Introduction
Malignant tumors represent a significant health
problem, and the incidence of and mortality associated with these
tumors have rapidly increased in China in recent decades. Among
them, gastrointestinal tumors remain a major cause of
cancer-related mortality worldwide (1). Chemotherapy serves as an essential
treatment option, and several meta-analyses have demonstrated that
chemotherapy has significant benefits for patients with gastric
cancer (GC) (2). Although new
therapeutic strategies are being developed rapidly, the prognosis
of patients with GC remains poor, mainly due to interindividual
variations in drug response. It has been predicted that genetic
variants may account for 20–95% of interindividual variation in
drug response (3). Therefore,
identifying polymorphisms in xenobiotic-metabolizing enzymes prior
to the administration of chemotherapy and pharmacogenetic markers
influencing drug response may help doctors to make more precise and
effective treatment choices for individual patients.
As first-line drugs, 5-fluorouracil (5-FU) and
platinum-based drugs are being widely used clinically. Numerous
studies have confirmed the close correlation between drug efficacy
with metabolic enzymes and gene polymorphisms in DNA repair enzymes
(4–6).
Among them, thymidylate synthase (TYMS) and excision repair
cross-complementing 1 (ERCC1) were the earliest identified
(5,7).
TYMS is an enzyme that plays a significant role in converting
deoxyuridine-5′-monophosphate (dUMP) to
deoxythymidine-5′-monophosphate (dTMP). Studies illustrated that
the level of TYMS expression in patients with colorectal
cancer receiving 5-FU-based chemotherapy was association with
clinical responsiveness (8,9). The TYMS promoter comprises a
28-bp tandem repeat in the 5′-untranslated enhanced region (5′-UTR)
that usually presents as a double-(2R) or triple-tandem repeat
(3R).
ERCC1 is a key rate-limiting enzyme in the multistep
nucleotide excision repair process that participates in
single-strand annealing repair and the homologous repair of
double-strand breaks. ERCC1 is highly conserved, and it is crucial
for the removal of DNA adducts caused by platinum compounds
(10,11). A common C→T polymorphism at codon 118
of ERCC1 has been identified as a meaningful predictor of
outcome in patients with colorectal cancer who received
platinum-based chemotherapy (12).
C/C, T/T and C/T are the most common genotypes.
At present, tumor tissue is often used to detect
TYMS and ERCC1 polymorphisms, but there are several
limitations in using tissue for this purpose. It was previously
reported that TYMS and ERCC1 polymorphisms could be
detected in the blood of patients with colorectal and rectal
cancers (13), but it is unclear
whether they could be detected in the blood of patients with GC and
whether the polymorphisms are consistent in tumor tissue and
peripheral blood. The aim of this study was to assess TYMS
and ERCC1 polymorphisms in the cancer tissue and peripheral
blood of patients with GC to verify whether the results of these
samples were consistent, thereby exploring the feasibility of using
peripheral blood to detect gene polymorphisms instead of tumor
tissue. On this basis, peripheral blood was collected from patients
with gastrointestinal tumors who received 5-FU- and platinum-based
chemotherapy to determine whether the different polymorphisms in
TYMS and ERCC1 may be predictive of the outcome of
chemotherapy in these patients. In this study, we evaluated the
effects of TYMS and ERCC1 polymorphisms on the
efficacy of chemotherapy in patients with gastric tumors using
peripheral venous blood, in order to provide a simple laboratory
evaluation measurement to individualize patient treatment.
Materials and methods
Patient information
Forty-three patients who underwent surgery in the
Department of Gastrointestinal Surgery of the First Affiliated
Hospital of Zhengzhou University, China, between February and
August 2012 were selected, comprising 31 males and 12 females.
Pathological examination confirmed that all cancer tissues were
gastric adenocarcinoma, and none of the patients received
radiotherapy or chemotherapy prior to surgery. Additionally, 76
patients with gastrointestinal cancer who received 5-FU- and
platinum-based chemotherapy were also enrolled. This study was
conducted in accordance with the declaration of Helsinki, and with
approval from the Ethics Committee of Zhengzhou University. Written
informed consent was obtained from all participants.
Extracting genomic DNA
Peripheral blood and tumor tissue genomic DNA was
extracted using a Blood Genomic mini kit (ComWin Biotech Co,
Beijing, China) and UNQ-10 Column Animal Genomic DNA isolation kit
(Sangon Biotech, Shanghai, China).
