Introduction
Cutaneous melanoma is one of the most aggressive
human malignancies, with an increasing incidence worldwide.
Currently, this incidence is high among young patients and is
accompanied with an increased mortality rate, despite latest
advances in therapy. The lack of adequate treatment strategies for
melanoma is an important public health concern, and remains a
challenge for the dermatologist, the pathologist and the
oncologist. Of note, melanoma is one of the few tumors that can
present spontaneous tumor regression due to complex interactions
between the cancer cells and the host; this process occurs
relatively frequently (10–35% of cases), with an even higher
incidence in thin melanomas (60% of cases with a Breslow index of
<0.75 mm) (1–6).
Tumor regression in melanoma involves the
destruction of tumor cells by the host immune system, whereas its
histopathological appearance is characterized by the infiltration
of inflammatory cells, such as lymphocytes with melanophages, and
by vascular hyperplasia and fibrosis. Indeed, several morphological
types of regression may occur [partial regression (PR), segmental
regression (SR) and complete regression]. We have previously
investigated the morphological characteristics of regression in
melanoma and correlated these to the expression of matrix
metalloproteinases (MMPs) (6).
The role of regression in the prognosis of melanoma
has not yet been well-established, with some authors supporting a
favorable prognosis, whereas others have argued that tumor
regression in melanoma may be a marker for a poor prognosis
(7–16). An unfavorable prognosis in melanoma
with regression is supported by bibliographic cases of
histopathologically verified complete tumor regression with the
simultaneous presence of lymphatic and/or visceral metastases
(17,18). Among the patients at the Department of
Pathology, Colentina University Hospital (Bucharest, Romania), we
detected 3 additional cases of completely regressed melanomas whose
identification was based on presenting metastases. These are very
dramatic cases with a significant psychological impact both on the
patients and their families, as well as on the medical staff.
However, to the best of our knowledge, we are unaware of the
existence of melanoma cases with complete regression without
metastasis as a clinical presentation; nobody will biopsy cutaneous
scars, even if there is a history of a previously spontaneously
vanishing tumor in the same spot, if the person is otherwise
healthy.
In view of the existing controversies on the
prognostic significance of regression in melanoma, it may be
helpful to assume that we can encounter both progression and
regression in the same tumor. This process would be the result of
complex interactions between tumor cells and the host immune
system, with the final outcome depending on the efficacy of the
defense mechanisms. It seems that the most important mechanism
involved in the process of tumor regression is the action of
cytotoxic T lymphocytes and natural killer cells (19,20).
Importantly, during the progression of this insidious disease, both
the inflammatory and angiogenic response have been shown to
correlate with the remodeling of the melanoma extracellular matrix
(ECM) (21). Moreover, alterations in
the melanoma microenvironment have been shown to correlate with
melanoma vertical progression (22),
as well as with the regulation of its key biological functions
(23–25). Alterations in the ECM may be
attributed to varying expression patterns between melanoma cells
and melanocytes (26), or to the
activity of proteolytic enzymes, including MMPs, which in addition
to the remodeling of the ECM, also shed cell membrane receptors and
release mediators, thus regulating ECM-melanoma cell interactions
(27).
In view of the fact that the interactions between
the tumor cells and the surrounding microenvironment play an
important role in the evolution of cutaneous melanoma, in this
study, we examined the expression of tissue inhibitors of
metalloproteinases (TIMPs) in regressed melanoma.
Materials and methods
We selected 93 cases of superficial spreading
melanoma (SSM) and nodular melanoma (NM) consecutively diagnosed
between January 2007 and December 2008 at the Department of
Pathology, Colentina University Hospital. All the specimens were
received for histopathological diagnosis. Fragments of tumor and/or
nontumoral tissue where harvested according to guidelines of
medical practice in pathology (Ministry of Health Regulation no.
1217/September 16, 2010 published in Official Monitor no.
723/October 29, 2010, annex 1); tissue fragments were routinely
processed and paraffin-embedded; 3 micron-thick sections were cut
and routinely stained with hematoxilin and eosin (H&E). Based
on the microscopic examination of H&E stained slides, the
histopathologic diagnosis of melanoma was established and several
histopathological characteristics were evaluated: Breslow index,
the Clark level of invasion into adjacent normal structures,
ulcerations, vascular invasion, perineural invasion, intratumoral
inflammatory infiltrates, cellular pleomorphism, the mitotic index,
intratumoral vascularity, satellite/in-transit metastasis, as well
as lymphatic or visceral metastases when specific biopsies were
performed.
This study was approved by the Ethics Committee of
Colentina University Hospital, and written informed consent was
obtained from all patients for the use of the human specimes for
experimental purposes.
For the purpose of this study, additional sections
were prepared and immunohistochemical analyses for TIMP1, TIMP2 and
TIMP3 were duly performed. The specific details of the primary
antibodies used are listed in Table
I. The detection system used was Novolink Polymer
(Leica/Novocastra, Leica Biosystems, Wetzlar, Germany) with DAB as
a chromogen. A semiquantitative score to record the level of
staining was utilized as follows: absent (−), faint positivity (+),
moderate positivity (++) and intense positivity (+++).
 | Table I.Primary antibodies used in this
study. |
Table I.
