Introduction
Breast cancer is the most common cancer detected in
Chinese women in the past 20 years; the incidence rate is rising,
and therefore the early detection, diagnosis and treatment are
important for the survival and life quality of these patients
(1–6).
When assessing the tumorigenic process of breast cancer, the World
Health Organization (WHO) histopathological grade is an important
indicator that can be used to evaluate the malignant behavior and
prognosis of breast cancer. However, histopathological grade can
often only be obtained following surgery, thus limiting its roles
in selecting the treatment options for breast cancer (7–10).
Magnetic resonance imaging (MRI) allows high soft tissue contrast,
multi-directions, multi-parameters and multi-functional imaging,
thus it may be used to estimate the lesion size, number, boundary
and internal structure more accurately than mammography and
ultrasound. Dynamic contrast enhanced MRI (DCE-MRI) is particularly
sensitive in revealing the morphological and hemodynamic features
of tumors, thus it has increasingly demonstrated its superiority in
the diagnosis of breast diseases (11–19). The
present study retrospectively analyzed the clinical data from
DCE-MRI of 92 patients, who were diagnosed with invasive breast
cancer using surgical resection or biopsy, with respect to the WHO
histopathological grade. The present study ultimately aimed to
realize a mechanism of the in vivo evaluation of biological
behavior and prognosis of breast cancer, thus facilitating the
development of treatment programs.
Materials and methods
Subjects
A total of 142 patients, who were diagnosed using
unilateral breast cancer by surgery or biopsy and who recieved
DCE-MRI in West China Hospital, Sichuan University, (Sichuan,
China) from June 2012 to December 2013, were collected, among which
were 127 cases of invasive ductal cancer, 92 cases of tumor-like
enhancement lesion, all females, aged 21 to 72 years old, with a
mean age of 47.15 years old. The patients did not receive any
clinical intervention prior to DCE-MRI examination, including
neoadjuvant chemotherapy, hormonal therapy or acupuncture. The
study was conducted in accordance with the Declaration of Helsinki
(20). This study was conducted with
approval from the Ethics Committee of Sichuan University. Written
informed consent was obtained from all participants.
MRI examination technique and
parameters
The Philips 3.0T MRI (Achieva, Phillips Medical
Systems, Netherlands) scanner was used, which was equipped with a
breast surface-dedicated phased array coil. The patient was placed
in the prone position against the dedicated phased array coil, the
breasts naturally hung in the cavity of coil, and remained still
during the scanning. The scanning sequence was as follows: i)
Fat-suppression T2WI sequence of fast inversion recovery fat
suppression sequence (SPAIR): TR 1900 ms, TE 120 ms, TI 150 ms; ii)
T1WI sequence of fast spin-echo imaging (TSE): TR 111 ms, TE 9 ms,
slice thickness 8 mm, with 20 layers; iii) dynamic contrast
enhanced scanning, used the fat suppression T1WI sequence of fast
spoiled gradient echo 3D imaging sequence (FLASH-3D): TR 4.2 ms, TE
2.1 ms, flip angle (FA) 100°, slice thickness 1.25 mm, 140 layers,
field of vision (FOV) 320×320 mm, matrix 336×336 pixels, each
scanning time 50.4 sec, and repeated 10 times; the high-pressure
syringe was used to inject Gd-DTPA 0.1 mmol/kg through the
hand-dorsal vein, with the flow rate as 2.5 ml/s; iv) post-DCE
high-resolution scanning, following DCE, the bilateral breasts were
examined with cross-sectional scanning using high-resolution
enhanced fat suppression T1WI sequences, TR 4.6 ms, TE 1.73 ms,
slice thickness 0.8 mm, FA 100, and the scanning time was 340
sec.
Image evaluation
The images were interpreted by two radiologists who
were blind to the results of surgical pathology. The MRI features
were described according to the American College of Radiology,
Breast Image-Reporting and Data System (ACR BI-RADS) (1). The number, location, size (expressed as
long diameter), shape, border and signal of the lesions were
recorded, in addition to the enhancement characteristics of early
lesions in DCE. Disagreement in features between the pathologists
were discussed in order to reach a consensus. MRI features
included: i) Tumor size. The delay-phase image was set as the
standard, the single lesion was expressed by its maximal diameter,
and the multiple lesions were expressed by the maximal diameter of
the largest lesion. Primary tumors (T) were divided by their sizes
according to the TNM staging of Union for International Cancer
Control (UICC) (21): ≤2 cm, 2~5 cm,
≥5 cm (4). ii) Gross shape: Round,
oval, lobulated and irregular. iii) Margin: Smooth, irregular and
spiculated. iv) Characteristics of internal enhancement:
Homogeneous enhancement, heterogeneous enhancement and ring-like
enhancement. v) Other signs: Accompanied with or without skin
thickening, nipple retraction, lymph node metastasis and clear
retromamary space.
