Introduction
Gastric cancer is a prevalent disease, with the
incidence rate being the fourth highest, and the mortality rate
being the second highest, amongst all types of human cancer
(1). The prognosis of advanced
gastric cancer remains poor, with median survival time currently
calculated at 7.9 months (2). The
presence of peritoneal carcinomatosis (PC) is a poor prognostic
factor in patients already suffering from advanced gastric cancer,
functioning as a primary cause of mortality in nearly half of all
patients (3). Until recently, no
standardized effective treatment for PC originating from gastric
origin had previously existed. Surgery alone has been demonstrated
to provide no substantial benefit for prognosis, with a median
survival time of 1 month (4).
Therefore, surgery alone is not typically recommended as a
treatment for patients with PC. Furthermore, systemic chemotherapy
has also been proven to be ineffective against PC, with a median
survival time of 6 months when used alone (5). Due to the effect of the peritoneal-blood
barrier, when cytotoxic or biological agents are administered
systemically, the concentration of such agents in the peritoneal
cavity is less than one-third to one-fourth of the concentration
observed in the blood and thus, numerous patients presenting with
PC do not respond to systemic chemotherapy (6).
Intraperitoneal administration can deliver a higher
concentration of cytotoxic or biological agents to the peritoneal
cavity, [up to several fold higher compared with intravenous
administration (7)], and can deliver
them directly to the tumor site, therefore decreasing harmful
systemic exposure. IPC is accepted as a standard treatment for
gynecological tumors, resulting in marked improvements in patient
survival (8). The efficacy of IPC in
PC of gastric origin has not yet been fully understood, due to the
rapid development of the disease and the short patient survival
times. However, IPC has been considered as a potential therapy for
PC (1,9), with studies demonstrating a median
survival time of 6.1–11.5 months after IPC treatment in patients
with PC originating from gastric cancer (3,10–12). Despite this, the median overall
survival (OS) time of patients receiving IPC treatment varies,
indicating that there may be confounding factors also affecting
survival. Certain studies have reported favorable outcomes for
patients who underwent a complete resection, including an improved
physical status and the absence of ascites (11,13,14). The
present study reviewed the use of IPC in treating PC originating
from gastric cancer, and aimed to identify any factors correlating
with the effect of IPC.
Materials and methods
Study population
Patients with PC originating from gastric cancer
received IPC treatment between June 2007 and October 2012, at the
Cancer Center, West China Hospital of Sichuan University (Chengdu,
China). All patients were confirmed to have synchronous peritoneal
metastasis of gastric cancer upon histopathological analysis of
surgery or biopsy specimens, or by analysis of the ascites cytology
at first diagnosis. Criteria for eligible patients were as follows:
Patients ≥18 years of age, who presented with an adenocarcinoma of
gastric origin [including signet ring cell (SRC) type] and who
demonstrated no visceral metastasis (except ovarian metastasis).
Patients had received at least one cycle of intraperitoneal
chemotherapy and demonstrated an Eastern Cooperative Oncology Group
(ECOG) score of 0–2 (15). Patients
were excluded if they had a previous history of malignant disease
or if they had previously received palliative chemotherapy.
Patients were also excluded if they demonstrated the occurrence of
peritonitis, intestinal obstruction, intestinal perforation or
uncontrolled infection prior to treatment.
Data collection
Data regarding age, gender, ascites, ECOG
performance status (PS) score, sites of primary disease, tumor
differentiation, tumor markers [carcinoembryonic antigen (CEA),
carbohydrate antigen 19–9 (CA19-9) and carbohydrate antigen 72–4
(CA72-4)] and stage of disease were collected. Symptomatic ascites
was defined as a volume of ≥500 ml identified during surgery or as
estimated by computed tomography scan. Data regarding surgery and
IPC were also collected. According to the surgical records, those
who did not receive cytoreductive surgery were classified as the
non-surgery group. Patients who had undergone complete resection of
primary gastric lesions and cytoreductive surgery of PC were
classified as the surgery group. Within the surgery group, complete
resection surgery (CCR-0) indicated no remains of macroscopic
residual cancer, whilst incomplete resection surgery (CCR-1)
indicated residual nodules of PC. OS was calculated from the
initial date of surgery, or first IPC, to mortality or the last
recorded date of follow-up.
Statistics analysis
Kaplan-Meier methods were used to estimate the
survival distributions stratified by pertinent clinical and
histopathological variables. The log-rank test was used to perform
univariate analysis of prognostic factors. Cox's proportional
hazards regression model was used to perform multivariate analysis
of prognostic predictors. P<0.05 was considered to indicate a
statistically significant difference. SPSS software, version 17
(SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Patients and clinical data
A total of 57 patients presenting with advanced
gastric cancer achieved the inclusion criteria, with a median age
of 51 years. The median follow-up time was 12.4 months (range, 1–56
months). A total of 3 patients received IPC treatment alone, whilst
54 patients received IPC treatment alongside systemic chemotherapy.