Polymerase chain reaction (PCR)
The primer pair used for detecting TYMS was as
follows: F, 5′-GCGGAAGGGGTCCTGCCA-3′; and R,
5′-CGTGCGGTCGTCCTTCCTG-3′. The volume of the PCR reaction mixture
(Sangon Biotech) was 25 µl, and PCR amplification was performed
using the following procedure: pre-denaturation at 95°C for 5 min,
followed by 40 cycles at 95°C for 30 sec, 63°C for 30 sec, and 72°C
for 30 sec, with a final extension at 72°C for 5 min. PCR products
(5 µl) were subjected to electrophoresis, and the results were
analyzed using a gel imaging system (UVP, Upland, CA, USA).
PCR-restriction fragment length
polymorphism
The primer pair for ERCC1 was as follows: F,
5′-TGTGGTTATCAAGGGTCATCC-3′; and R, 5′-CAGTCCAGAACACTGGGACAT-3′.
The volume of the PCR reaction mixture was 25 µl, and PCR
amplification was performed using the following procedure:
pre-denaturation at 95°C for 5 min, followed by 40 cycles at 95°C
for 30 sec, 63°C for 30 sec, and 72°C for 30 sec, with a final
extension at 72°C for 5 min. PCR products (10 µl) and
HindIII incision enzyme (2 µl; Promega, Madison, WI, USA)
were added to the reaction tube and incubated for 4 h in a water
bath at 37°C, and the reaction was finally terminated via 5 min of
heating at 65°C. The digested products were subjected to
electrophoresis, and the results were analyzed using a gel imaging
system (UVP).
Statistics analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform the statistical analysis. Comparisons between
the two groups were performed using the Chi-squared test with
correction for continuity. Correlations between sample groups were
evaluated using Spearman's test, and Fisher's exact test was
performed to analyze the correlation between genotype and
chemotherapeutic efficacy.
Results
Correlation analysis
Among the 43 patients who underwent surgery, there
was no significant correlation between TYMS and ERCC1 genotypes and
patient gender, tumor location, differentiation, number of
metastatic sites and carcinoembryonic antigen levels (P>0.05,
