Introduction
Bladder cancer is the fourth most common malignancy
in males and the ninth most common in females in the United States.
In total, 74,690 cases were diagnosed in 2014 (1). Although the cancer can be treated
effectively using transurethral resection (TUR), patients are at
risk of recurrence or progression to muscle-invasive disease or
metastasis (2,3).
Tumor biology and progression, and the therapeutic
response are affected by the tumor microenvironment (4,5), including
stromal cells, infiltrating leukocytes and blood vessels, which all
contribute to the tumor stroma (5).
Tumor-associated macrophages (TAMs) are one of the main components
of this tumor stroma, and contribute to the progression of a number
of cancer types (5,6). The induction of angiogenesis was
initially hypothesized to be via tumor cells themselves. However,
certain stromal components are likely to be involved in regulating
the behavior of tumors also (7,8). For
example, macrophages play a significant role in tumor angiogenesis
and inflammatory reactions (9,10). Taken
together, these observations suggest that TAMs are important for
tumor angiogenesis and invasion, and that they affect the
prognosis.
Macrophages can alter their phenotype depending on
environmental signals (11), and can
be divided into classically-activated macrophages (M1) and
alternatively-activated macrophages (M2) (12). M1-macrophages are pro-inflammatory and
tumoricidal, whereas M2-macrophages release anti-inflammatory
molecules, regulate tissue remodeling and reduce inflammation
(13). In the tumor microenvironment,
the majority of TAMs exhibit an M2-like phenotype (6), exert anti-inflammatory and protumor
effects, and facilitate tumor growth, angiogenesis,
immunosuppression and matrix remodeling (14). As such, a detailed assessment of
macrophage phenotypes in the tumor, stroma and microenvironment is
required in order to fully comprehend the manner in which M2
macrophages affect tumor behavior. Nevertheless, previous reports
have been based solely on the expression of cluster of
differentiation (CD)68, a macrophage lineage marker that does not
discriminate between the M1 and M2 phenotypes, thereby generating
bias with regard to resultant observations (15,16).
The current study evaluated the overall TAM
population (CD68; M1 and M2 macrophages) and the M2 phenotype
(based on CD163 expression) in the stroma and tumor tissue of
bladder cancer patients. The objective of the study was to
determine the association among TAMs (including CD163 expression),
microvessel counts (MVCs), pathological outcome, tumor grade and
invasiveness.
Materials and methods
Tissue samples
Surgical specimens were obtained from 21 patients
(19 males and 2 females; mean age, 74.0 years; range, 50–89 years)
with bladder cancer, including 17 non-muscle invasive cancers (Ta
and T1) and 4 invasive bladder cancers (T2), who underwent
transurethral resection at the Department of Urology, Tokyo
Metropolitan Hiroo Hospital (Tokyo, Japan). Written informed
consent was obtained from all the patients and all procedures used
in the present study were approved by the Ethical Committee of
Tokyo Metropolitan Hiroo Hospital. All tumors were transitional
cell carcinomas. None of the patients had received chemotherapy,
radiotherapy or any medication that may have otherwise affected the
macrophage count prior to surgery. The profiles of the patients
from whom tumor samples were obtained are summarized in Table I. Solitary tumors were present in 12
individuals and multiple tumors in 9 subjects. Primary and
recurrent tumors were exhibited in 12 and 9 individuals,
respectively. The cancer stage was determined on the basis of the
tumor-node-metastasis classification system [International Union
Against Cancer (17)]: 11 cases were
Ta, 6 were T1 and 4 were T2. The tumor was grade I in 2 patients,
grade II in 11 subjects and grade III in 8 individuals. A
pathologist evaluated the tissue samples pathologically (stage and
grade). Tumor recurrence was examined using computed tomography
scans, cystoscopy, urinary cytology and intravenous pyelography.
The mean follow-up period in the present study was 6.5 months
(range, 1–42 months).
 | Table I.Clinical and pathological features of
the 21 patients with bladder cancer. |
Table I.