WHO histopathological grade
All specimens were examined histologically with
hematoxylin-eosin (HE) staining, then evaluated for tumor ductal
shape, nuclear atypia and nuclear splitting number according to the
WHO histopathological grading method of invasive breast cancer
(9), which is divided into 3 grades:
Grade 1, well-differentiated; grade 2, moderately differentiated;
and grade 3, poorly differentiated.
Statistical analysis
SPSS statistical software, version 19.0 (IBM SPSS,
Armonk, NJ, USA) was used to perform statistical analysis. The
statistical method used was the χ2 test, with the
significance level set as α=0.05.
Results
Characteristics of lesion
distribution
Among the 92 patients, the lesion presented in the
right breast of 42 patients (45.65%), and 50 cases presented in the
left breast (53.35%), and 9 cases exhibited multiple lesions
(9.78%), while 83 cases exhibited a single lesion (90.22%).
DCE-MRI signs of lesions
Among the 92 patients, 29 cases presented with a
tumor diameter of ≤2.0 cm (31.52%), 53 cases were between 2~5 cm
(57.61%), and 10 cases were ≥5.0 cm (10.87%); 3 lesions were round
(3.26%), 7 cases were oval (7.61%), 33 cases were lobulated
(35.87%), and 49 cases were irregular (53.26%). 11 cases exhibited