A total of 32 patients underwent cytoreductive surgery, with 11
achieving a CCR-0 resection and 21 achieving a CCR-1 resection. The
other 25 non-surgery patients received either IPC alone or combined
with systemic chemotherapy. Poorly-differentiated tumors were
demonstrated to be common (~60%), whilst SRC adenocarcinoma
accounted for 70.2% of all patients (40 patients). The median
number of cycles of IPC administered was 3 (range, 1–6), whilst the
median number of cycles of systemic chemotherapy administered was 5
(range, 1–27). A total of 20 patients (35.1%) presented with
symptomatic ascites at initial diagnosis (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Value |
|---|
| Total patients, n
(%) | 57 (100.0) |
| Gender ratio
(male:female), n | 29:28 |
| Median age (range),
years | 52.1 (21–80) |
| Tumor
differentiation, n (%) |
|
| High | 0 (0.0) |
|
Moderate | 6 (10.5) |
| Poor | 34 (59.7) |
| Not
reported | 17 (29.8) |
| Type of
adenocarcinoma, n (%) |
|
| nSRC | 17 (29.8) |
| SRC | 40 (70.2) |
| Symptomatic ascites,
n (%) |
|
| Yes | 20 (35.1) |
| No | 37 (64.9) |
| Performance status
(ECOG), n (%) |
|
| 0 | 2 (3.5) |
| 1 | 31 (54.4) |
| 2 | 24 (42.1) |
| Location of primary
tumor, n (%) |
|
|
Corpus | 26 (45.6) |
|
Atrium | 19 (33.3) |
|
Fundus | 10 (17.6) |
|
Unknown | 2 (3.5) |
| Status of surgery, n
(%) |
|
| Resected
(surgery) | 32 (56.1) |
|
CCR-0 | 11 (19.3) |
|
CCR-1 | 21 (36.8) |
|
Undissected (non-surgery) | 25 (43.9) |
| Systemic
chemotherapy, n (%) |
|
|
Paclitaxel-containing | 31 (54.4) |
|
Oxaliplatin-containing | 42 (73.7) |
|
Fluorouracil-containing | 37 (64.9) |
| IPC agents, n
(%) |
|
|
Cisplatin-containing | 43 (75.4) |
|
5-Fluorouracil-containing | 29 (50.9) |
OS
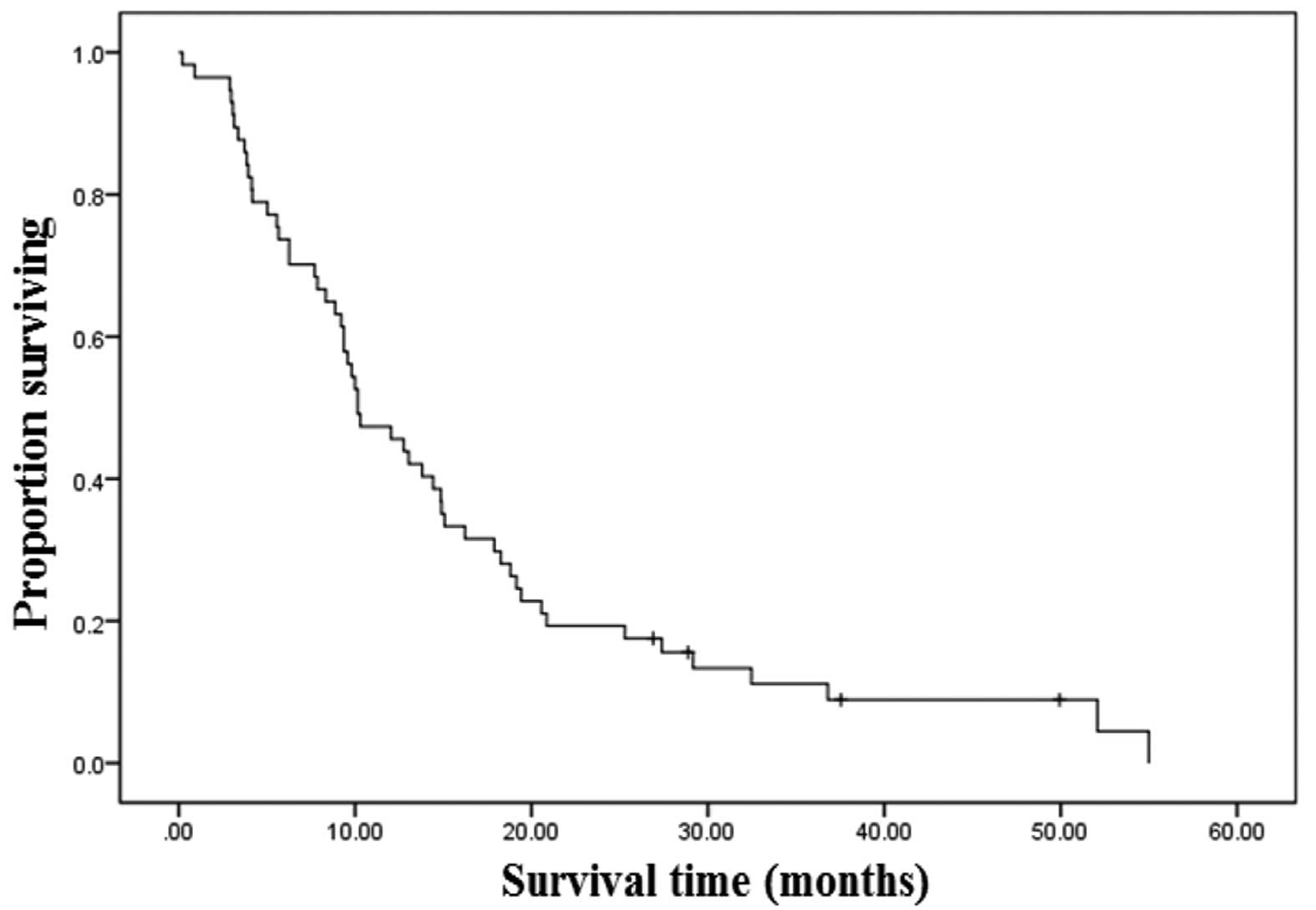
The median survival time for all 57 patients was
calculated as 10.1 months [95% confidence interval (CI), 6.8–13.5;
Fig. 1]. The OS rates at 1, 2 and 3
years were 46, 19 and 12%, respectively. In total, 4 of the 57