Clinical and pathological features of
the 21 patients with bladder cancer.
| Characteristics | Value |
|---|
| Median age (range),
years | 74 (50–89) |
| Gender (male:female),
n | 19:2 |
| Pathological T stage,
n (%) |
|
| pTa | 11 (52.4) |
| pT1 | 6
(28.6) |
| pT2 | 4
(19.0) |
| Tumor grade, n
(%) |
|
| I | 2 (9.5) |
| II | 11 (52.4) |
| III | 8
(38.1) |
| No. of tumors
(%) |
|
| 1 | 12 (57.1) |
|
>1 | 9
(42.9) |
| Recurrence status,
n (%) |
|
|
Primary | 12 (57.1) |
|
Recurrent | 9
(42.9) |
| CIS presence, n
(%) |
|
|
None | 16 (76.2) |
|
Presence | 5
(23.8) |
| Recurrent after
TUR, n (%) |
|
|
None | 12 (57.1) |
|
Recurrence | 9
(42.9) |
| Treatment after
TUR, n (%) |
|
|
None | 11 (52.4) |
|
BCG | 5
(23.8) |
| 2nd
TUR | 1 (4.8) |
| Total
cystectomy | 4
(19.0) |
TAM count
Sections of the surgical specimens were stained with
hematoxylin and eosin for histomorphological evaluation. In the
bladder cancer samples, TAM and M2 macrophages were assessed using
monoclonal mouse anti-human CD68 (catalog no., M0876; clone, PG-M1;
Dako, Glostrup, Denmark) and monoclonal mouse anti-human CD163
(catalog no., CD163-L-CE; clone, 10D6; Novocastra™; Leica
Biosystems, Nusslock, Germany) antibodies at a dilution of 1:100 in
phosphate-buffered saline. Tissue sections were deparaffinized in
xylene and then rehydrated using graded alcohol solutions.
Endogenous peroxidase activity was blocked by immersing the slides
in 3% H2O2 for 10 min. Next, the tissues were
incubated in 0.125% trypsin at 37°C for 20 min, followed by
incubation with the primary CD68 and CD163 antibodies for 60 min at
room temperature. The tissues were then incubated with horseradish
peroxidase-conjugated secondary antibodies (Histofine®
Simple Stain Mouse MAX PO; Nichirei Biosciences Inc., Tokyo, Japan)