Table I).
 | Table I.Correlation of TYMS and
ERCC1 genotypes with clinicopathological factors in gastric
cancer patients. |
Table I.
Correlation of TYMS and
ERCC1 genotypes with clinicopathological factors in gastric
cancer patients.
|
| TYMS |
| ERCC1 |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
factors | 3R/3R | 2R/2R, 2R/3R | P-value | C/C | T/T, C/T | P-value |
|---|
| Gender |
|
| 0.651 |
|
| 0.293 |
| Male | 22 | 9 |
| 25 | 6 |
|
|
Female | 9 | 3 |
| 11 | 1 |
|
| Tumor location |
|
| 0.778 |
|
| 0.885 |
| Stomach
fundus | 3 | 1 |
| 4 | 0 |
|
| Gastric
body | 16 | 7 |
| 18 | 5 |
|
|
Antrum | 11 | 4 | | 13 | 2 |
|
|
Other | 1 | 0 |
| 1 | 0 |
|
|
Differentiation |
|
| 0.417 |
|
| 0.466 |
|
High/medium | 9 | 2 |
| 10 | 1 |
|
|
Low | 22 | 10 |
| 26 | 6 |
|
| Metastatic
sites |
|
| 0.489 |
|
| 0.717 |
| 0 | 7 | 2 |
| 8 | 1 |
|
| 1 | 16 | 9 |
| 20 | 5 |
|
| 2 | 6 | 1 |
| 7 | 0 |
|
| ≥3 | 2 | 0 |
| 1 | 1 |
|
Detection and analysis of TYMS and
ERCC1 genotypes
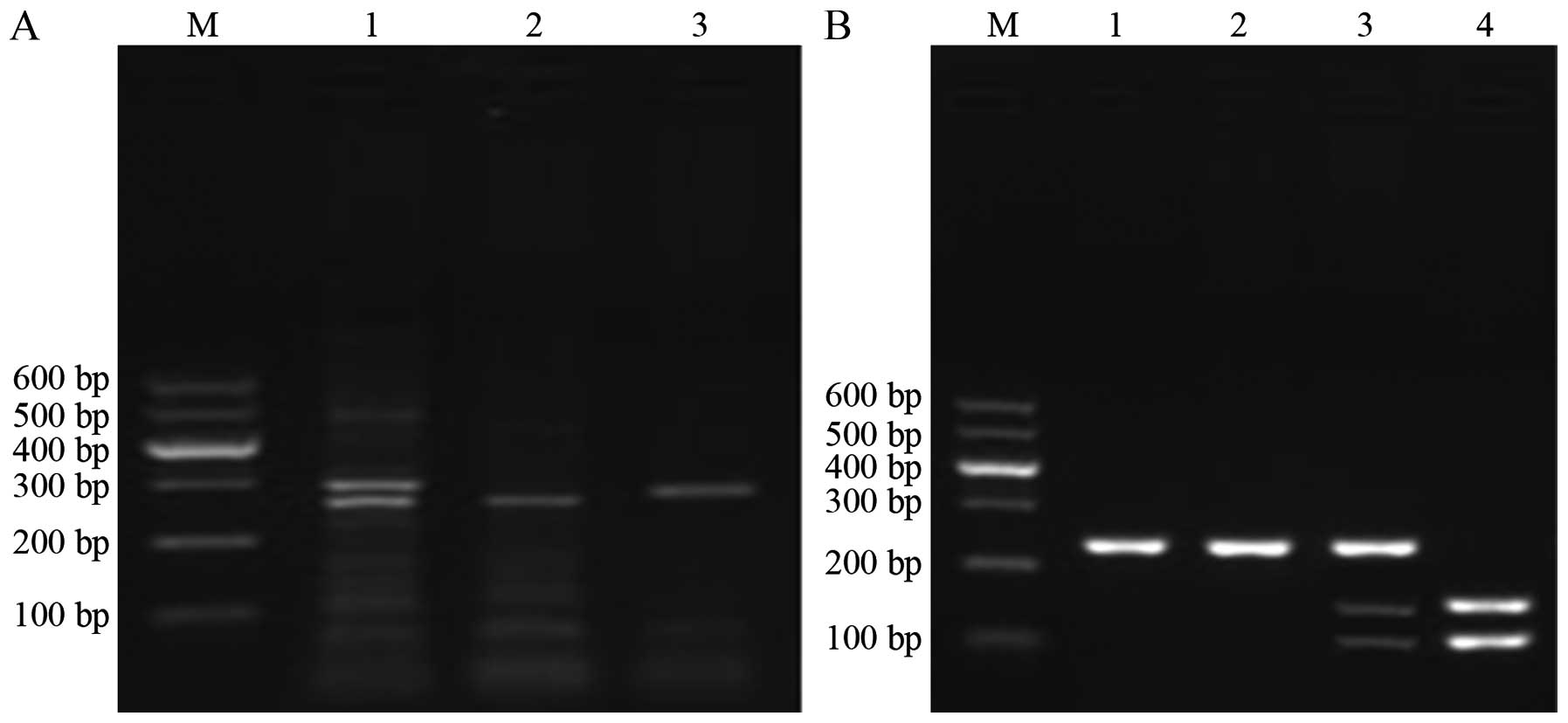
In Fig. 1A and B, the
amplification fragments produced according to the TYMS and ERCC1
genotypes are presented. Tables II
and III illustrate that the
distributions of TYMS and ERCC1 genotypes in peripheral blood and
tumor tissue samples were consistent. The detection rates of the
3R/3R and 2R/2R or 2R/3R genotypes of TYMS were 72.09% (31/43) and
27.91% (12/46), respectively, and the detection rates of the C/C
and T/T or C/T genotypes of ERCC1 were 81.39% (35/43) and 18.61%
(8/43), respectively.
 | Table II.Genotype frequencies of TYMS in
peripheral blood and tumor tissues. |
Table II.
Genotype frequencies of TYMS in
peripheral blood and tumor tissues.
|
| TYMS in peripheral
blood |
|
|
|
|---|
|
|
|
|
|
|
|---|
| TYMS in tumor
tissue | 3R/3R | 2R/2R, 2R/3R | Total | Chi-squared | P-value |
|---|
| 3R/3R | 31 | 0 | 31 | 38.175 | <0.01 |
| 2R/2R, 2R/3R | 0 | 12 | 12 |
|
|
| Total | 31 | 12 | 43 |
|
|
 | Table III.Genotype frequencies of ERCC1 in
peripheral blood and tumor tissue. |
Table III.