Primary antibodies used in this
study.
| No. | Primary
antibody | Clone | Catalogue no. | Host | Source |
Pre-treatmenta | Dilution |
|---|
| 1 | TIMP1 | 6F6a | NCL-TIMP1–485 | Mouse | Leica, Wetzlar,
Germany | HIER, EDTA citrate,
pH 8 | 1:300 |
| 2 | TIMP2 | 46E5 | NCL-TIMP2–487 | Mouse | Leica, Wetzlar,
Germany | HIER, EDTA citrate,
pH 8 | 5:240 |
| 3 | TIMP3 | 18D12b | NCL-TIMP3 | Mouse | Leica, Wetzlar,
Germany | HIER, EDTA citrate,
pH 8 | 0.5:1,000 |
We separated our cases into 2 groups, one of
melanoma with regression, and the other of melanoma without
regression [absence of regression (AR)]. We further stratified the
cases with regression based on the respective morphologic type as
follows: i) SR, complete regression of tumor cells with the
simultaneous preservation of the proliferating cells in other parts
of the melanoma; ii) PR, partial disappearance of tumor cells with
partial replacement by inflammatory cells, melanophages and
fibrosis; and iii) SR-PR, the simultaneous presence of SR and RP
areas, observed in a few cases (7).
In total, we analyzed 3 categories of tumor tissue
segments: for the subgroup of the melanoma with regression, we
analyzed areas with regression [regressed component (RC) of the
melanoma with regression], as well as areas without regression
[non-regressed component (NRC) of the melanoma with regression],
whereas one type of tumor tissue of the AR subgroup was examined.
The level of expression recorded for each marker was compared
between the NRC versus the RC in the same tumor, as well as in the
NRC versus AR among different tumors. For the better appreciation
of the differences between the NRC and RC, components we subtracted
the immunohistochemical score for the RC from that of the NRC
(i.e., NRC +++ and RC ++: NRC/R = +1; NRC ++ and RC +++: NRC/RC =
−1).
Statistical analysis was performed using the EXCEL
and EPIINFO programs; a P-value of <0.05 was considered to
indicate a statistically significant difference (calculated by the