for 30 min at room temperature. After color development with
diaminobenzidine, the slides were counterstained with hematoxylin
and mounted using aqueous mounting media. The immunoglobulin
fraction of normal mouse serum was used as a negative control. For
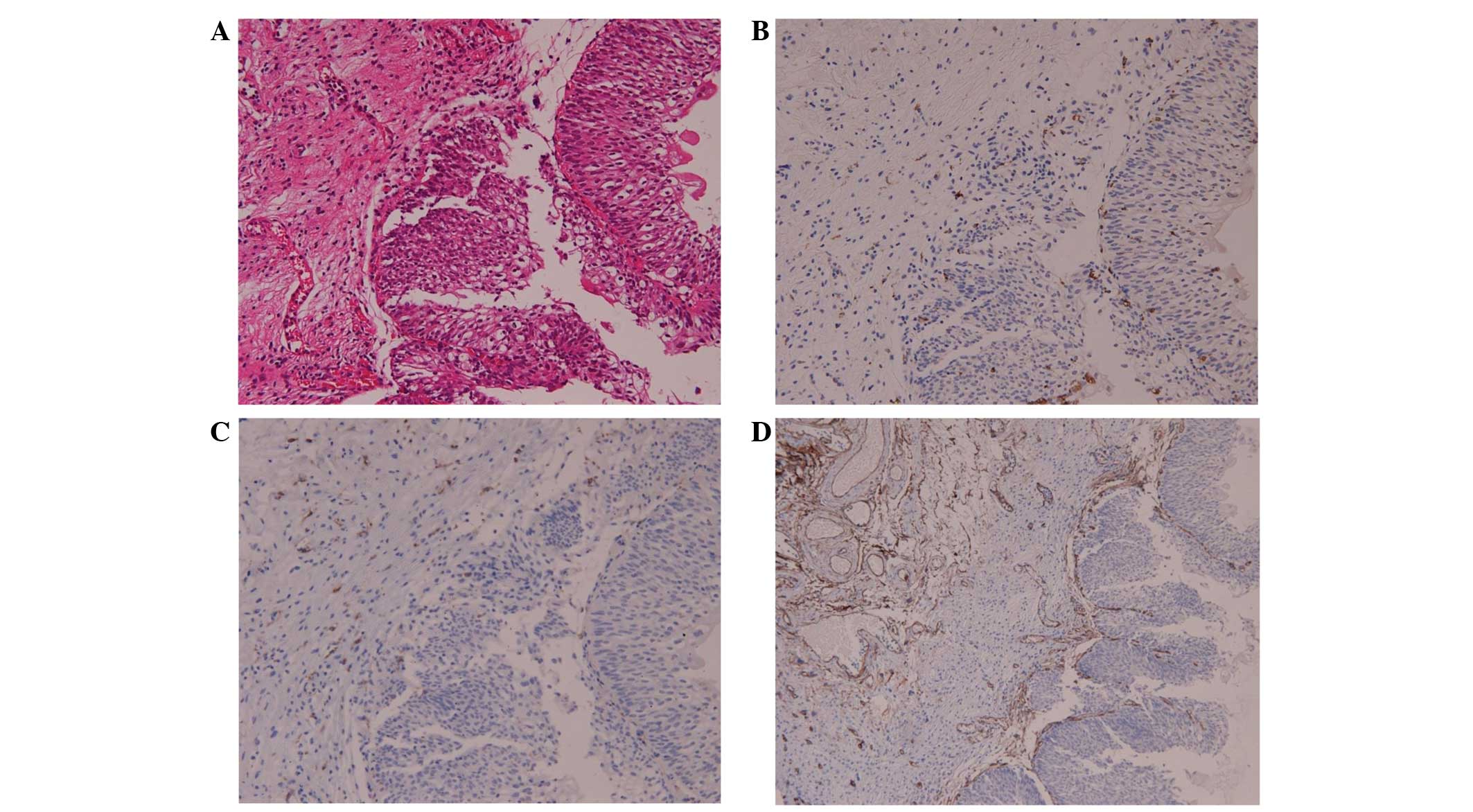
TAM counts, three areas with the highest density of macrophages
were analyzed at low magnification; macrophages were then counted
at ×200 magnification (20X objective and 10X ocular pieces)
(Fig. 1A).
MVCs
Tumor-associated angiogenesis was determined using
MVCs according to the method described by Weidner et al,
with slight modifications (18).
Anti-CD34 antibodies (monoclonal mouse anti-human CD34; clone QB
end 10; Dako) were specifically used rather than anti-factor VIII
antibodies to detect endothelial cells in the bladder tumors.
Vessels were counted in three areas of maximal neovascularization
(hot spots) in which the highest number of discrete microvessels
was stained within the tumor and the stroma at ×100 magnification
(Fig. 1B).
Statistical analysis
All statistical analyses were performed using
StatView (ver. 5.0; SAS Institute Inc., Cary, NC, USA).
Mann-Whitney U tests and Student's t-tests were used to evaluate
the correlations among TAM, MVCs and clinicopathological features,
including age, recurrence status, carcinoma in situ (CIS)
presence, recurrent after TUR and treatment after TUR. The
correlations among angiogenic factors, MVCs and TAMs were
determined by Spearman's rank correlation tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patterns of macrophage infiltration
and MVC
The present study first evaluated the localization
of macrophages within the tumor specimens. The patterns of
macrophage infiltration and MVCs according to TAM and CD34 counts
and ratios are shown in Table II.