the smooth margin (11.96%), 47 cases were irregular (51.09%), and
34 cases were spiculated (36.96%). A total of 15 cases exhibited
homogeneous enhancement of early lesions (16.30%), 40 cases
exhibited heterogeneous enhancement (43.48%), and 37 cases
exhibited ring-like enhancement (40.22%).
WHO histopathological grade
A total of 5 cases were classified as grade 1
(1.09%), 30 cases were classified as grade 2 (32.61%), and 57 cases
were classified as grade 3 (61.96%).
DCE-MRI features correlate with WHO
histopathological grade (Table
I)
As presented in Table
I, the tumor size, shape and enhancement characteristics of
early lesions were associated with the WHO histopathological grade
(P=0.012, P=0.004, P=0.000, respectively), namely the larger the
tumor diameter, the higher the WHO histopathological grade. Round
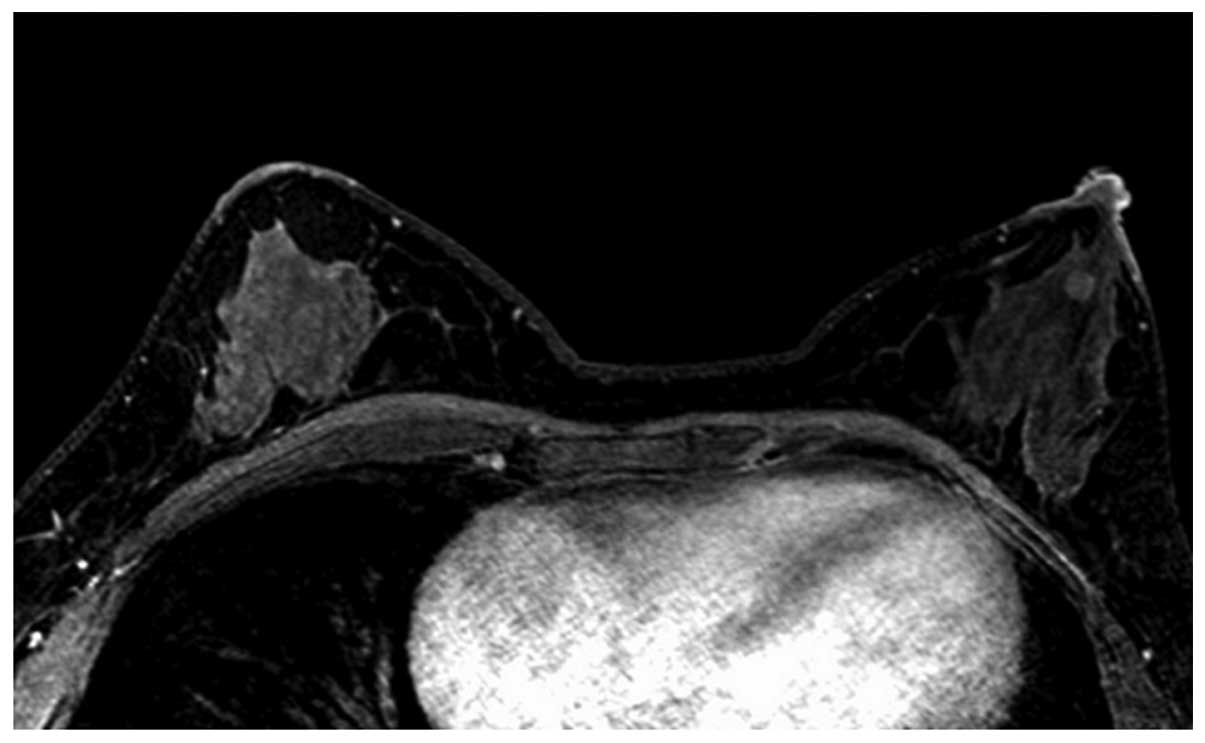
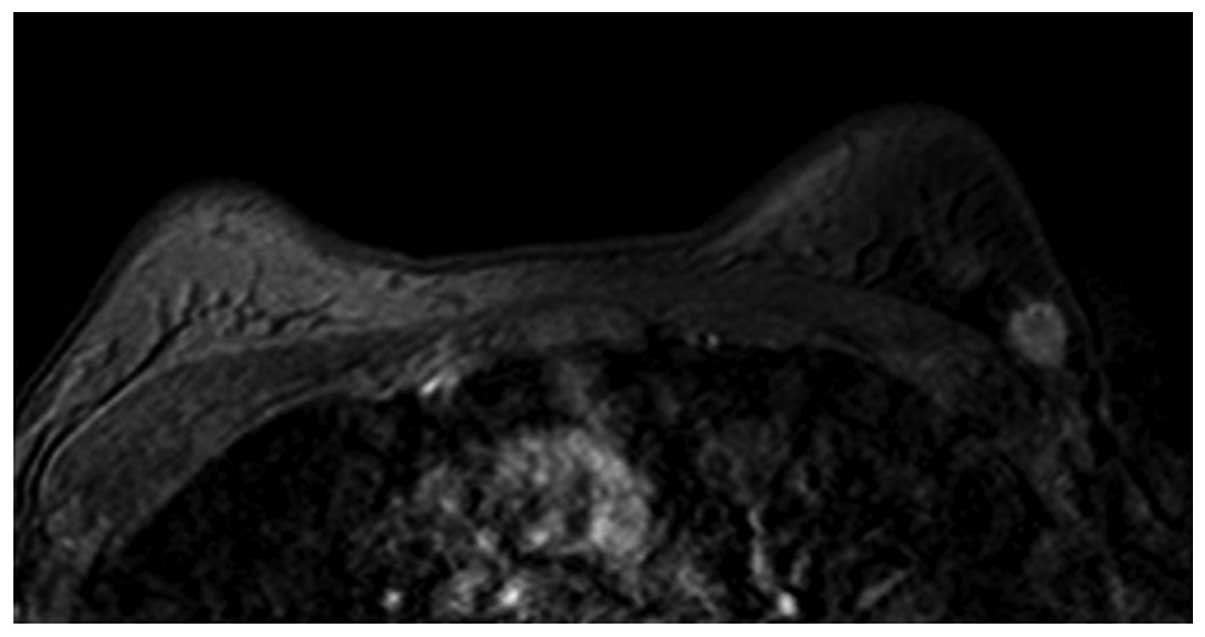
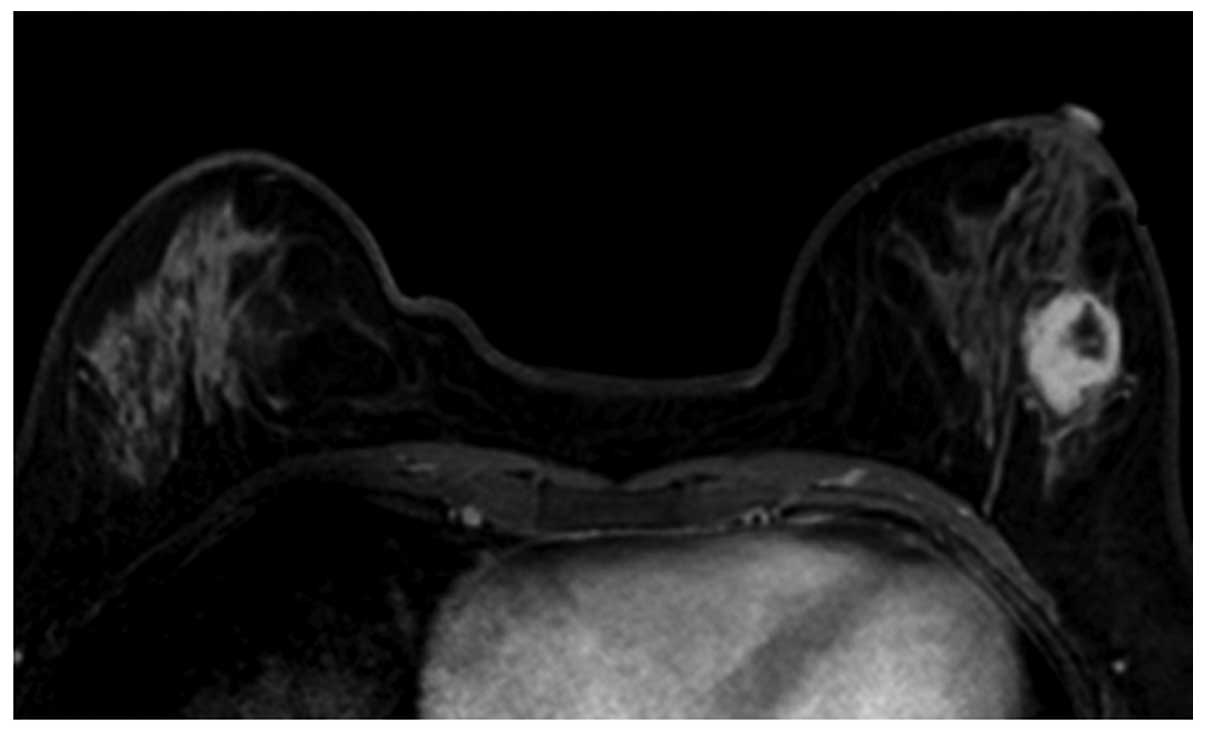
(Fig. 1) and oval (Fig. 2) masses were a relatively lower WHO
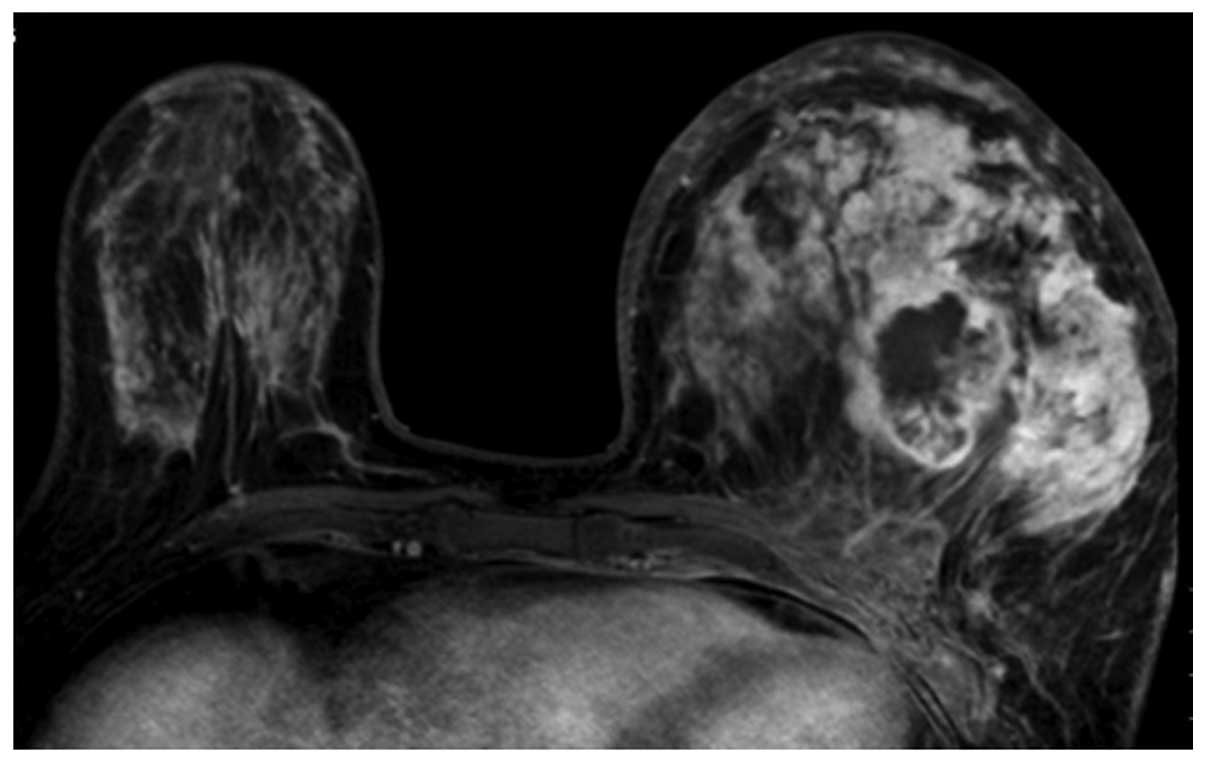
histopathological grade, while the lobulated and irregular masses
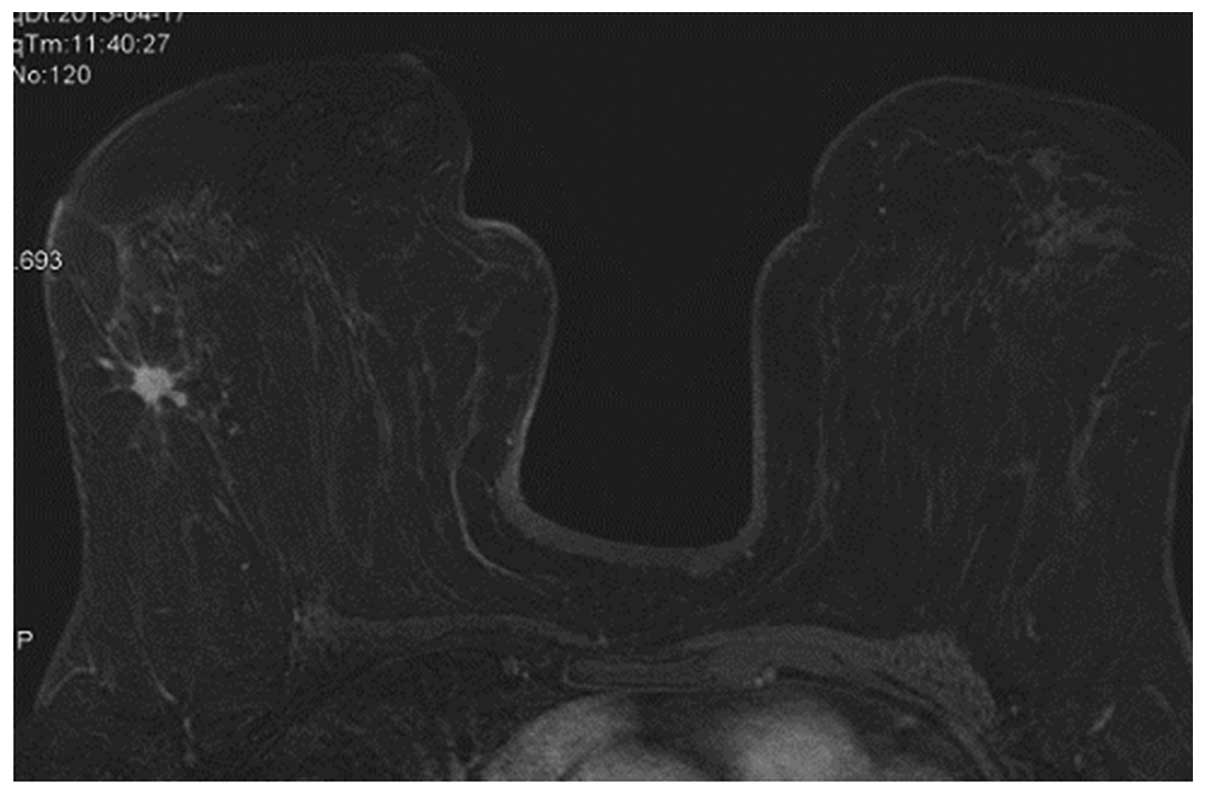
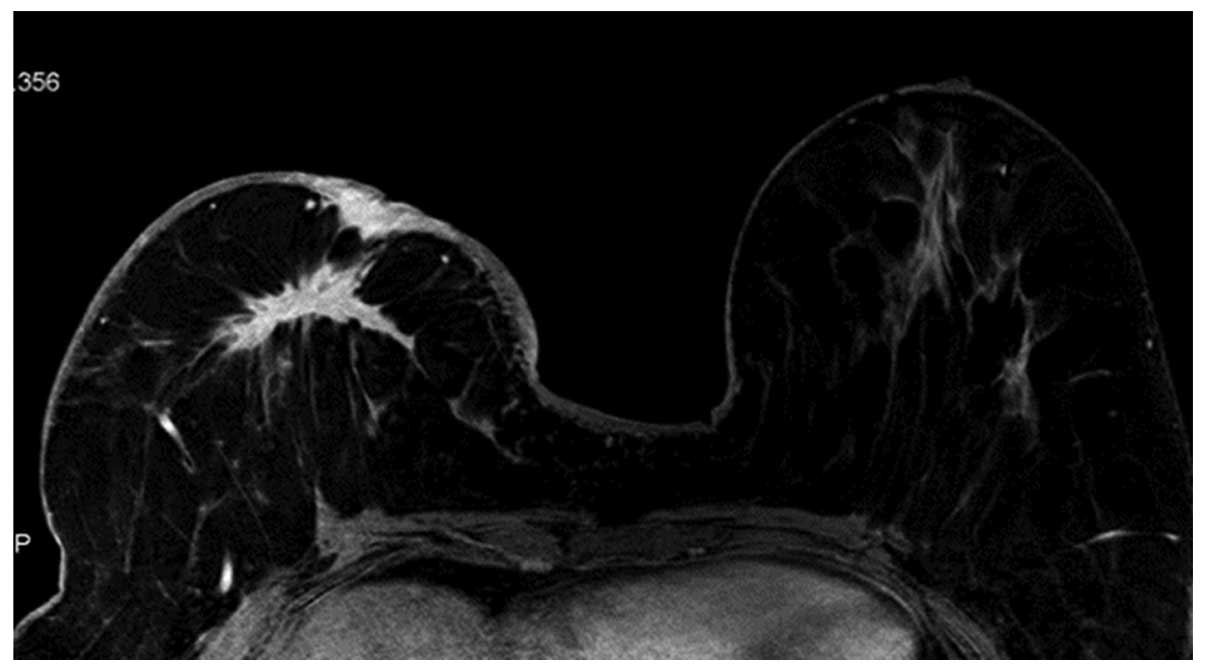
were higher WHO histopathological grades (Figs. 3 and 4).