χ2test).
Results
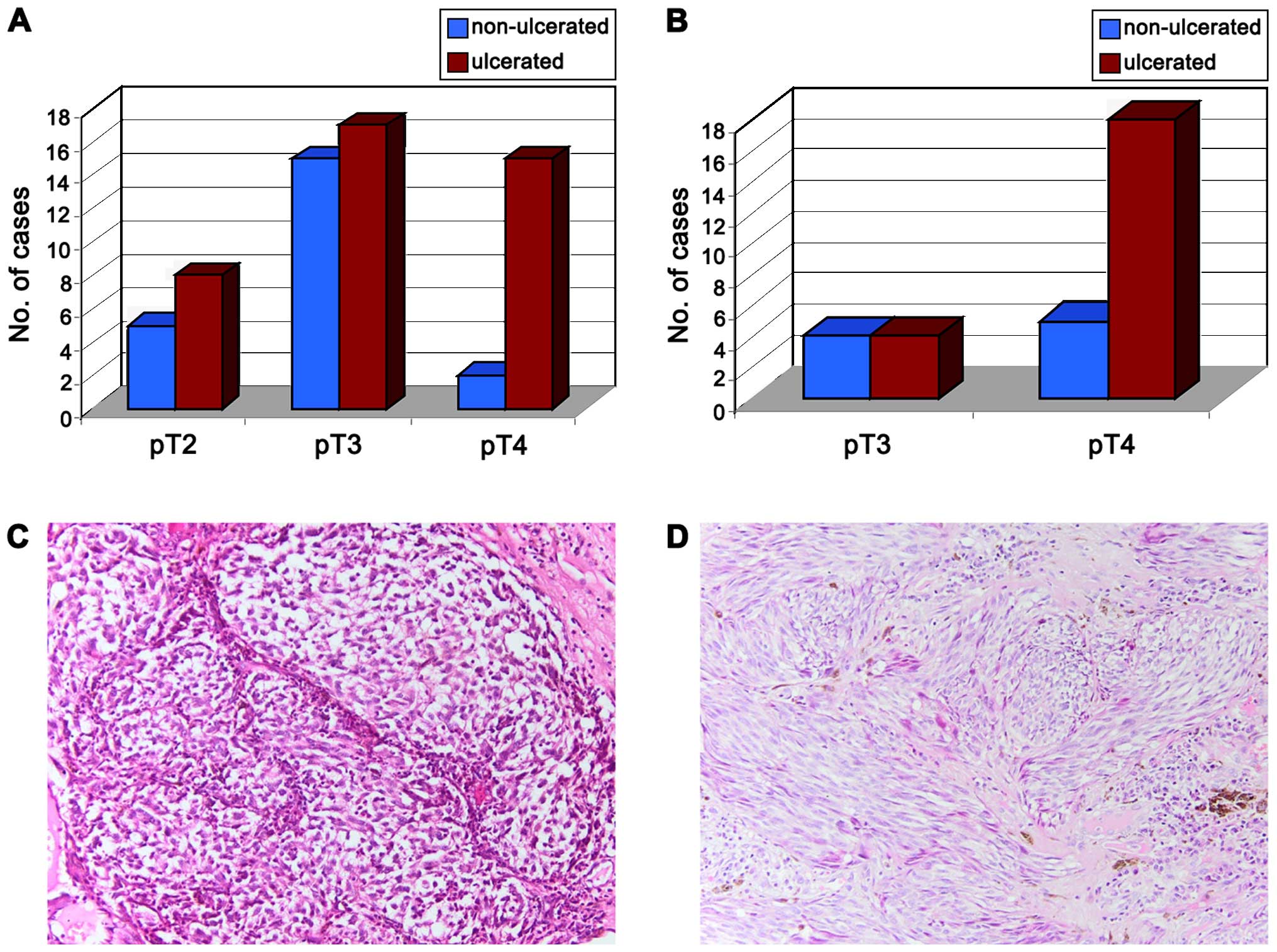
In total, we analyzed a group of 93 melanoma
specimens including 62 SSMs (66.66%) and 31 NMs (33.33%). The
majority of the tumors were at the pT4 stage (86.02%) and/or
ulcerated (66.67%). In both tumor types, the majority of cases were
at the pT3 and pT4 stage (79.03% of SSMs and all cases of NMs)
(Fig. 1).
Regression was present in the SSM cases; 39 SSMs
(62.90%) presented regression: 13 cases (33.33%) of SR, 17 cases
(43.58%) of PR and 9 cases (23.07%) with areas of SR-PR in
different parts of the tumor.
TIMP expression in the NRC component
compared with the AR cases
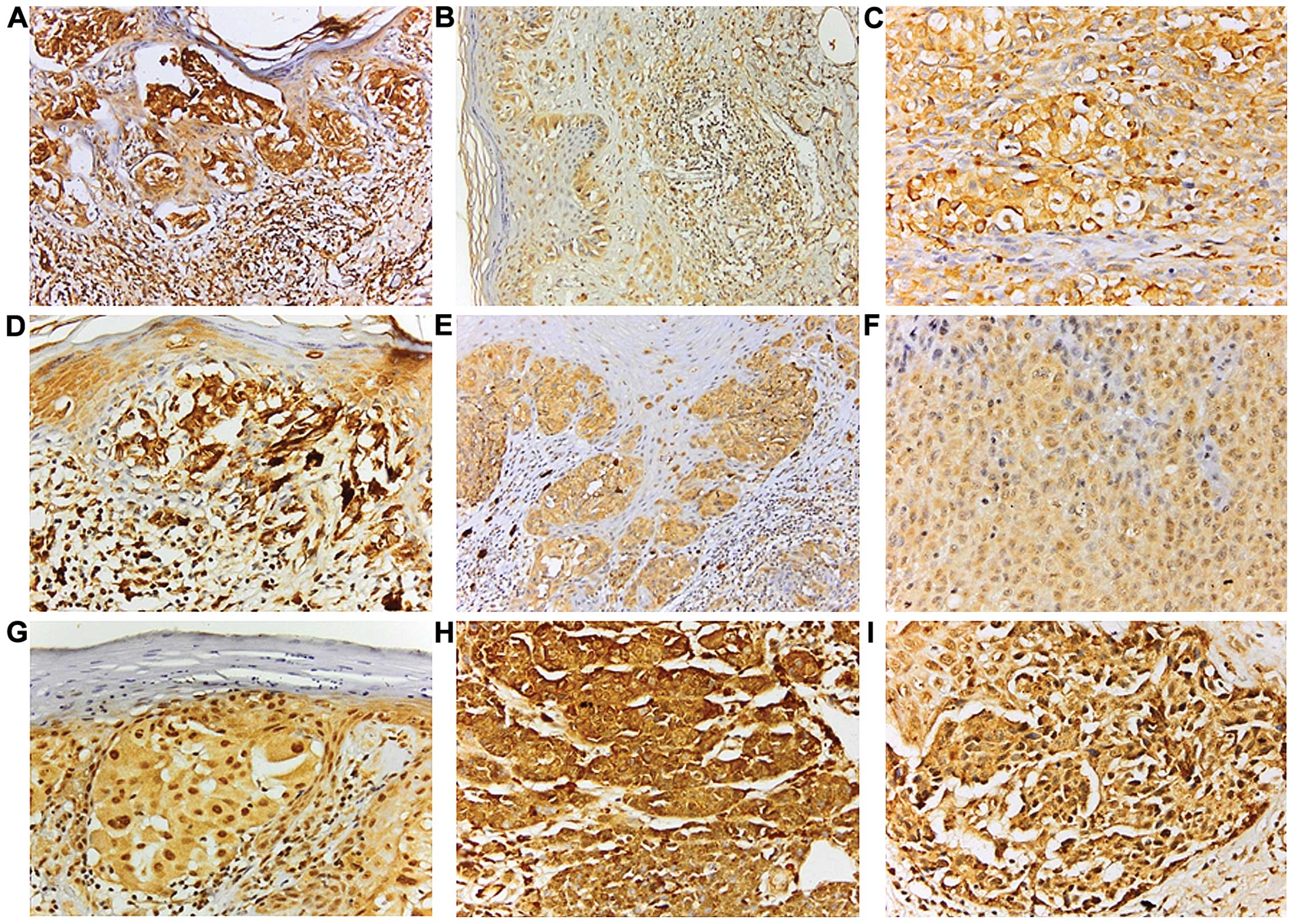
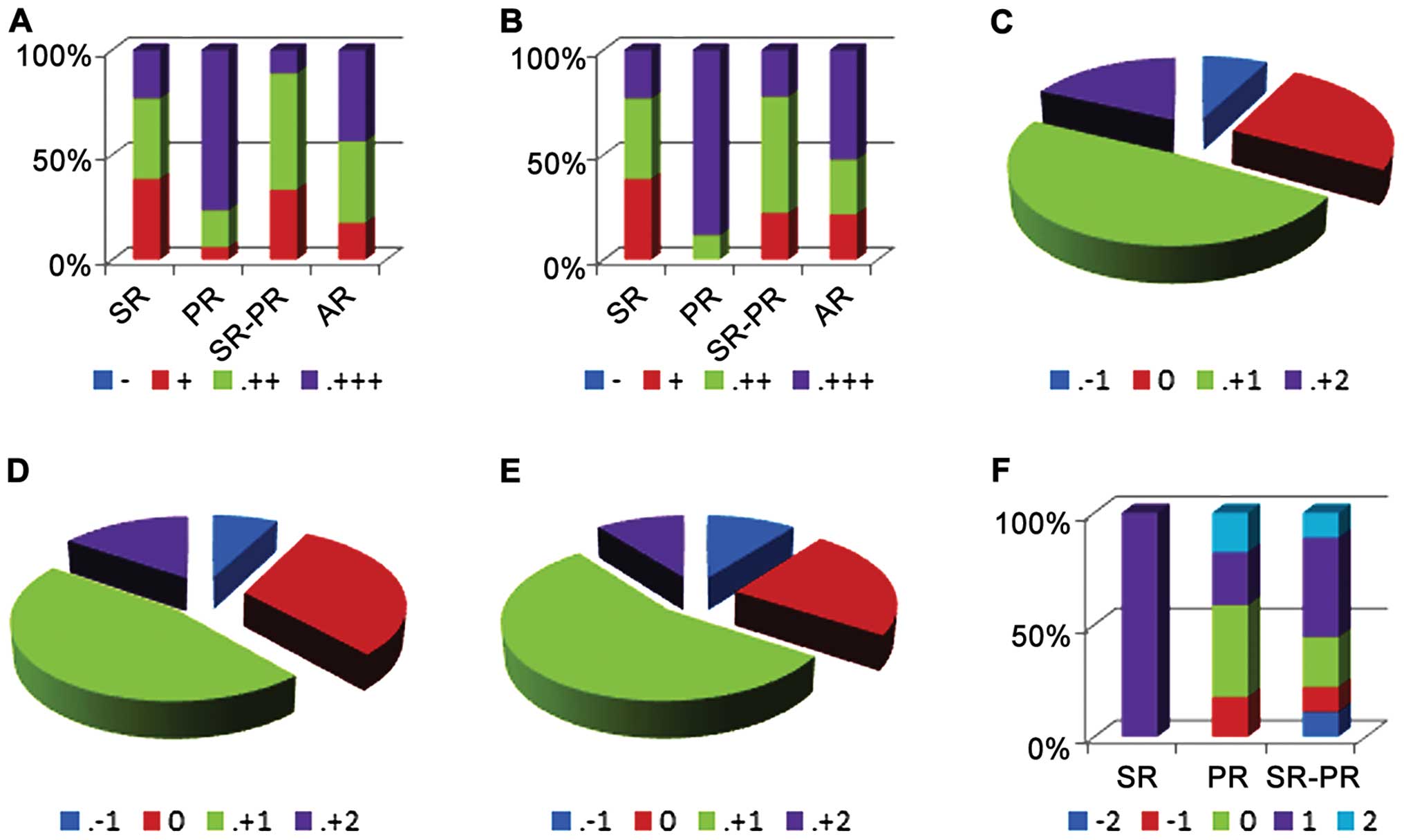
The TIMP1, TIMP2 and TIMP3 expression levels were
similar in both the NRC and AR cases. There was obvious TIMP1
overexpression in PR cases [76.47% cases with PR were intensely
positive (+++) for TIMP1 compared to 23.07% of SR cases that had
the same feature (PR/SR, P=0.011); Figs.