Genotype frequencies of ERCC1 in
peripheral blood and tumor tissue.
|
| ERCC1 in peripheral
blood |
|
|
|
|---|
|
|
|
|
|
|
|---|
| ERCC1 in tumor
tissue | C/C | T/T, C/T | Total | Chi-squared | P-value |
|---|
| C/C | 35 | 0 | 35 | 38.750 | <0.01 |
| T/T, C/T | 0 | 8 | 8 |
|
|
| Total | 35 | 8 | 43 |
|
|
Correlation between TYMS polymorphisms
and chemotherapeutic efficacy
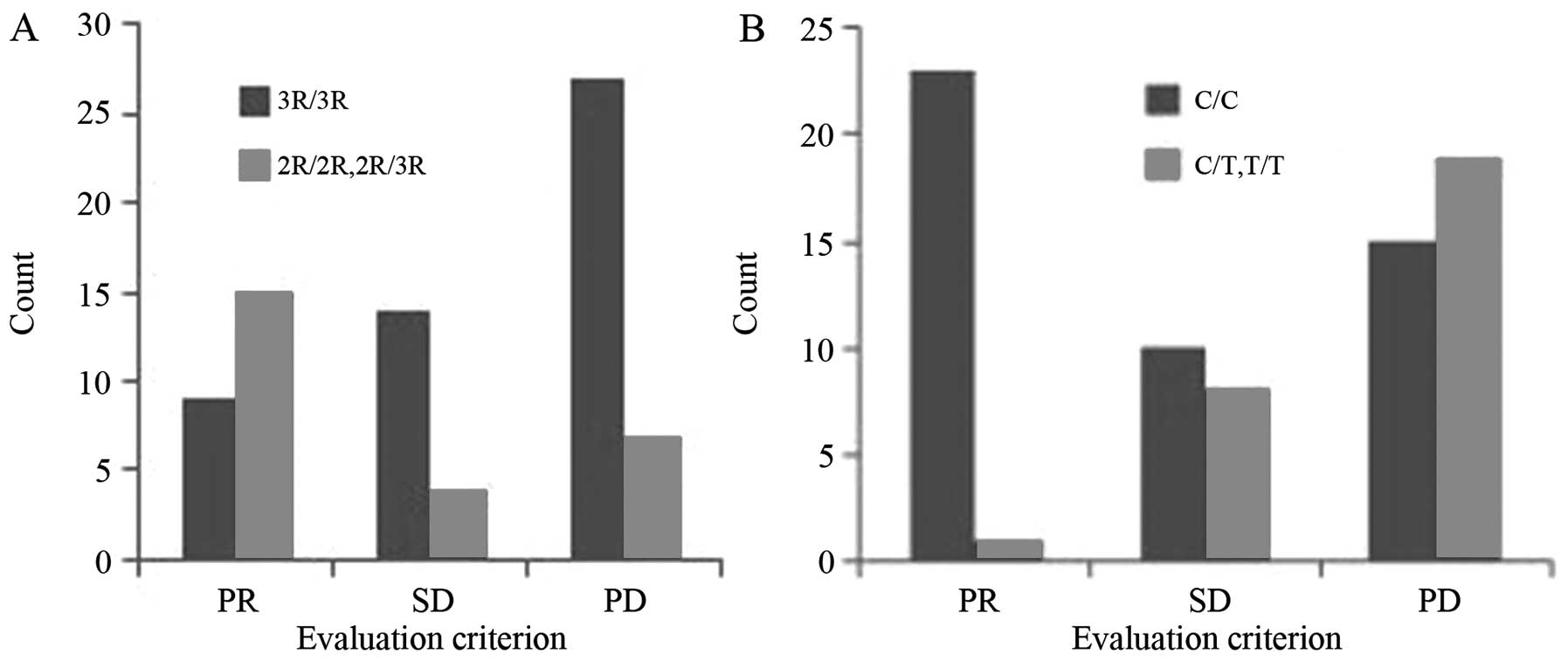
Among the patients who received chemotherapy, the
3R/3R genotype was present in 65.79% of patients (50/76), vs.
34.21% for the 2R/2R and 2R/3R genotypes (26/76). In total, no
complete responses (CRs), 9 partial responses (PRs), 14 cases of
stable disease (SD) and 27 cases of progressive disease (PD) were
recorded among the patients with the 3R/3R genotype, producing an
overall response rate (ORR) of 18.00% (9/50). Conversely, 0 CRs, 4
PRs, 4 cases of SD and 7 cases of PD were noted among the patients
with the 2R/2R or 2R/3R genotype, producing an ORR of 57.69%
(15/26; Fig. 2A). Spearman's analysis
uncovered a correlation between TYMS genotypes (5′-UTR) and
chemotherapeutic efficacy. Fisher's exact test demonstrated that
the 2R/3R and 2R/2R genotypes were associated with better
chemotherapeutic efficacy than the 3R/3R genotype (Table IV).
 | Table IV.Comparison of chemotherapy efficacy
of TYMS and ERCC1 genotypes. |
Table IV.