CD68+ and CD163+ macrophages were present in
both the tumor stroma and tumor islets. The tumor stroma included
the papillary axis, lymphoid aggregates and the stroma. The mean
count of CD68+ macrophages was 110.0 in the stroma, 83.6
in the tumor and 193.7 in total, whereas the mean number of
CD163+ macrophages was 85.0 in the stroma, 48.1 in the
tumor and 133.1 in total. The mean ratio of
CD163+/CD68+ macrophages was 0.49 in the
tumor, 0.72 in the associated stroma and 0.66 in total. Next, the
microvessel density (MVD) within the tumor specimens was examined
by staining with anti-CD34 antibodies. CD34 was present in the
total tumor and its associated stroma; the mean CD34 count was 36.
The mean CD68+/CD34+ and
CD163+/CD34+ ratios in the tumor were 7.13
and 4.57.
 | Table II.Pattern of macrophage infiltration
and MVCs according to TAM (CD68 and CD163) and CD34 counts and
ratios. |
Table II.
Pattern of macrophage infiltration
and MVCs according to TAM (CD68 and CD163) and CD34 counts and
ratios.
| Factor | Count/ratio in the
tumor, mean (range) | Count/ratio in the
stroma, mean (range) | Total count/ratio,
mean (range) |
|---|
| CD68 | 83.6 (3–272) | 110.0 (23–482) | 193.6 (53–547) |
| CD163 | 48.1 (1–167) | 85.0 (3–407) | 133.1 (28–445) |
|
CD163+/68+ | 0.49
(0.10–0.89) | 0.72
(0.37–1.00) | 0.66
(0.25–0.95) |
| CD34 |
|
| 36.0 (8–87) |
|
CD68+/34+ |
|
| 7.13
(1.01–20.00) |
|
CD163+/34+ |
|
| 4.57
(0.93–13.13) |
Correlation among clinicopathological
characteristics and TAMs and MVCs in bladder cancer
Next, the correlation among the clinicopathological