The heterogeneous enhancement (Fig.
5) and ring-like enhancement (Fig.
6) presented as higher WHO histopathological grade, while those
with homogeneous enhancement (Fig. 2)
presented with lower WHO histopathological grade. The status of the
lesion margin, whether smooth (Fig.
1), irregular (Fig. 6) or
Spiculated (Fig. 5), was not
associated with the WHO histopathological grade (P>0.05).
Discussion
The features revealed from the DCE-MRI scans were
diverse and complex and were informed by histopathological features
of tumors such as different growth patterns, growth rates and
malignant degrees. Theoretically, the relationships between the
lesions' imaging features and histopathological features may be
used to performed the non-invasive prediction of tumor invasion,
thus guiding the treatment selection and improving the prognosis
for patients (22–26).
The T staging is based on the size of the tumor. A
previous study demonstrated that survival rates for breast cancer
patients was negatively correlated with their tumor sizes. As the T
stage increases, the metastasis rate to the lymph nodes increases,
and the degree of differentiation becomes worse, indicating the
poor prognosis of tumors (17). In
the present study, the lesions were categorized according to the
size of primary tumors (T) in the UICC TNM staging, among which 29
cases exhibited a tumor diameter of ≤2.0 cm (31.52%), 53 cases
exhibited a 2–5 cm diameter (57.61%), and 10 cases exhibited a
diameter of ≥5.0 cm in (10.87%). Tumor size was associated with WHO
histopathological grade (P<0.05); as the tumor diameter
increased, the degree of differentiation increased.
The tumor shape may reflect the growth pattern and
biological characteristics of the tumor to a certain extent.
According to the standard of ACR BI-RADS-MRI (2013) (1), tumor shapes may be divided into 4 types:
i) Round, referring to the spherical growth of lesions; ii) oval,
referring to the oval growth of lesions; iii) lobulated, referring
to the edge of lump or nodule appeared the wave-like outline; iv)
irregular, referring to the uneven outline of lesions (non-round,
oval and lobulated). A lobulated shape results from unbalanced
tumor growth rates in all directions and constraints by breast
support structure; the tumor growth pattern is in a conglomerate
type or expansive type. In the present study, among the 92 cases,
the irregular pattern was the most commonly observed (49 cases,
53.26%), and the majority of tumors were WHO histopathologic grade
3 (57 cases, 61.96%). Tumors with a round pattern predominantly
presented as WHO histopathological grade 1, while the lobulated and
irregular lesions presented with a higher WHO histopathological
grade.
The tumor margins may be divided into 3 types: i)
Smooth, referring to the clear margin; ii) irregular, uneven
margin, round or uneven (non-smooth, non-spiculated); iii)
speculated, characterized by radial lines, and with a ‘starry-like’
or ‘crab foot-like’ appearance. Clear margins indicated that the
tumor exhibited the extrapolated growth pattern; irregular margins
indicated that the tumor exhibited invasive growth patterns; and
spiculated margins are widely considered as the typical signs of
malignant tumor, indicating that the tumor cells spread in all
directions around or stimulated the proliferation of breast
condulets and the surrounding fibrous tissues; there may also be
the invasion of cancer cells, resulting in pure ductal hyperplasia
and fibroplasia (6,7,10). Tozaki
et al (11) analyzed 171
lesions of breast masses, and determined that the malignant feature
that had the highest positive predictive value was the presence of
a speculated margin (100%). The speculated margin may appear in a
large proportion of tumors, particularly peripheral lung cancer;
however, there remains a controversy about whether there is a
correlation between the presence of a speculated margin in breast
cancer tumors and the malignant degree. Lamb et al (10) performed ultrasound and mammography
X-ray studies, and demonstrated that a speculated margin appeared
more commonly in lesions with lower histopathological grade, which
represents lower levels of tumor invasion: The authors considered
that the speculated margin was the result of reactive hyperplasia
of tumor interstitial fibrous connective tissues, which may limit
the spread of tumor cells, and it may also be an early protective
mechanism against cancer. Lee et al (6) also hypothesized that the speculated
margin was more prone to appear in well-differentiated tumors,
indicating an improved prognosis in patients. Paradiso et al
(18) also reported that tumors with
speculated margins exhibited lower aggression, and that endocrine
therapy exhibited better results in these tumors. The results of
the present study indicated that the tumor margin was not
associated to the WHO histopathological grade. Whether the
speculated margin is a protective mechanism requires further
study.