Comparison of chemotherapy efficacy
of TYMS and ERCC1 genotypes.
|
| TYMS
genotypes (n) |
|
|
| ERCC1
genotypes (n) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Chemotherapy
efficacy | 3R/3R | 2R/3R, 2R/2R | Total | F | P-value | C/C | C/T, T/T | Total | F | P-value |
|---|
| Effective | 9 | 15 | 24 |
| <0.01 | 23 | 1 | 24 | 19.89 | <0.01 |
| Ineffective | 41 | 11 | 52 | 14.53 |
| 25 | 27 | 52 |
|
|
| Total | 50 | 26 | 76 |
|
| 48 | 28 | 76 |
|
|
Correlation between ERCC1
polymorphisms and chemotherapeutic efficacy
Among the patients who received chemotherapy, 63.16%
(48/76) carried the C/C genotype, compared with 36.84% (28/76) for
the T/T and C/T genotypes. In total, 0 CRs, 23 PRs, 10 cases of SD
and 15 cases of PD were recorded among patients with the C/C
genotype, giving an ORR of 47.91% (23/48). No CRs, 1 PR, 8 cases of
SD and 19 cases of PD were noted among patients with the T/T or C/T
genotype, resulting in an ORR of 3.57% (1/28; Fig. 2B). Spearman's analysis revealed a
correlation between ERCC1 genotypes and chemotherapeutic efficacy.
Fisher's exact test demonstrated that the C/C genotype was
associated with better chemotherapeutic efficacy than the C/T and
T/T genotypes (Table IV).
Discussion
The TYMS gene is located on the 18th
chromosome (18p 11.32). The gene is 16 kb long, and its main
transcription initiation site is located 160–180 bp upstream of the
initiation codon sequence. Previous studies have revealed that
TYMS contains a polymorphism, which is a 28-bp tandem repeat
in the 5′-UTR, generally 2R or 3R. Thus, the most common genotypes
are 3R/3R, 2R/3R and 2R/2R(14).
TYMS influences chemotherapeutic efficacy through its
protein product, which regulates folic acid circulation and
converts dUMP into dTMP, with the latter serving as the unique
source of nucleotides for DNA synthesis and repair. Thus,
restraining TYMS could lead to a decrease in dTMP levels and
promote chromosome breakage in cells, resulting in cell death. In
addition, TYMS is a significant target of 5-FU, and the
expression level of this enzyme in the body influences the drug's
antitumor effect. The 5′-UTR polymorphism of TYMS influences
the stability and translation efficiency of mRNA, in turn
influencing the expression level of TYMS and promoting
interindividual variation in sensitivity to chemotherapeutics.
With regard to gastrointestinal tumors, previous
studies have demonstrated that TYMS expression was higher in
patients carrying the 3R/3R genotype than in those carrying the
2R/2R or 2R/3R genotype. In addition, clinical manifestations were
more pronounced in the former than the latter (15), which suggested that 3R/3R was a poor
prognostic factor in adjuvant chemotherapy for tumors. A study by
Morganti et al (16) of 48
patients with gastrointestinal cancer demonstrated that the mRNA
expression level of TYMS in patients carrying the 3R/3R
genotype was notably higher than that of 2R/2R or 2R/3R carriers.
In 2005, Yawata et al (17)
determined that forward copies of TYMS could be regarded as
a predictive index for evaluating sensitivity to 5-FU.
Subsequently, Brody et al (14) and Watson et al (15) suggested that the 5′-UTR 28-bp repeat
nucleotide fragment polymorphism in TYMS could influence the
efficacy of 5-FU in vivo. Huang et al (18) studied 116 patients with GC who
received 5-FU-based chemotherapy, and drew the same conclusion.
With regard to gastric cancer, Cui et al
(19) noted that Chinese patients
with gastrointestinal tumors who carry the 2R/3R genotype exhibited
greater sensitivity to 5-FU than their counterparts who carried the
3R/3R genotype.
Villafranca et al (20) studied 65 patients with rectal cancer
and demonstrated that the downstaging rate was higher for patients
with the 2R/3R or 2R/2R genotype than for those with the 3R/3R
genotype, and the three-year survival rates for the 2R/3R or 2R/2R
genotype and the 3R/3R genotype were 81 and 41%, respectively.