characteristics and the CD68+ and CD163+
macrophages, and CD34+ microvessels, was assessed. The
correlations between clinical variables and macrophage counts are
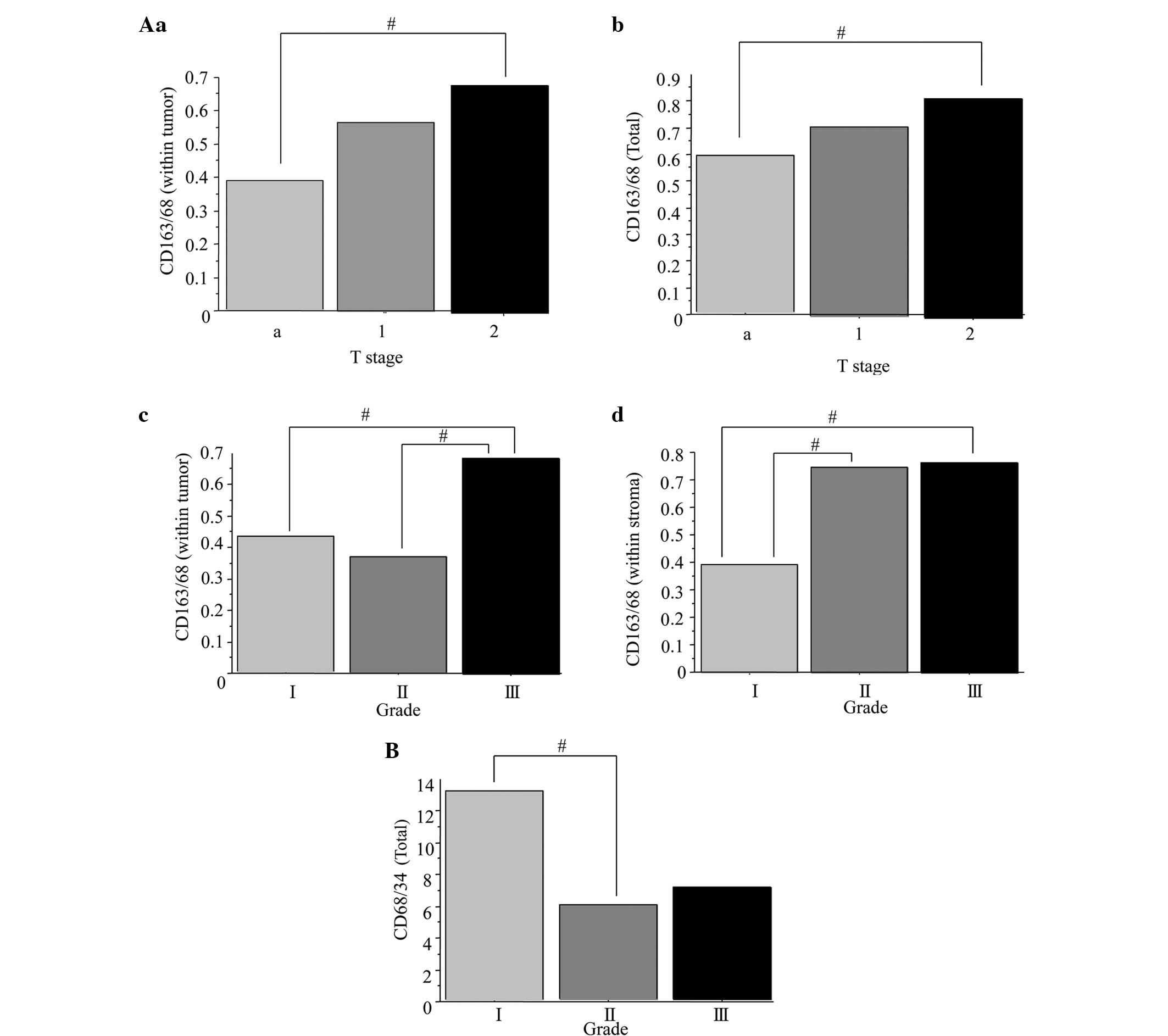
presented in Fig. 2. The mean
CD163+/CD68+ ratio in the stage T2 bladder
tumors (0.68) was significantly higher than that in the stage Ta
tumors (0.39) (Fig. 2Aa; P=0.0384).
The mean ratio of CD163+/CD68+ was
significantly higher in the total tumor tissues of the stage T2
bladder cancers (0.71) compared with that in the stage Ta cancers
(0.56) (Fig. 2Ab; P=0.0474). The mean
CD163+/CD68+ ratio was significantly higher
in the grade III bladder tumors (0.69) than in the grade I (0.43)
and II tumors (0.37) (Fig. 2Ac;
P=0.0320). The mean ratio of CD163+/CD68+
within the tumor stroma of grade I (0.39) bladder cancers was
significantly lower compared with that in the grade II (0.75) and
III (0.76) tumors (Fig. 2Ad;
P=0.0451). The mean CD68+/CD34+ ratio was
significantly higher in the total tumors of the grade I bladder
cancers (13.2) than in the grade II (5.8) specimens (Fig. 2B; P=0.0492). These results suggested
that the higher ratios of CD163+/CD68+
macrophages within the stroma, tumor and total tissues were
correlated with a higher tumor stage and grade. In addition, the
low CD68+/CD34+ microvessel ratio was
correlated with a higher tumor stage. Conversely, no correlations
were found between age, multifocality, recurrence status, CIS
presence, recurrence after TUR and treatment after TUR (data not
shown).