In the present study, the breast lesions presented
with 3 enhancement patterns: i) Homogeneous enhancement, referring
to the even consistent enhancement in the entire lesions; ii)
heterogeneous enhancement, meaning an absence of characteristic
mottle-like diffused enhancement; iii) ring-like enhancement, where
the tumor's margin enhancement was much more apparent. When the
tumor grew to a certain size, particularly in highly malignant
breast cancer cases, the internal blood supply may be deficient,
liquefactive necrosis and signs of minor bleeding may occur inside
the parenchyma, which may lead to mixed signals in MRI conventional
scanning. In enhanced scanning, because the tumor's internal
structures are uneven, concentric enhancement which penetrated from
the margin to the center would appear, which is an important
diagnostic feature of breast cancer, with the diagnostic
sensitivity as 100%. It is widely accepted that ring enhancement is
an important morphological sign to distinguish between benign and
malignant tumors. Buadu et al (23) performed histopathological analysis
investigating the ring enhancement of breast lesions, and the
results demonstrated that the accumulation of microvessels around
the tumor margin was the main cause of DCE-MRI margin enhancement.
Kuhl et al (25) demonstrated
that nearly two-thirds of breast cancer cases would present with
ring enhancement, the tumor's margin ring enhancement was
associated with its histopathological characteristics: Partial
areas around the tumor had dense angiogenesis, thus the
permeability would be increased, the proliferation of tumor cells
was active and the interstitial substances would be rich, so that
the contrast agent could enter early; while the center of the tumor
may have hemorrhage, necrosis, cystic changes and central fibrosis,
the densities of tumor blood vessels would be low, and the contrast
agent distribution would be lower; the adjacent tissues were
predominantly the normal breast glandular tissues, although they
may be associated with such changes as atypical hyperplasia,
adenosis and cysts. The densities of microvessels were
significantly lower than those in the tumor center and
tumor-adjacent tissues (25). A
previous study demonstrated that the enhancement features of the
breast cancer tissue were associated with its tissue
differentiation, the proliferation abilities of breast cells
increased from low to high in homogeneous enhancement, ring
enhancement and heterogeneous enhancement, respectively (17). Lee et al (6) demonstrated that the presence of ring
enhancement alone may indicate the high-differentiation of tumors
and relatively larger lesions. However, Mussurakis et al
(27) reported that ring enhancement
was not related with the histopathological prognostic factors. The
results of the present study indicated that the DCE-MRI enhancement
patterns of tumors were related to the histopathological grades,
and that ring enhancement and heterogeneous enhancement often
occurred in the high-level breast cancer.
In conclusion, DCE-MRI signs exhibited certain
associations with the WHO histopathological grades, and MRI
features could be used to evaluate the biological behaviors and
prognosis of lesions, thus providing guidance for the clinical
treatment.
References
|
1
|
Morris EA, Comstock CE and Lee CH: ACR
BI-RADS®. Magnetic Resonance Imaging. In: ACR
BI-RADS® Atlas, Breast Imaging Reporting and Data
System. American College of Radiology (Reston, VA). 1–173.
2013.
|
|
2
|
Trimboli RM, Verardi N, Cartia F,
Carbonaro LA and Sardanelli F: Breast cancer detection using double
reading of unenhanced MRI including T1-weighted, T2-weighted STIR
and diffusion-weighted imaging: A proof of concept study. AJR Am J
Roentgenol. 203:674–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oseledchyk A, Kaiser C, Nemes L, Döbler M,
Abramian A, Keyver-Paik MD, Leutner C, Schild HH, Kuhn W and Debald
M: Preoperative MRI in patients with locoregional recurrent breast
cancer: Influence on treatment modalities. Acad Radiol.
21:1276–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saslow D, Boetes C, Burke W, Harms S,
Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, et al:
American cancer society guidelines for breast screening with MRI as
an adjunct to mammography. CA Cancer J Clin. 57:75–89. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An YY, Kim SH and Kang BJ: Characteristic
features and usefulness of MRI in breast cancer in patients under
40 years old: Correlations with conventional imaging and prognostic
factors. Breast Cancer. 21:302–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko
ES and Moon WK: Correlation between high resolution dynamic MR
features and prognostic factors in breast cancer. Korean J Radiol.
9:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szabó BK, Aspelin P, Kristoffersen Wiberg
M, Tot T and Boné B: Invasive breast cancer: Correlation of dynamic
MR features with prognostic factors. Eur Radiol. 13:2425–2435.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim TH, Kang DK, Yim H, Jung YS, Kim KS
and Kang SY: Magnetic resonance imaging patterns of tumor
regression after neoadjuvant chemotherapy in breast cancer
patients: Correlation with histopathological response grading
system based on tumor cellularity. J Comput Assist Tomogr.