Pullarkat et al (21), Marsh
et al (22), Park et al
(23) and Matasui et al
(24) observed that 2R/2R or 2R/3R
carriers were more sensitive to 5-FU than 3R/3R carriers. In the
present study, the ORR for 3R/3R carriers was 18%, which was
significantly lower than the rate of 57.69% observed for 2R/3R or
2R/2R carriers. This illustrated that the repetitive elements
polymorphism of TYMS could be regarded as a predictor of
tumor downstaging and a new modality for predicting the effect of
5-FU-based chemotherapy.
ERCC1 is located on the 19th chromosome (19q
13.2). The gene, which is 16 kb long, contains 10 exons, and the
most common and meaningful polymorphism is a C→T transition at the
118th codon on the fourth exon. The three resulting genotypes are
C/C, C/T and T/T. Substantial research has demonstrated that
patients carrying the C/C genotype were more sensitive to platinum
drugs, which means that this genotype could reduce the
transcriptional efficiency and protein expression level of ERCC1 in
cells and weaken the protein's DNA repair activity (25–28).
Furthermore, individual sensitivity to platinum drugs was affected.
Moreover, DNA repair capacity is the molecular basis by which the
effects of platinum drugs are altered, and it plays a significant
part in platinum resistance mechanisms. ERCC1 is a key enzyme
involved in DNA damage repair, which is significantly correlated
with resistance to platinum drugs.
With regard to gastrointestinal tumors, Won et
al demonstrated that using the ERCC1 C118T polymorphism
to predict the toxicity of chemotherapy was feasible (29). Liu et al also confirmed that
ERCC1 polymorphisms could predict the effect based on
oxaliplatin-based therapy (30). In
2011, Yin et al noted in their study that ERCC1 C118T
could be a predictor of the efficacy of oxaliplatin-based therapy
(12).
Liu et al stated that patients with GC who
carry the C/C genotype could receive a survival benefit from
platinum-based chemotherapy (30).
Ruzzo et al suggested that the C/T genotype is associated
with better chemotherapeutic efficacy than the T/T genotype;
however, in that article the author identified the small sample
size of the study, retrospective nature of the experiment, and
heterogeneity of clinical situations as possible explanations for
the divergent conclusion (31).
Ruzzo et al also observed that patients with
colorectal cancer who carry the C/C genotype exhibit greater
chemosensitivity than those who carry the C/T or T/T genotype
(32). However, several studies
contradict this finding, and Viguier et al noted that
patients with colorectal cancer who carry the T/T genotype are more
sensitive to 5-FU and platinum (33).
By studying the correlation between ERCC1
genotypes and platinum drugs, the present study revealed that the
ORR of C/C carriers (47.91%) was notably lower than that of T/T or
T/C carriers (57.69%). A significant association existed between
ERCC1 genotypes and the efficacy of platinum-based
chemotherapy, which indicated that chemosensitizing genotypes could
be used to evaluate the effects of platinum-based chemotherapy.
Therefore, adjusting the dosage of chemotherapeutics according to
patients' genotypes and selectively using agents to overcome drug
resistance associated with high gene expression may greatly improve
chemotherapeutic efficacy.
However, in the clinic, the efficacy of drug
treatment in certain 2R/3R or 2R/2R and C/C carriers was not fully
consistent with the expected effect, which suggested that other
possible factors including age, diet and organ function may
influence chemotherapeutic efficacy.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carrato A, Gallego-Plazas J and
Guillen-Ponce C: Adjuvant therapy of resected gastric cancer is
necessary. Semin Oncol. 32(6 Suppl 9): S105–S108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalow W, Tang BK and Endrenyi L:
Hypothesis: comparisons of inter- and intra-individual variations
can substitute for twin studies in drug research. Pharmacogenetics.
8:283–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adleff V, Hitre E, Köves L, Orosz Z,
Hajnal A and Kralovánszky J: Heterozygote deficiency in thymidylate
synthase enhancer region polymorphism genotype distribution in
Hungarian colorectal cancer patients. Int J Cancer. 108:852–856.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graziano F, Kawakami K, Watanabe G, Ruzzo
A, Humar B, Santini D, Catalano V, Ficarelli R, Merriman T, Panunzi
S, et al: Association of thymidylate synthase polymorphisms with
gastric cancer susceptibility. Int J Cancer. 112:1010–1014. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandola MV, Stoehlmaeher J, Zhang W,
Groshen S, Yu MC, Iqbal S, Lenz HJ and Ladner RD: A 6 bp
polymorphism in the thymidylate synthase gene causes message
instability and is associated with decreased intratumoral TS mRNA
levels. Pharmacogeneties. 14:319–327. 2004. View Article : Google Scholar
|
|
7
|
Chen J, Hunter DJ, Stampfer MJ, Kyte C,
Chan W, Wetmur JG, Mosig R, Selhub J and Ma J: Polymorphism in the
thymidylate synthase promoter enhancer region modifies the risk and
survival of colorectal cancer. Cancer Epidemiol Biomarkers Prev.