2A–C and 3A]. In additoin, the PR
cases exhibited intense positivity for TIMP2 as compared to cases
with other types of regression or AR cases (PR/SR, P=0.009;
PR/SR-PR, P=0.002; PR/AR, P=0.037; Figs.
2D–F and 3B]. No differences were
noted in TIMP3 expression among the different types of regression
(Fig. 2G–I).
TIMPS expression in regressed and
non-regressed areas in the same tumor
All TIMPs had a similar pattern of expression in
melanoma with regression: some cases exhibited a loss of expression
of TIMPs in the NRC versus the RC (TIMP1 and TIMP2, 7.69% of cases;
TIMP3, 12.82% of cases), some presented with the overexpression of
TIMPs in the NRC versus the RC (TIMP1, 66,66%; TIMP2, 61.53%;
TIMP3, 64.10%), whereas almost a quarter of the cases had similar
TIMP expression (TIMP1, 25.41%; TIMP2, 30.76%; TIMP3, 23.07%)
(Fig. 3C and E). There were no
differences observed between the NRC and RC in TIMP1 or TIMP2
expression according to the type of regression; TIMP3 was
overexpressed in all SR cases when comparing the NRC to the RC
component (P=0.007; Fig. 3F).
Discussion
ECM remodeling has been shown to closely correlate
with the progression of melanoma (22,27).
Numerous biomolecules are involved in this process with TIMPs being
one of the key regulators. The functions of TIMPs are complex as
they participate in the progression of melanoma at several levels.
Thus, TIMPs participate in the regulation of MMP activity (28–30). This
function is perpetrated by both the N-terminal and C-terminal
domain of TIMPs; the N-terminal domain binds to the active site of
MMPs, thus preventing the access of substrates to the catalytic
site of MMP, while the C-terminal domain binds to the
hemopexin-like domain of pro-MMP-9 and pro-MMP-2 (31). TIMPs are involved in the cell adhesion
process and in the subsequent modulation of cell growth through
several mechanisms, including the regulation of cytoskeletal
organization, the direct interaction with cell adhesion molecules
or regulating the expression of specific ECM components (32).
TIMPs exhibit anti-angiogenic activities by
inhibiting MMP-dependent angiogenesis. TIMP3 affects angiogenesis
through the direct regulation of vascular endothelial growth factor
receptor-2 (VEGFR2) activity and the inhibition of VEGF-A mitogenic
effects, whereas the effects of TIMP2 are more complex, as it
inhibits both endothelial cell growth and the migration of these
cells (33,34). The latter is accomplished through
TIMP2 interaction with α3β receptor or by enhancing the
reversion-inducing-cystein-rich protein with Kazal motif (RECK)
expression, which inhibits several downstream biomolecules, such as
MMP-2, MMP-9, MT1-MMP, ADAM10, the final effect being the loss of
cell migration (33,34). TIMPs have also been reported to
modulate apoptosis with different outcomes, either exhibiting
pro-apoptotic activity [TIMP3 through the stabilization of tumor
necrosis factor receptor 1 (TNFR-I) and Fas] or anti-apoptotic
activity (TIMP1 and TIMP2) (33,34).
Importantly, TIMP expression has been shown to be associated with
other pathological and toxicological conditions (35,36).
We have previously investigated MMP-1, MMP-2, MMP-3,
MMP-9, MMP-11 and MMP-13 expression in melanomas with and without
regression (6). Indeed, the
differential expression of the MMPs, MMP-1 and MMP-11, in
non-regressed tumors as compared to melanoma without regression,
with a possible improvement in prognosis, for the former group of
cases, was reported (6).
In the present study, we examined TIMP1, TIMP2 and
TIMP3 expression in regressed versus non-regressed melanoma. We
failed to identify differences in the expression of the 3 TIMPs
(TIMP1, TIMP2 and TIMP3) between the NRC and AR or between the RC
and NRC. However, interesting results were obtained when particular
forms of regression were analyzed. Our examination of the
morphological features of melanoma regression suggest that the
destruction of tumor cells in melanoma regression may present
different histological phenotypes, indicating a different spectrum
of alteration, with SR seeming to bestow a more favorable potential
(6).
In the present study, TIMP3 was overexpressed in all
SR cases in the NRC versus the RC tumor segments. Of note, a recent
study on metastatic lymph node melanoma demonstrated an inverse
negative correlation of TIMP3 expression with intratumor vessel
density (37). Das et al
(37) thus concluded that ‘TIMP3 gene
silencing by promoter methylation is associated with a poor
outcome’. Moreover, TIMP3 gene promoter methylation has been shown
to positively correlate with melanoma brain metastases, incurring a
poor prognosis (poor disease-free survival and overall survival)
(38).
Our present findings however, demonstrate that PR
cases overexpressed TIMP2 in the NRC more often than AR or in the
NRC of melanomas with other types of regression. A recently
published study, in line with the above, demonstrated that the
overexpression of TIMP2 suppressed the proliferation of melanoma
cell lines by inhibiting the activation of the Wnt/β-catenin
pathway (39). In this study, TIMP1
was overexpressed in the NRC of PR cases as compared to AR cases.