Correlation between TAM counts and
MVCs in bladder cancer
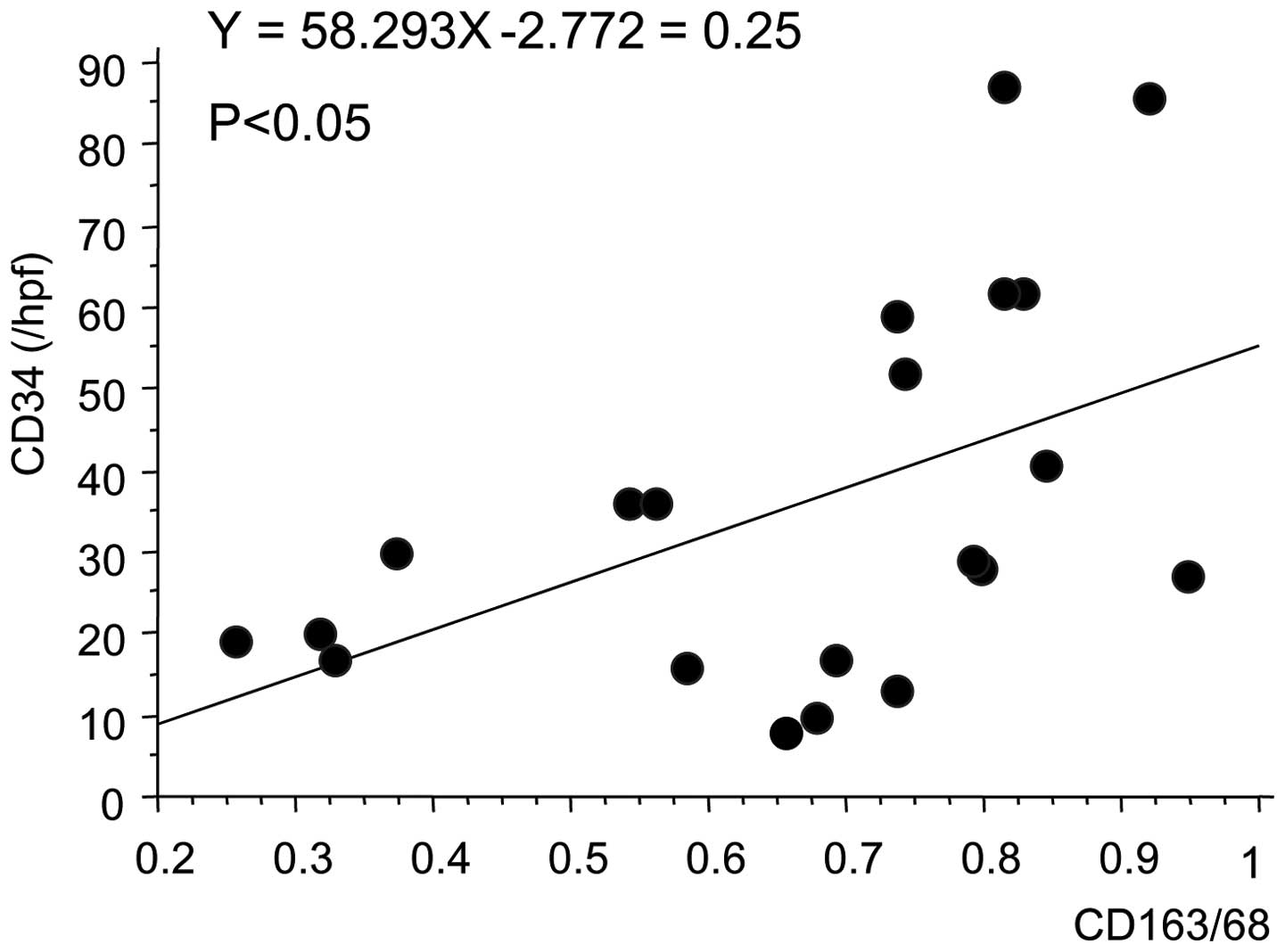
Finally, the correlation between TAM counts and MVCs
was examined using Spearman's correlation test. There was a
positive correlation (r2=0.25; P=0.0209) between TAM
counts (CD163+/CD68+ ratio) and MVCs (CD34
count) in bladder cancer, suggesting that TAM may be involved in
tumor angiogenesis in bladder cancers (Fig. 3; P=0.0209).