patients are currently alive without recurrence and had a median
follow-up time of 33 months when the study finished. A total of 7
patients were lost to follow-up, whilst 5 patients survived for
more than 3 years; all of these patients presented with no
symptomatic ascites and received on average 12.6 cycles of systemic
chemotherapy. Furthermore, 4 of these patients underwent a CCR-0
resection and only 1 demonstrated SRC adenocarcinoma.
Factors correlating with OS in
univariate analysis
A log-rank test was conducted to investigate the
possible prognostic factors of OS. A total of 16 factors were
analyzed, consisting of gender, primary disease site, ECOG PS
score, symptomatic ascites, elevated tumor markers (CEA, CA19-9 and
CA72-4), status of resection, histological subtype of
adenocarcinoma, cycles of systemic chemotherapy, systemic
chemotherapy containing oxaliplatin, fluorouracil or paclitaxel,
cycles of IPC and IPC procedure containing cisplatin or
5-fluorouracil. Factors that favored an increased rate of survival
(Table II) included an ECOG PS score
of 0–1 [P=0.02; hazard ratio (HR), 1.91; 95% CI, 1.09–3.35], no
symptomatic ascites at primary diagnosis (P=0.005; HR, 2.86; 95%
CI, 1.60–5.10), complete resection of all gross disease (P=0.001;
HR, 2.74; 95% CI, 1.79–4.22), non-schistosomal rectal cancer (nSRC)
adenocarcinoma (P=0.037; HR, 1.96; 95% CI, 1.03–3.72), ≥4 cycles of
systemic chemotherapy (P=0.001; HR, 1.67; 95% CI, 0.15–0.48) and
systemic chemotherapy containing oxaliplatin (P=0.01; HR, 2.70; 95%
CI, 1.43–5.09) and fluorouracil (P=0.006; HR, 2.24; 95% CI,
1.24–4.06). Systemic chemotherapy containing paclitaxel (P=0.07;
HR, 1.70; 95% CI, 0.96–3.00) was demonstrated to be numerically,
but not statistically different. Gender, primary disease site,
elevated tumor markers (CEA, CA19-9 and CA72-4), cycles of IPC and
IPC procedure containing cisplatin or 5-fluorouracil were not
significantly associated with OS.
 | Table II.Survival by univariate analysis. |
Table II.
Survival by univariate analysis.