This finding is difficult to evaluate, since the role of TIMP1 in
carcinogenesis is unclear. Since TIMP1 negatively regulates the
activity of several MMPs involved in ECM degradation, it is likely
that it inhibits tumor development and progression. In some tumors,
TIMP1 expression correlates with a less aggressive tumor behavior
and, conversely, factors that downregulate TIMP1 are associated
with an unfavorable prognosis (poor overall survival and short
disease-free interval) (32,40).
However, TIMP1 may also promote tumor progression by
intervening in several key cellular processes, including apoptosis
(anti-apoptotic activity) and anoikis resistance (41,42)
Specifically, the activation of the PI3K pathway resulting in the
assembly of a supramolecular complex containing TIMP1, CD63 and
β1-integrins, has been suggested (43). Several studies have correlated TIMP1
overexpression in melanoma with an unfavorable prognosis (31–33): some
authors suggest that increased serum TIMP1 levels correlate with a
poor prognosis in patients with unresectable stage melanoma
(43). Moreover, concomitant high
serum levels of TNFR-II, transforming growth factor (TGF)-α, TIMP1
and C-reactive protein (CRP) are associated with a poor outcome in
melanoma (44). Indeed, tumors
overexpressing TIMP1 progress more rapidly and have an increased
tendency towards metastases (45).
Particular types of regression may occur due to
different immunological mechanisms. Our previous studies on
inflammatory infiltrates in melanoma with regression suggest that
Langerhans cells are constantly associated with thinner tumors
(46), whereas the presence of
nodular infiltrates of Langerhans cells in areas of regression are
statistically associated with the presence of Langerhans cells in
the main tumor mass (47–49). Indeed, particular types of regression
(from an immunological point of view) may bestow a more favorable
prognosis than other forms of regression or AR.
In conclusion, further progress in understanding
cancer biology, as regards tumor cell proliferation, tumor
microenvironment and host response is in order (29,50,51).
However, differences in the biological behavior of tumors sharing
the same origin indicate, without equivoque, that the mechanisms
involved in tumorigenesis and progression are not yet completely
deciphered. Further studies are warranted, both to further analyze
the tumor omics, as well as to identify novel tumor biomarkers for
diagnosis and potential therapeutic targets.
In this study, we described the differences in the
expression of TIMP1, TIMP2 and TIMP3 in regressed and non-regressed
areas of melanoma with regression. TIMP3 was overexpressed in all
SR cases in the NRC as compared to the RC component. Moreover, a
tendency towards TIMP1 and TIMP2 overexpression in the NRC in
melanomas with PR as compared to AR cases was evident. These
findings support the hypothesis that the morphological differences
identified in the melanoma regression spectrum may correlate with
prognosis, thus explaining the controversial findings within the
literature concerning the biological and prognostic role of
regression.
Acknowledgements
The authors would like to thank the physicians from
the Colentina Hospital in Bucharest and the Dermato-Oncology
Excellence Centre in Bucharest for providing access to patients
diagnosed with cutaneous melanoma, as well as for their clinical
monitoring. This study was financially supported by the Sectorial
Operational Programme Human Resources Development, financed from
the European Social Fund and by the Romanian Government under the
contract nos. POSDRU/159/1.5/S/137390 and POSDRU/89/1.5/S/60746,
‘Carol Davila’ University of Medicine and Pharmacy, Bucharest for
Young Researchers grant no. 33891/2014 and the Executive Agency for
Higher Education, Research, Development and Innovation (UEFISCDI)
under the contract no. PN-II-PT-PCCA-2013-4-1407 (project no.
190).
References
|
1
|
Alquier-Bouffard A, Franck F,
Joubert-Zakeyh J, Barthélémy I, Mansard S, Ughetto S,
Aublet-Cuvelier B, Déchelotte PJ, Mondié JM, Souteyrand P and
D'incan M: Regression in primary cutaneous melanoma is not
predictive for sentinel lymph node micrometastasis. Ann Dermatol
Venereol. 134:521–525. 2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGovern VJ, Shaw HM and Milton GW:
Prognosis in patients with thin malignant melanoma: Influence of
regression. Histopathology. 7:673–680. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramova L, Slingluff CL Jr and Patterson
JW: Problems in the interpretation of apparent ‘radial growth
phase’ malignant melanomas that metastasize. J Cutan Pathol.
29:407–414. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zurac S, Negroiu G, Petrescu S, Andrei R,
Tebeica T, Popp C, Musţată R, Neagu M, Constantin C, Solovan C, et
al: Spectrum of morphologic alterations of regression in cutaneous
melanoma - potential for improving disease prognosis. Rom J Intern
Med. 50:145–153. 2012.PubMed/NCBI
|
|
6
|
Andrei R, Zurac S, Socoliuc C and
Staniceanu F: Variation in expression of metalloproteinases in
cutaneous melanoma with regression, possible indicator of tumor
heterogeneity. DermatoVenerol (Buc). 60:133–145. 2015.
|
|
7
|
Trau H, Kopf AW, Rigel DS, Levine J,
Rogers G, Levenstein M, Bart RS, Mintzis MM and Friedman RJ:
Regression in malignant melanoma. J Am Acad Dermatol. 8:363–368.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur C, Thomas RJ, Desai N, Green MA,
Lovell D, Powell BW and Cook MG: The correlation of regression in
primary melanoma with sentinel lymph node status. J Clin Pathol.