Discussion
The present study examined the correlations between
TAM and the predominance of M2-polarized macrophages in bladder
cancer, angiogenesis and clinicopathological features. It was
demonstrated that the density of M2-polarized macrophages was
significantly higher in invasive bladder cancers than in non-muscle
invasive cancers. In addition, the MVC distribution was higher in
higher grade bladder cancers, and the predominance of M2-polarized
macrophages correlated positively with MVCs in bladder cancer.
TAMs are a major component of tumor immune
infiltrates (18). A previous study
reported that there were more TAMs in invasive bladder cancers than
in non-muscle invasive cancers (19),
and that there was a positive correlation between TAMs and MVD.
These findings suggest that TAMs with an angiogenic or infiltrating
phenotype are differentially expressed in invasive or non-muscle
invasive bladder cancers. However, the process by which TAMs become
angiogenic or infiltrating is unclear. Hypoxia, a common process in
tumor tissues, may be one of the mechanisms that is involved in the
activation of TAMs. The migration of angiogenic factor-secreting
TAMs may be partially aided by reduced oxygen tension in tumor
tissues, which subsequently promotes tumor angiogenesis and
invasion. It is also possible that invasive bladder cancers
stimulate the deep migration of TAMs into tumors and the secretion
of a number of angiogenic factors.
However, previous findings have been based on the
analysis of CD68. TAMs assume an M2-like phenotype, which is
associated with tumor promotion (18,20).
Extensive TAM infiltration is typically associated with
angiogenesis and a poor prognosis in a variety of human cancers
(21–24). However, macrophages polarization
results in two distinct functional forms: M1 and M2 (5,6,25). Type 1 helper cells (Th1) are activated
by classical or M1 macrophages, and exhibit the ability to kill
pathogens and create interleukin (IL)-2, IL-12 and pro-inflammatory
cytokines for the promotion of responses such as cytotoxic T-cell
activation (25). By contrast, low
levels of IL-12 and high levels of IL-4 and IL-10 are expressed by
alternatively-activated M2 macrophages, promoting Th2 cytokines and
inhibiting Th1 responses (7).
However, multiple subtypes of M2-polarized macrophages are
associated with tumors, which may contribute to immunosuppression,
angiogenesis, cell invasion and metastasis depending on the
microenvironment (5,26). The identification of CD163+
macrophages has also been shown to be associated with a poor
prognosis in several types of cancer (27,28).
However, the current study is the first to suggest that the M2
subtype may be a characteristic of bladder tumors that are
invasive, with a high tumor grade and microvessels.
The current study also revealed a positive
correlation between TAM count and MVCs. This finding suggests that
TAMs have direct relevance to tumor angiogenesis through the
secretion of angiogenic factors in bladder cancer, although other
angiogenic factors from the tumor and stromal components may
exhibit a primary role in angiogenesis. It was previously reported
that tumor angiogenesis, determined according to MVD, was a
significant and independent prognostic indicator in patients with
breast and prostate cancer (29,30). In
addition, tumor angiogenesis (as determined using MVCs) was an
independent prognostic indicator in patients with invasive bladder
cancer (31). These findings support
the current conclusions, whereby TAM may be of value as a
prognostic factor in bladder cancer.
The limitations of the present study include the
small sample size and the fact that the study was retrospective and
observational, using only existing materials. Therefore the present
study did contain a comparison between healthy and cancerous
tissues. Accordingly, an interventional study should be performed,
using normal bladder tissue from patients as control samples, in
order to confirm the present findings.
The current study demonstrated an association
between the polarized M2 TAM phenotype and MVCs, and the
pathological outcome, tumor grade and invasiveness. Therefore, more
aggressive therapeutic strategies than TUR may be recommended in
patients who have a high M2 count and MVC. However, prior to
clinical use of this strategy, further studies with large
population sizes and healthy control groups are required in order
to determine the optimal treatment for such patients.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for reviewing the English language of
the original manuscript.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Rhijn BW, Burger M, Lotan Y, Solsona
E, Stief CG, Sylvester RJ, Witjes JA and Zlotta AR: Recurrence and
progression of disease in non-muscle-invasive bladder cancer: From
epidemiology to treatment strategy. Eur Urol. 56:430–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M: European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qian BZ and Pollard JW: Macrophage
diversity enhance stumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P and Mantovani
A: Macrophage polarization in tumor progression. Semin Cancer Biol.