|
|
|
| Years, n (%) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | n | Median survival
time, months | 0.5 | 1 | 2 | 3 | Hazard ratio | 95% CI | P-value |
|---|
| PS score |
|
|
|
|
|
| 1.91 | 1.09–3.35 | 0.02 |
|
0–1 | 33 | 12±2.3 | 28 (84.8) | 16 (48.5) | 10 (30.3) | 4 (12.1) |
|
|
|
| 2 | 24 | 9.2±2.8 | 14 (58.3) | 10 (41.7) | 1 (4.2) | 1 (4.2) |
|
|
|
| Resection
status |
|
|
|
|
|
| 2.74 | 1.79–4.22 | 0.001 |
|
Surgery | 32 | 14.9±1.3 | 29 (90.6) | 22 (68.8) | 11 (34.3) | 5 (15.6) |
|
|
|
|
CCR-0 | 11 | 32.4±10.9 | 11 (100.0) | 9 (81.8) | 6 (54.5) | 4 (36.4) |
|
|
|
|
CCR-1 | 21 | 13.8±1.3 | 18 (85.7) | 13 (61.9) | 5 (23.8) | 1 (4.76) |
|
|
|
|
Non-surgery | 25 | 6.3±2.3 | 13 (52.0) | 4 (16.0) | 0 (0.0) | 0 (0.0) |
|
|
|
| Symptomatic
ascites |
|
|
|
|
|
| 2.86 | 1.60–5.10 | 0.005 |
|
Yes | 20 | 4.2±2.4 | 10 (50.0) | 3 (15.0) | 1 (5.0) | 0 (0.0) |
|
|
|
| No | 37 | 14.9±1.9 | 32 (86.5) | 23 (62.2) | 10 (27.0) | 5 (13.5) |
|
|
|
| Type of
adenocarcinoma |
|
|
|
|
|
| 1.96 | 1.03–3.72 | 0.037 |
|
SRC | 40 | 9.8±0.6 | 29 (72.5) | 17 (42.5) | 5 (12.5) | 1 (2.5) |
|
|
|
|
nSRC | 17 | 17.9±5.6 | 13 (76.5) | 9 (52.9) | 6 (35.3) | 4 (23.5) |
|
|
|
| Systemic
chemotherapy |
|
|
|
|
|
|
|
|
|
|
Oxaliplatin-containing |
|
|
|
|
|
| 2.70 | 1.43–5.09 | 0.01 |
|
Yes | 42 | 12.8±1.8 | 35 (83.3) | 23 (54.8) | 11 (26.2) | 5 (11.9) |
|
|
|
|
No | 15 | 5.6±1.5 | 7 (46.7) | 4 (26.7) | 0 (0.0) | 0 (0.0) |
|
|
|
|
Fluorouracil-containing |
|
|
|
|
|
| 2.24 | 1.24–4.06 | 0.006 |
|
Yes | 37 | 12.8±2.2 | 28 (75.7) | 19 (51.4) | 10 (27.0) | 5 (13.5) |
|
|
|
|
No | 20 | 8.3±3.9 | 11(55.0) | 7 (35.0) | 1 (5.0) | 0 (0.0) |
|
|
|
| Cycles
of systemic chemotherapy |
|
|
|
|
|
| 1.67 | 0.15–0.48 | 0.001 |
|
≥4 | 29 | 17.9±3.6 | 29 (100.0) | 20 (69.0) | 10 (34.5) | 4 (13.8) |
|
|
|
|
<4 | 28 | 5.6±1.4 | 13 (46.4) | 6 (21.4) | 1 (3.6) | 1 (3.6) |
|
|
|
Factors correlating with OS on
multivariate analysis
A multivariate Cox regression was conducted to
determine independent predictors of OS. Only four factors were
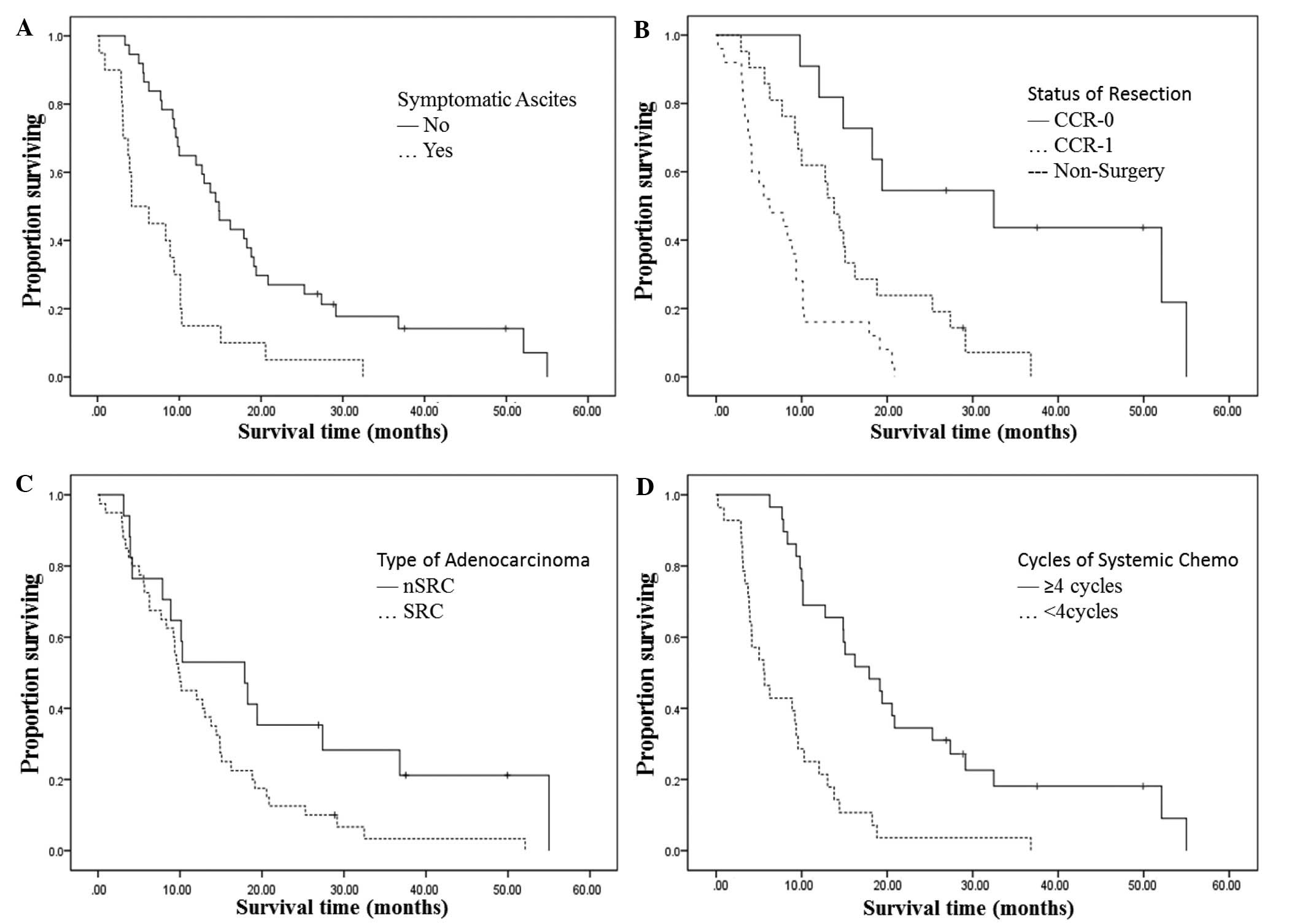
demonstrated to be statistically different. Patients with no
symptomatic ascites at surgery or primary diagnosis experienced a
median OS time of 14.9±1.9 months compared with 4.2±2.4 months for
patients who did present with symptomatic ascites (P=0.003; HR,
3.89; 95% CI, 1.58–9.64). The median OS time was 14.9±1.3 months