61:297–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw HM, Rivers JK, McCarthy SW and
McCarthy WH: Cutaneous melanomas exhibiting unusual biologic
behavior. World J Surg. 16:196–202. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guitart J, Lowe L, Piepkorn M, Prieto VG,
Rabkin MS, Ronan SG, Shea CR, Tron VA, White W and Barnhill RL:
Histological characteristics of metastasizing thin melanomas: A
case-control study of 43 cases. Arch Dermatol. 138:603–608. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blessing K and McLaren KM: Histological
regression in primary cutaneous melanoma: Recognition, prevalence
and significance. Histopathology. 20:315–322. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paladugu RR and Yonemoto RH: Biologic
behavior of thin malignant melanomas with regressive changes. Arch
Surg. 118:41–44. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oláh J, Gyulai R, Korom I, Varga E and
Dobozy A: Tumour regression predicts higher risk of sentinel node
involvement in thin cutaneous melanomas. Br J Dermatol.
149:662–663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Socrier Y, Lauwers-Cances V, Lamant L,
Garrido I, Lauwers F, Lopez R, Rochaix P, Chevreau C, Payoux P,
Viraben R, et al: Histological regression in primary melanoma: Not
a predictor of sentinel lymph node metastasis in a cohort of 397
patients. Br J Dermatol. 162:830–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fontaine D, Parkhill W, Greer W and Walsh
N: Partial regression of primary cutaneous melanoma: Is there an
association with sub-clinical sentinel lymph node metastasis? Am J
Dermatopathol. 25:371–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liszkay G, Orosz Z, Péley G, Csuka O,
Plótár V, Sinkovics I, Bánfalvi T, Fejõs Z, Gilde K and Kásler M:
Relationship between sentinel lymph node status and regression of
primary malignant melanoma. Melanoma Res. 15:509–513. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emanuel PO, Mannion M and Phelps RG:
Complete regression of primary malignant melanoma. Am J
Dermatopathol. 30:178–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
High WA, Stewart D, Wilbers CRH, Cockerell
CJ, Hoang MP and Fitzpatrick JE: Completely regressed primary
cutaneous malignant melanoma with nodal and/or visceral metastases:
A report of 5 cases and assessment of the literature and diagnostic
criteria. J Am Acad Dermatol. 53:89–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klebanoff CA, Acquavella N, Yu Z and
Restifo NP: Therapeutic cancer vaccines: Are we there yet? Immunol
Rev. 239:27–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faries MB and Morton DL: Therapeutic
vaccines for melanoma: Current status. BioDrugs. 19:247–260. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Kempen LC, van Muijen GN and Ruiter
DJ: Stromal responses in human primary melanoma of the skin. Front
Biosci. 10:2922–2931. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nikitovic D, Mytilinaiou M, Berdiaki A,
Karamanos NK and Tzanakakis GN: Heparan sulfate proteoglycans and
heparin regulate melanoma cell functions. Biochim Biophys Acta.
1840:2471–2481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chalkiadaki G, Nikitovic D, Berdiaki A,
Katonis P, Karamanos NK and Tzanakakis GN: Heparin plays a key
regulatory role via a p53/FAK-dependent signaling in melanoma cell
adhesion and migration. IUBMB Life. 63:109–119. 2011.PubMed/NCBI
|
|
24
|
Chalkiadaki G, Nikitovic D, Katonis P,
Berdiaki A, Tsatsakis A, Kotsikogianni I, Karamanos NK and
Tzanakakis GN: Low molecular weight heparin inhibits melanoma cell
adhesion and migration through a PKCa/JNK signaling pathway
inducing actin cytoskeleton changes. Cancer Lett. 312:235–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikitovic D, Assouti M, Sifaki M, Katonis
P, Krasagakis K, Karamanos NK and Tzanakakis GN: Chondroitin
sulfate and heparan sulfate-containing proteoglycans are both
partners and targets of basic fibroblast growth factor-mediated
proliferation in human metastatic melanoma cell lines. Int J
Biochem Cell Biol. 40:72–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sifaki M, Assouti M, Nikitovic D,
Krasagakis K, Karamanos NK and Tzanakakis GN: Lumican, a small
leucine-rich proteoglycan substituted with keratan sulfate chains
is expressed and secreted by human melanoma cells and not normal
melanocytes. IUBMB Life. 58:606–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moro N, Mauch C and Zigrino P:
Metalloproteinases in melanoma. Eur J Cell Biol. 93:23–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slootweg PJ and Zurac S: Prognostic and
predictive value of epithelial to mesenchymal transition-associated
markers in oral squamous cell carcinoma. Curr Proteomics.