36:200–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of
histopathological grade in breast cancer: Experience from a large
study with long-term follow-up. Histopathology. 19:403–410. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamb PM, Perry NM, Vinnicombe SJ and Wells
CA: Correlation between ultrasound characteristics, mammographic
findings and histopathological grade in patients with invasive
ductal carcinoma of the breast. Clin Radiol. 55:40–44. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tozaki M, Igarashi T and Fukuda K:
Positive and negative predictive values of BI-RADS-MRI descriptors
for focal breast masses. Magn Reson Med Sci. 5:7–15. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitz AM, Loo CE, Wesseling J, Pijnappel
RM and Gilhuijs KG: Association between rim enhancement of breast
cancer on dynamic contrast-enhanced MRI and patient outcome: Impact
of subtype. Breast Cancer Res Treat. 148:541–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Goethem M, Schelfout K, Dijckmans L,
Van Der Auwera JC, Weyler J, Verslegers I, Biltjes I and De
Schepper A: MR mammography in the pre-operative staging of breast
cancer in patients with dense breast tissue: Comparison with
mammography and ultrasound. Eur Radiol. 14:809–816. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He D, Ma D and Jin E: Dynamic MRI-derived
parameters for hot and cold spots: Correlation with breast cancer
histopathology. J BUON. 17:57–64. 2012.PubMed/NCBI
|
|
15
|
Buadu LD, Murakami J, Murayama S,
Hashiguchi N, Sakai S, Masuda K, Toyoshima S, Kuroki S and Ohno S:
Breast lesions: Correlation of contrast medium enhancement patterns
on MR images with histopathologic findings and tumor angiogenesis.
Radiology. 200:639–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsubayashi RN, Fujii T, Yasumori K,
Muranaka T and Momosaki S: Apparent diffusion coefficient in
invasive ductal breast carcinoma: Correlation with detailed
histologic features and the enhancement ratio on dynamic
contrast-enhanced MR images. J Oncol. 2010:pii: 821048. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su MY, Baik HM, Yu HJ, Chen JH, Mehta RS
and Nalcioglu O: Comparison of choline and pharmacokinetic
parameters in breast cancer measured by MR spectroscopic imaging
and dynamic contrast enhanced MRI. Technol Cancer Res Treat.
5:401–410. 2006.PubMed/NCBI
|
|
18
|
Paradiso A, Mangia A, Barletta A, Marzullo
F, Ventrella V, Racanelli A, Schittulli F and De Lena M:
Mammography and morphobiologic characteristics of human breast
cancer. Tumori. 79:422–426. 1993.PubMed/NCBI
|
|
19
|
Liu H and Peng W: MRI morphological
classification of ductal carcinoma in situ (DCIS) correlating with
different biological behavior. Eur J Radiol. 81:214–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ellis IO, Schnitt SJ, Sastre-Garau X,
Bussolati G, Tavassoli FA, Eusebi V, Peterse JL, Mukai K, Tabár L,
Jacquemier J, et al: Tumors of the Breast. WHO Classification of
Tumours of the Breast. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH and
van de Vijver MJ: 4:(4th). IARC Press. (Lyon, France). 34–38.
2012.
|
|
22
|
Matsubayashi R, Matsuo Y, Edakuni G, Satoh
T, Tokunaga O and Kudo S: Breast masses with peripheral rim
enhancement on dynamic contrast-enhanced MR images: Correlation of
MR findings with histologic features and expression of growth
factors. Radiology. 217:841–848. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buadu LD, Murakami J, Murayama S,
Hashiguchi N, Sakai S, Toyoshima S, Masuda K, Kuroki S and Ohno S:
Patterns of peripheral enhancement in breast masses: Correlation of
findings on contrast medium enhanced MRI with histologic features
and tumor angiogenesis. J Comput Assist Tomogr. 21:421–430. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Partridge SC, Stone KM, Strigel RM,
DeMartini WB, Peacock S and Lehman CD: Breast DCE-MRI: Influence of
postcontrast timing on automated lesion kinetics assessments and
discrimination of benign and malignant lesions. Acad Radiol.
21:1195–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuhl CK, Schild HH and Morakkabati N:
Dynamic bilateral contrast-enhanced MR imaging of the breast:
Trade-off between spatial and temporal resolution. Radiology.
236:789–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CH, Yin FF, Horton J and Chang Z:
Review of treatment assessment using DCE-MRI in breast cancer
radiation therapy. World J Methodol. 4:46–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mussurakis S, Gibbs P and Horsman A:
Peripheral enhancement and spatial contrast uptake heterogeneity of
primary breast tumours: Quantitative assessment with dynamic MRI. J
Comput Assist Tomogr. 22:35–46. 1998. View Article : Google Scholar : PubMed/NCBI
|