for patients who underwent surgery, and 6.3±2.3 months for those
who did not undergo surgery (P=0.010; HR, 3.49; 95% CI, 1.34–9.04).
For patients with a CCR-0 resection, the median survival time was
32.5 months in comparison to 13.8±1.3 months for those with a CCR-1
resection. Patients with nSRC adenocarcinoma experienced a median
OS time of 17.9±5.6 months, which was longer than the 9.8±0.6
months observed in patients with SRC adenocarcinoma (P=0.024; HR,
2.94, 95% CI, 1.15–7.47). Those individuals who received ≥4 cycles
of systemic chemotherapy experienced a median OS time of 17.9±3.6
months, while patients who had received <4 cycles of systemic
chemotherapy only experienced a median OS time of 5.6±1.4 months
(P=0.001; HR, 0.13; 95% CI, 0.06–0.32) (Table III and Fig. 2).
 | Table III.Independent predictive
clinicopathologic factors in Cox's regression. |
Table III.
Independent predictive
clinicopathologic factors in Cox's regression.
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|---|
| Parameters | Hazard ratio | Lower | Upper | P-value |
|---|
| Symptomatic ascites
(yes vs. no) | 3.89 | 1.58 | 9.64 | 0.003 |
| Status of resection
(CCR-1 vs. CCR-0) | 3.49 | 1.34 | 9.04 | 0.010 |
| Type of
adenocarcinoma (SRC vs. nSRC) | 2.94 | 1.15 | 7.47 | 0.024 |
| Cycles of systemic
chemotherapy (≥4 vs. <4) | 0.13 | 0.06 | 0.32 | 0.001 |
Adverse effects
The grade 3 to 4 treatment-related adverse effects
morbidity rate was 22.8% (13 patients). Severe bone marrow
suppression was noted in 8 patients (14.3%), severe nausea and
vomiting in 3 patients (5.3%), and severe diarrhea in 2 patients
(3.5%). Obstruction of the digestive tract was observed in 5
patients (8.8%) and all instances were considered as progression of
the disease rather than as the adverse effects of IPC.
Puncture-associated complaints were primarily mild distention and
abdominal pain. Peri-operative mortality (mortality during the 3
months after surgery) in the patients who underwent surgery was
3.1% (1 patient), with this patient succumbing 87 days after
surgery.
Discussion
PC originating from gastric cancer is a prevalent
disease, which occurs in 5–20% of patients being assessed for
potentially curative resection and accounts for more than half of
the mortalities in those patients (11,16).
Patients presenting with PC have a relatively short survival time,
yet there is not a standard treatment for the disease (12,17).
