pancreatic cancer. Cancer Res. 63:2649–2657. 2003.PubMed/NCBI
|
|
12
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology. 73:215–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chua W, Charles KA, Baracos VE and Clarke
SJ: Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in
patients with advanced colorectal cancer. Br J Cancer.
104:1288–1295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim
KH and Kim HJ: Prognostic significance of neutrophil lymphocyte
ratio and platelet lymphocyte ratio in advanced gastric cancer
patients treated with FOLFOX chemotherapy. BMC Cancer. 13:3502013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Absenger G, Szkandera J, Pichler M, Stotz
M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H,
Stojakovic T and Gerger A: A derived neutrophil to lymphocyte ratio
predicts clinical outcome in stage II and III colon cancer
patients. Br J Cancer. 109:395–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Absenger G, Szkandera J, Stotz M,
Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H,
Samonigg H and Gerger A: Preoperative neutrophil-to-lymphocyte
ratio predicts clinical outcome in patients with stage II and III
colon cancer. Anticancer Res. 33:4591–4594. 2013.PubMed/NCBI
|
|
17
|
Martin HL, Ohara K, Kiberu A, Van Hagen T,
Davidson A and Khattak MA: Prognostic value of systemic
inflammation-based markers in advanced pancreatic cancer. Intern
Med J. 44:676–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szkandera J, Stotz M, Eisner F, Absenger
G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R,
Alzoughbi W, Ress AL, et al: External validation of the derived
neutrophil to lymphocyte ratio as a prognostic marker on a large
cohort of pancreatic cancer patients. PLoS One. 8:e782252013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Proctor MJ, McMillan DC, Morrison DS,
Fletcher CD, Horgan PG and Clarke SJ: A derived neutrophil to
lymphocyte ratio predicts survival in patients with cancer. Br J
Cancer. 107:695–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farrell JJ, Elsaleh H, Garcia M, Lai R,
Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, et
al: Human equilibrative nucleoside transporter 1 levels predict
response to gemcitabine in patients with pancreatic cancer.
Gastroenterology. 136:187–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haas M, Heinemann V, Kullmann F, Laubender
RP, Klose C, Bruns CJ, Holdenrieder S, Modest DP, Schulz C and
Boeck S: Prognostic value of CA 19–9, CEA, CRP, LDH, and bilirubin
levels in locally advanced and metastatic pancreatic cancer:
Results from a multicenter, pooled analysis of patients receiving
palliative chemotherapy. J Cancer Res Clin Oncol. 139:681–689.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsunaga S, Ikeda M, Shimizu S, Ohno I,
Furuse J, Inagaki M, Higashi S, Kato H, Terao K and Ochiai A: Serum
levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in
patients with advanced pancreatic cancer. Br J Cancer.
108:2063–2069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eto K, Kawakami H, Kuwatani M, Kudo T, Abe
Y, Kawahata S, Takasawa A, Fukuoka M, Matsuno Y, Asaka M and
Sakamoto N: Human equilibrative nucleoside transporter 1 and Notch3
can predict gemcitabine effects in patients with unresectable
pancreatic cancer. Br J Cancer. 108:1488–1494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldstein D, El-Maraghi RH, Hammel P,
Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S,
Tabernero J, Teixeira L, et al: Nab-paclitaxel plus gemcitabine for
metastatic pancreatic cancer: Long-term survival from a phase III
trial. J Natl Cancer Inst. 107:doi: 10.1093/jnci/dju413. 2015.
View Article : Google Scholar
|