18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J and Klagsbrun M: Angiogenic
factor. Science. 235:442–446. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
9
|
Sunderkötter C, Steinbrink K, Goebeler M,
Bhardwaj R and Sorg C: Macrophages and angiogenesis. J Leukoc Biol.
55:410–422. 1994.PubMed/NCBI
|
|
10
|
Leek RD, Harris AL and Lewis CE: Cytokine
network in solid human tumors regulation of angiogenesis. J Leukoc
Biol. 56:423–435. 1994.PubMed/NCBI
|
|
11
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murray PJ and Wynn TA: Obstacles and
opportunities for understanding macrophage polarization. J Leukoc
Biol. 89:557–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR
and Yang SM: Macrophages in tumor microenvironments and the
progression of tumors. Clin Dev Immunol. 2012:9480982012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang X: Tumor-associated macrophages as
potential diagnostic and prognostic biomarkers in breast cancer.
Cancer Lett. 332:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shabo I and Svanvik J: Expression of
macrophage antigens by tumor cells. Adv Exp Med Biol. 714:141–150.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours (UICC) (6th). Wiley-Blackwell.
Hoboken, NY: 2002.
|
|
18
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanada T, Nakagawa M, Emoto A, Nomura T,
Nasu N and Nomura Y: Prognostic value of tumor-associated
macrophage count in human bladder cancer. Int J Urol. 7:263–269.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siveen KS and Kuttan G: Role of
macrophages in tumor progression. Immunol Lett. 123:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Talmadge JE, Donkor M and Scholar E:
Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer
Metastasis Rev. 26:373–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koide N, Nishio A, Sato T, Sugiyama A and
Miyagawa S: Significance of macrophage chemoattractant protein-1
expression and macrophage infiltration in squamous cell carcinoma
of the esophagus. Am J Gastroenterol. 99:1667–1674. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lissbrant IF, Stattin P, Wikstrom P,
Damber JE, Egevad L and Bergh A: Tumor associated macrophages in
human prostate cancer: Relation to clinicopathological variables
and survival. Int J Oncol. 17:445–451. 2000.PubMed/NCBI
|
|
24
|
Ohno S, Ohno Y, Suzuki N, Kamei T, Koike
K, Inagawa H, Kohchi C, Soma G and Inoue M: Correlation of
histological localization of tumor-associated macrophages with
clinicopathological features in endometrial cancer. Anticancer Res.
24:3335–3342. 2004.PubMed/NCBI
|
|
25
|
Mantovani A and Locati M: Tumor-associated
macrophages as a paradigm of macrophage plasticity, diversity and
polarization: Lessons and open questions. Arterioscler Thromb Vasc
Biol. 33:1478–1483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shimura S, Yang G, Ebara S, Wheeler TM,
Frolov A and Thompson TC: Reduced infiltration of tumor-associated
macrophages in human prostate cancer: Association with cancer
progression. Cancer Res. 60:5857–5861. 2000.PubMed/NCBI
|
|
27
|
Medrek C, Pontén F, Jirström K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH,
Wang XZ, Zhao YW and Wei YQ: Prognostic significance of
tumor-associated macrophages in solid tumor: A meta-analysis of the
literature. PLoS One. 7:e509462012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostatic carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
31
|
Bochner BH, Cote RJ, Weidner N, Groshen S,
Chen SC, Skinner DG and Nichols PW: Angiogenesis in bladder cancer:
Relationship between microvessel density and tumor prognosis. J
Natl Cancer Inst. 87:1603–1612. 1995. View Article : Google Scholar : PubMed/NCBI
|