Currently, PC that originates from gastric cancer is typically
treated with the use of palliative systemic chemotherapy only,
however, the literature regarding the efficacy of this treatment
when used alone is limited, with a recorded median OS time of only
6 months (5). In the present study,
patients undergoing systematic chemotherapy, including a proportion
of patients who did not receive surgery (43.9%), demonstrated a
median OS time of 10.1 months. Although certain studies have
reported longer survival times, metachronism PC was also included
in those studies, possibly skewing the results (18,19);
however, in the current study, all patients presented with PC at
primary diagnosis or during surgery. Considering this, the median
OS time of 10.1 months established in the present study was a
relatively long survival time when compared with published data
from other studies investigating patients with PC originating from
gastric cancer.
In the present study, SRC adenocarcinoma was
confirmed as an independent factor associated with a reduced OS
time in PC patients who had been treated with IPC. Similar results
have been demonstrated in tumors with gynecological and colorectal
origins (20,21). In the present study, the median OS
time was 18.3 months for patients with nSRC adenocarcinoma compared
with 9.8 months for patients with SRC. SRC adenocarcinoma was
present in 70.2% of all patients in the current study, whilst only
accounting for 10.2–15.6% of all gastric cancer cases globally
(22,23). This could be explained by the idea
that SRC adenocarcinoma metastasizes earlier during development,
therefore causing PC to arise more frequently than nSRC
adenocarcinoma. SRC adenocarcinoma of the stomach is associated
with an improved prognosis when compared with nSRC adenocarcinoma
in early gastric cancer, however, it is associated with a poor
prognosis in comparison to nSRC adenocarcinoma at an advanced
disease stage (22,24). A further reason for the poor prognosis
associated with SRC may be that SRC adenocarcinoma appears to be
more resistant to currently used cytotoxic or biological agents,
with more frequent drug resistance-associated gene mutations
observed in SRC adenocarcinoma (25,26).
In the present study, resection status was one of
the most significant prognostic factors for survival (P<0.001)
as determined by multivariate analysis. The median OS time was
observed as 14.9±1.3 months for patients who underwent surgery, and
6.3±2.3 months for those who did not undergo surgery (P=0.010).
Similar results were reported by Scaringi et al (3), with a median survival time of 6.6 months
in patients with PC originating from gastric cancer who had been
treated by cytoreductive surgery and hyperthermia IPC. Glehen et
al (11) also reported a median
survival time of 9.2 months in 159 patients who had been treated by
cytoreductive surgery combined with perioperative IPC. The effect
of IPC treatment on survival when used alone in patients with PC
originating from gastric cancer has not yet been reported. In the
present study, the median OS time was only 6.3±2.3 months for those
with undissected primary tumors, similar to that observed in
patients with PC who received only systemic chemotherapy (median OS
time, 6 months) (5). The synergistic
effects of cytoreductive surgery to remove the macroscopic tumor
and of IPC to eradicate microscopic residual diseases are major
advantages of this combined approach. In the surgery group from the
current study, the median OS time for CCR-0 patients (32.4±10.9
months) was considerably higher than that demonstrated for the
CCR-1 patients (13.8±1.3 months). Similar results were reported by
Yonemura et al in a study of 107 patients; the median
survival time after complete cytoreduction was 15.5 months, and the
median survival time following incomplete cytoreduction was 7.9
months (12). Glehen et al
conducted an analysis of 159 patients from 15 institutions. For
patients treated by CCR-0 surgery, the median survival time was 15
months, compared with 6 or 4 months for CCR-1 or CCR-2 patients,
respectively (11). Even in patients
where a CCR-0 cannot be undertaken, a nearly complete resection or
CCR-1 can still achieve a longer survival time compared with that
of patients with large residual tumor nodules left (10). This implies that a more radical
surgery strategy may be taken into consideration. However, a
thorough and precise assessment prior to surgery should be
addressed (27).
Symptomatic ascites is an independent factor
associated with poor survival. In the present study, patients with
ascites at primary diagnosis or surgery experienced a median
survival time of 4.2 months, compared with 14.9 months for patients
without the presence of symptomatic ascites. Numerous studies have
reported a median survival time of 4–33 weeks for patients
presenting with symptomatic ascites (28). This therefore indicates that ascites
should be included in the regular evaluation prior to cytoreductive
surgery in order to make a fully informed judgment. Symptomatic
ascites often complicates PC and may severely impair quality of
life (29). However, despite the
improvement of survival in those patients being limited, IPC has
demonstrated a high effectiveness in symptom control, with it being
reported that >80% of ascites was controlled following
manipulation (30,31). This was observed typically following
the use of conventional treatments, including repeated
paracenteses, diuretics and systemic chemotherapy. However,
although they may initially be successful, these therapeutics
strategies lose their efficacy over time, so cannot be considered
as a long-term fix (29).