10:218–227. 2013. View Article : Google Scholar
|
|
31
|
Duan JX, Rapti M, Tsigkou A and Lee MH:
Expanding the activity of tissue inhibitors of metalloproteinase
(TIMP)-1 against surface-anchored metalloproteinases by the
replacement of its C-terminal domain: Implications for anti-cancer
effects. PLoS One. 10:e01363842015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baker AH, Edwards DR and Murphy G:
Metalloproteinase inhibitors: Biological actions and therapeutic
opportunities. J Cell Sci. 115:3719–3727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kovatsi L, Batzios S, Nikolaou K, Fragou
D, Njau S, Tsatsakis A, Karakiulakis G and Papakonstantinou E:
Alterations in serum MMP and TIMP concentrations following chronic
heroin abuse. Toxicol Mech Methods. 23:377–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsarouhas K, Soufla G, Apostolakis S,
Zaravinos A, Panagiotou M, Khoury M, Hassoulas JA, Tsatsakis AM and
Spandidos DA: Transcriptional regulation of TIMPs in ascending
aorta aneurysms. Thromb Res. 126:399–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Das AM, Koljenović S, Oude Ophuis CM, van
der Klok T, Galjart B, Nigg AL, van Cappellen WA, Noordhoek Hegt V,
Dinjens WN, Atmodimedjo PN, et al: Association of TIMP3 expression
with vessel density, macrophage infiltration and prognosis in human
malignant melanoma. Eur J Cancer. 53:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gonzalez-Gomez P, Bello MJ, Alonso ME,
Amiñoso C, Lopez-Marin I, De Campos JM, Isla A, Gutierrez M and Rey
JA: Promoter methylation status of multiple genes in brain
metastases of solid tumors. Int J Mol Med. 13:93–98.
2004.PubMed/NCBI
|
|
39
|
Xia Y and Wu S: Tissue inhibitor of
metalloproteinase 2 inhibits activation of the β-catenin signaling
in melanoma cells. Cell Cycle. 14:1666–1674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wen X, Wu JQ, Peng W, Feng JF and Tang JH:
MicroRNA-377 predicts poor clinical outcome of gastric cancer and
induces tumorigenesis by targeting multiple tumor-suppressor genes.
Oncol Rep. 34:203–210. 2015.PubMed/NCBI
|
|
41
|
Toricelli M, Melo FH, Peres GB, Silva DC
and Jasiulionis MG: Timp1 interacts with beta-1 integrin and CD63
along melanoma genesis and confers anoikis resistance by activating
PI3-K signaling pathway independently of Akt phosphorylation. Mol
Cancer. 12:222013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chunhacha P, Sriuranpong V and
Chanvorachote P: Epithelial-mesenchymal transition mediates anoikis
resistance and enhances invasion in pleural effusion-derived human
lung cancer cells. Oncol Lett. 5:1043–1047. 2013.PubMed/NCBI
|
|
43
|
Kluger HM, Hoyt K, Bacchiocchi A, Mayer T,
Kirsch J, Kluger Y, Sznol M, Ariyan S, Molinaro A and Halaban R:
Plasma markers for identifying patients with metastatic melanoma.
Clin Cancer Res. 17:2417–2425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tarhini AA, Lin Y, Yeku O, LaFramboise WA,
Ashraf M, Sander C, Lee S and Kirkwood JM: A four-marker signature
of TNF-RII, TGF-α, TIMP-1 and CRP is prognostic of worse survival
in high-risk surgically resected melanoma. J Transl Med. 12:192014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ricca TI, Liang G, Suenaga AP, Han SW,
Jones PA and Jasiulionis MG: Tissue inhibitor of metalloproteinase
1 expression associated with gene demethylation confers anoikis
resistance in early phases of melanocyte malignant transformation.
Transl Oncol. 2:329–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zurac S, Negroiu G, Andrei R, Petrescu S,
Tebeica T, Petre M, Neagu M, Constantin C, Chitu V, Salavastru C,
et al: Inflammatory infiltrate in melanoma with regression as
prognostic parameter. Virchows Arch. 463:1272013.
|
|
47
|
Neagu M, Constantin C and Zurac S: Immune
parameters in the prognosis and therapy monitoring of cutaneous
melanoma patients: Experience, role, and limitations. BioMed Res
Int. 2013:1079402013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neagu M: The immune system - a hidden
treasure for biomarker discovery in cutaneous melanoma. Adv Clin
Chem. 58:89–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bulman A, Neagu M and Constantin C:
Immunomics in skin cancer - improvement in diagnosis, prognosis and
therapy monitoring. Curr Proteomics. 10:202–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Spandidos DA: A unified theory for the
development of cancer. Biosci Rep. 6:691–708. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Spandidos DA: The cancer story. Cancer
Biol Ther. 3:1184–1186. 2004. View Article : Google Scholar : PubMed/NCBI
|