12:958–962. 2003.PubMed/NCBI
|
|
8
|
Cascinu S, Aschele C, Barni S, Debernardis
D, Baldo C, Tunesi G, Catalano V, Staccioli MP, Brenna A, Muretto P
and Catalano G: Thymidylate synthase protein expression in advanced
colon cancer: correlation with the site of metastasis and the
clinical response to leucovorin-modulated bolus 5-fluorouracil.
Clin Cancer Res. 5:1996–1999. 1999.PubMed/NCBI
|
|
9
|
Davies MM, Johnston PG, Kaur S and
Allen-Mersh TG: Colorectal liver metastasis thymidylate synthase
staining correlates with response to hepatic arterial floxuridine.
Clin Cancer Res. 5:325–328. 1999.PubMed/NCBI
|
|
10
|
Raymond E, Faivre S, Woynarowski JM and
Chaney SG: Oxaliplatin: mechanism of action and antineoplastic
activity. Semin Oncol. 25(2 Suppl 5): S4–S12. 1998.
|
|
11
|
Altaha R, Liang X, Yu JJ and Reed E:
Excision repair cross complementing group 1: gene expression and
platinum resistance. Int J Mol Med. 14:959–970. 2004.PubMed/NCBI
|
|
12
|
Yin M, Yan J, Martinez-Balibrea E,
Graziano F, Lenz HJ, Kim HJ, Robert J, Im SA, Wang WS,
Etienne-Grimaldi MC and Wei Q: ERCC1 and ERCC2 polymorphisms
predict clinical outcomes of oxaliplatin-based chemotherapies in
gastric and colorectal cancer: a systemic review and meta-analysis.
Clin Cancer Res. 17:1632–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin M, Yan J, Martinez-Balibrea E,
Graziano F, Lenz HJ, Kim HJ, Robert J, Im SA, Wang WS,
Etienne-Grimaldi MC and Wei Q: ERCC1 and ERCC2 polymorphisms
predict clinical outcomes of oxaliplatin-based chemotherapies in
gastric and colorectal cancer: A systemic review and meta-analysis.
Clin Cancer Res. 17:1632–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brody JR, Hucl T, Gallmeier E, Winter JM,
Kern SE and Murphy KM: Genomic copy number changes affecting the
thymidylate synthase (TYMS) gene in cancer: a model for patient
classification to aid fluoropyrimidine therapy. Cancer Res.
66:9369–9373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watson RG, Muhale F, Thorne LB, Yu J,
O'Neil BH, Hoskins JM, Meyers MO, Deal AM, Ibrahim JG, Hudson ML,
et al: Amplification of thymidylate synthetase in metastatic
colorectal cancer patients pretreated with 5-fluorouracil-based
chemotherapy. Eur J Cancer. 46:3358–3364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morganti M, Ciantelli M, Giglioni B,
Putignano AL, Nobili S, Papi L, Landini I, Napoli C, Valanzano R,
Cianchi F, et al: Relationships between promoter polymorphisms in
the thymidylate synthase gene and mRNA levels in colorectal
cancers. Eur J Cancer. 41:2176–2183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yawata A, Kim SR, Miyajima A, Kubo T,
Ishida S, Saito Y, Nakajima Y, Katori N, Matsumoto Y, Fukuoka M, et
al: Polymorphic tandem repeat sequences of the thymidylate synthase
gene correlates with cellular-based sensitivity to fluoropyrimidine
antitumor agents. Cancer Chemother Pharmacol. 56:465–472. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang ZH, Hua D and Li LH: The
polymorphisms of TS and MTHFR predict survival of gastric cancer
patients treated with fluorouracil-based adjuvant chemotherapy in
Chinese population. Cancer Chemother Pharmacol. 63:911–918. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui YH, Liu TS, Zhuang RY, Gao HJ and Li
H: Polymorphism of thymidylate synthase gene and chemosensitivity
of 5-fluorouracil regimen in metastatic gastrointestinal cancer. J
Dig Dis. 10:118–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villafranca E, Okruzhnov Y, Dominguez MA,
García-Foncillas J, Azinovic I, Martínez E, Illarramendi JJ, Arias
F, Martínez Monge R, Salgado E, et al: Polymorphisms of the
repeated sequences in the enhancer region of the thymidylate
synthase gene promoter may predict downstaging after preoperative
chemoradiation in rectal cancer. J Clin Oncol. 19:1779–1786.