Systemic chemotherapy has been regarded as an
effective treatment strategy for patients with gastric cancer
undergoing multidisciplinary treatment. Partial and complete
responses following systemic chemotherapy for PC of gastric origin
have been reported (32,33). In the present study, 54 patients
(94.7%) received systemic chemotherapy. Those who had the chance to
receive ≥4 cycles of systemic chemotherapy experienced a median OS
time of 17.9±3.6 months. This was a significant improvement in
comparison to patients who received <4 cycles (5.6±1.4 months)
(P=0.001). As previously mentioned, systemic chemotherapy alone did
not appear to produce a significant improvement in the OS of
gastric cancer patients with PC (5).
Regarding the results of the current study, it is believed that on
the basis of local control of IPC, systemic chemotherapy may be an
important modality for survival benefits. The results of the
current study also indicate that the use of systemic chemotherapy
should not be ceased too early into treatment without the
observation of disease progression or evidence of resistance.
However, regarding the evidence that increased cycles of systemic
chemotherapy may be the consequence of a better response for
chemotherapy, further investigation is required to confirm its
effect.
It should be noted that the present study was
conducted with a small sample size, and the differing regimens of
chemotherapy and IPC agents have made the investigation difficult.
Despite this, to the best of our knowledge, the current study is
the first analysis that evaluates the effect of IPC on survival in
patients with PC originating from gastric cancer, as well as
evaluating the correlation of patient characteristics with OS. We
believe that the results from the present study may provide
important information that may aid in clinical practice for
patients with PC.
In conclusion, IPC is one of the most important
treatments administered to patients presenting with the peritoneal
spread of gastric cancer. A complete resection of all gross
disease, receiving an increased number of cycles of systemic
chemotherapy (≥4 cycles) and certain clinical pathological factors,
including nSRC adenocarcinoma and no symptomatic ascites, are all
advantageous factors for an extended OS time in PC patients.
References
|
1
|
Yan TD, Black D, Savady R and Sugarbaker
PH: Systematic review on the efficacy of cytoreductive surgery
combined with perioperative intraperitoneal chemotherapy for
peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol.
24:4011–4019. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gill RS, Al-Adra DP, Nagendran J, Campbell
S, Shi X, Haase E and Schiller D: Treatment of gastric cancer with
peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A
systematic review of survival, mortality, and morbidity. J Surg
Oncol. 104:692–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scaringi S, Kianmanesh R, Sabate JM,
Sabate JM, Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM,
Flamant Y and Msika S: Advanced gastric cancer with or without
peritoneal carcinomatosis treated with hyperthermic intraperitoneal
chemotherapy: A single western center experience. Eur J Surg Oncol.
34:1246–1252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in nongynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cotte E, Colomban O, Guitton J, Tranchand
B, Bakrin N, Gilly FN, Glehen O and Tod M: Population
pharmacokinetics and pharmacodynamics of cisplatinum during
hyperthermic intraperitoneal chemotherapy using a closed abdominal
procedure. J Clin Pharmacol. 51:9–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi CR, Mocellin S, Pilati P, Foletto M,
Quintieri L, Palatini P and Lise M: Pharmacokinetics of
intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am.
12:781–794. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chua TC, Robertson G, Liauw W, Farrell R,
Yan TD and Morris DL: Intraoperative hyperthermic intraperitoneal
chemotherapy after cytoreductive surgery in ovarian cancer
peritoneal carcinomatosis: Systematic review of current results. J
Cancer Res Clin Oncol. 135:1637–1645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esquivel J, Elias D, Baratti D, Kusamura S
and Deraco M: Consensus statement on the loco regional treatment of
colorectal cancer with peritoneal dissemination. J Surg Oncol.
98:263–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang XJ, Li Y and Yonemura Y:
Cytoreductive surgery plus hyperthermic intraperitoneal
chemotherapy to treat gastric cancer with ascites and/or peritoneal
carcinomatosis: Results from a Chinese center. J Surg Oncol.
101:457–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glehen O, Gilly FN, Arvieux Cotte E,
Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F and Elias
D: Association Française de Chirurgie: Peritoneal carcinomatosis
from gastric cancer: A multi-institutional study of 159 patients
treated by cytoreductive surgery combined with perioperative
intraperitoneal chemotherapy. Ann Surg Oncol. 17:2370–2377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yonemura Y, Kawamura T, Bandou E,
Takahashi S, Sawa T and Matsuki N: Treatment of peritoneal
dissemination from gastric cancer by peritonectomy and
chemohyperthermic peritoneal perfusion. Br J Surg. 92:370–375.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glehen O, Gilly FN, Boutitie F, Bereder
JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S and Elias
D: French Surgical Association: Toward curative treatment of
peritoneal carcinomatosis from nonovarian origin by cytoreductive
surgery combined with perioperative intraperitoneal chemotherapy: A
multi-institutional study of 1,290 patients. Cancer. 116:5608–5618.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen P, Hawksworth J, Lovato J, Loggie BW,
Geisinger KR, Fleming RA and Levine EA: Cytoreductive surgery and
intraperitoneal hyperthermic chemotherapy with mitomycin C for
peritoneal carcinomatosis from nonappendiceal colorectal carcinoma.
Ann Surg Oncol. 11:178–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoo CH, Noh SH, Shin DW, Choi SH and Min
JS: Recurrence following curative resection for gastric carcinoma.
Br J Surg. 87:236–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cashin PH, Graf W, Nygren P and Mahteme H:
Intraoperative hyperthermic versus postoperative normothermic
intraperitoneal chemotherapy for colonic peritoneal carcinomatosis:
A case-control study. Ann Oncol. 23:647–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cavaliere F, De Simone M, Virzi S, Deraco
M, Rossi CR, Garofalo A, Di Filippo F, Giannarelli D, Vaira M, et
al: Prognostic factors and oncologic outcome in 146 patients with
colorectal peritoneal carcinomatosis treated with cytoreductive
surgery combined with hyperthermic intraperitoneal chemotherapy:
Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol.
37:148–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen P, Levine EA, Hall J, Case D, Russell
G, Fleming R, McQuellon R, Geisinger KR and Loggie BW: Factors
predicting survival after intraperitoneal hyperthermic chemotherapy
with mitomycin C after cytoreductive surgery for patients with
peritoneal carcinomatosis. Arch Surg. 138:26–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao C, Yan TD, Black D and Morris DL: A
systematic review and meta-analysis of cytoreductive surgery with
perioperative intraperitoneal chemotherapy for peritoneal
carcinomatosis of colorectal origin. Ann Surg Oncol. 16:2152–2165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kunisaki C, Shimada H, Nomura M, Matsuda
G, Otsuka Y and Akiyama H: Therapeutic strategy for signet ring
cell carcinoma of the stomach. Br J Surg. 91:1319–1324. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otsuji E, Yamaguchi T, Sawai K and
Takahashi T: Characterization of signet ring cell carcinoma of the
stomach. J Surg Oncol. 67:216–220. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ,
Kim SK and Lee JH: Clinicopathological characteristics of signet
ring cell carcinoma of the stomach. ANZ J Surg. 74:1060–1064. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu PG and Weiss LM: Immunohistochemical
characterization of signet-ring cell carcinomas of the stomach,
breast, and colon. Am J Clin Pathol. 121:884–892. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakatsuru S, Yanagisawa A, Ichii S, Tahara
E, Kato Y, Nakamura Y and Horii A: Somatic mutation of the APC gene
in gastric cancer: Frequent mutations in very well differentiated
adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet.
1:559–563. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hall JJ, Loggie BW, Shen P, Beamer S,
Douglas Case L, McQuellon R, Geisinger KR and Levine EA:
Cytoreductive surgery with intraperitoneal hyperthermic
chemotherapy for advanced gastric cancer. J Gastrointest Surg.
8:454–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loggie BW, Fleming RA, McQuellon RP,
Russell GB and Geisinger KR: Cytoreductive surgery with
intraperitoneal hyperthermic chemotherapy for disseminated
peritoneal cancer of gastrointestinal origin. Am Surg. 66:561–568.
2000.PubMed/NCBI
|
|
29
|
Facchiano E, Scaringi S, Kianmanesh R,
Sabate JM, Castel B, Flamant Y, Coffin B and Msika S: Laparoscopic
hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment
of malignant ascites secondary to unresectable peritoneal
carcinomatosis from advanced gastric cancer. Eur J Surg Oncol.
34:154–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sayag AC, Gilly FN, Carry PY, Perdrix JP,
Panteix G, Brachet A, Banssillon V and Braillon G: Intraoperative
chemohyperthermia in the management of digestive cancers. A general
review of literature. Oncology. 50:333–337. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilly F, Carry P, Bracket A, Sayag AC,
Panteix G, Salle B, Bienvenu J, Banssillon V, Burgard G, Manchon M,
et al: Treatment of malignant peritoneal effusion in digestive and
ovarian cancer. Med Oncol Tumor Pharmacother. 9:177–181.
1992.PubMed/NCBI
|
|
32
|
Inokuchi M, Yamashita T, Yamada H, Kojima
K, Ichikawa W, Nihei Z, Kawano T and Sugihara K: Phase I/II study
of S-1 combined with irinotecan for metastatic advanced gastric
cancer. Br J Cancer. 94:1130–1135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yano M, Shiozaki H, Inoue M, Tamura S,
Doki Y, Yasuda T, Fujiwara Y, Tsujinaka T and Monden M: Neoadjuvant
chemotherapy followed by salvage surgery: Effect on survival of
patients with primary noncurative gastric cancer. World J Surg.
26:1155–1159. 2002. View Article : Google Scholar : PubMed/NCBI
|