2001.PubMed/NCBI
|
|
21
|
Pullarkat ST, Stoehlmacher J, Ghaderi V,
Xiong YP, Ingles SA, Sherrod A, Warren R, Tsao-Wei D, Groshen S and
Lenz HJ: Thymidylate synthase gene polymorphism determines response
and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 1:65–70.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marsh S, McKay JA, Cassidy J and McLeod
HL: Polymorphism in the thymidylate synthase promoter enhancer
region in colorectal cancer. Int J Oncol. 19:383–386.
2001.PubMed/NCBI
|
|
23
|
Park DJ, Stoehlmacher J, Zhang W, Tsao-Wei
D, Groshen S and Lenz HJ: Thymidylate synthasegene polymorphism
predicts response to capecitabine in advanced colorectal cancer.
Int J Colorectal Dis. 17:46–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matasui T, Omura K, Kawakami K, Morita S
and Sakamoto J: Genotype of thymidylate synthase likely to affect
efficacy of adjuvant 5-FU based chemotherapy in colon cancer. Oncol
Rep. 16:1111–1115. 2006.PubMed/NCBI
|
|
25
|
Kakimoto M, Uetake H, Osanai T, Shirota Y,
Takagi Y, Takeshita E, Toriya Y, Danenberg K, Danenberg PV and
Sugihara K: Thymidylate synthase and dihydropyrimidine
dehydrogenase gene expression in breast cancer predicts 5-FU
sensitivity by a histocultural drug sensitivity test. Cancer Lett.
223:103–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ulrich CM, Bigler J, Velicer CM, Greene
EA, Farin FM and Potter JD: Searching expressed sequence tag
databases: discovery and confirmation of a common polymorphism in
the thymidylate synthase gene. Cancer Epidemiol Biomarkers Prev.
9:1381–1385. 2000.PubMed/NCBI
|
|
27
|
Mandola MV, Stoehlmacher J, Muller-Weeks
S, Cesarone G, Yu MC, Lenz HJ and Ladner RD: A novel single
nucleotide polymorphism within the 5′ tandem repeat polymorphism of
the thymidylate synthase gene abolishes USF1 binding and alters
transcriptional activity. Cancer Res. 63:2898–2904. 2003.PubMed/NCBI
|
|
28
|
Ulrich CM, Bigler J, Bostick R, Fosdick L
and Potter JD: Thymidylate synthase promoter polymorphism
interaction with folate intake and risk of colorectal adenomas.
Cancer Res. 62:3361–3364. 2002.PubMed/NCBI
|
|
29
|
Won DY, Kim SH, Hur H, Jung H and Jeon HM:
Chemotherapeutic responsibility according to polymorphism of ERCC1,
XRCC1 and GSPT1 in gastric cancer patients receiving oxaliplatin
based chemotherapy. J Korean Surg Soc. 8:350–356. 2010. View Article : Google Scholar
|
|
30
|
Liu YP, Ling Y, Zhang YP and Liu BR:
Predictive values of platinum related gene polymorphisms in gastric
cancer patients on oxaliplatin-based adjuvant chemotherapy.
Zhonghua Yi Xue Za Zhi. 91:256–259. 2011.(In Chinese). PubMed/NCBI
|
|
31
|
Ruzzo A, Graziano F, Kawakami K, Watanabe
G, Santini D, Catalano V, Bisonni R, Canestrari E, Ficarelli R,
Menichetti ET, et al: Pharmacogenetic profiling and clinical
outcome of patients with advanced gastric cancer treated with
palliative chemotherapy. J Clin Oncol. 24:1883–1891. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruzzo A, Graziano F, Loupakis F, Rulli E,
Canestrari E, Santini D, Catalano V, Ficarelli R, Maltese P,
Bisonni R, et al: Pharmacogenetic profiling in patients with
advanced colorectal cancer treated with first-line FOLFOX-4
chemotherapy. J Clin Oncol. 25:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Viguier J, Boige V, Miquel C, Pocard M,
Giraudeau B, Sabourin JC, Ducreux M, Sarasin A and Praz F: ERCC1
codon 118 polymorphism is a predictive factor for the tumor
response to oxaliplatin/5-fluorouracil combination chemotherapy in
patients with advanced colorectal cancer. Clin Cancer Res.
11:6212–6217. 2005. View Article : Google Scholar : PubMed/NCBI
|