Introduction
Multiple myeloma (MM) is a clonal malignant disease
of the plasma cells, characterized by the production of a
monoclonal immunoglobulin, anemia, lytic bone lesions and renal
failure (1). MM is divided into
symptomatic, smouldering multiple myeloma and monoclonal gammopathy
of undetermined significance (2).
Myeloma-associated amyloidosis is found in 12–15% of myeloma
patients, among whom, 30% exhibit asymptomatic disease (3). The amyloid substance, which originates
from immunoglobulin light chains (always of λ type) produced by
myeloma cells, is deposited in various tissues, leading to organ
dysfunction, affecting the heart, liver, kidney, nervous system and
gastrointestinal tract (3,4). The diagnosis of MM-associated
amyloidosis is confirmed based on positive amyloid tissue staining
with Congo red and immunoglobulin light chain subtyping, evidence
of a monoclonal plasma cell disorder and amyloid-related organ
dysfunction (5). In addition, novel
techniques are available for the early diagnosis, including
immunofixation electrophoresis, bone marrow core biopsies with
cluster of differentiation (CD)138 staining, and detection of the
levels of circulating plasma cells, cytogenetic subtypes and
abnormal plasma cell immunophenotype (2,3). Recent
novel agents, including lenalidomide, bortezomib and dexamethasone
combination therapy, demonstrate favorable tolerability for this
disease. It has been reported that MM-associated amyloidosis is an
independent high-risk prognostic factor, while a similar 1-year
survival rate (~80%) has been found in amyloid light-chain (AL)
amyloidosis patients with or without concurrent myeloma (1,3,6). MM-associated amyloidosis has complex and
diverse clinical manifestations. Occasionally, the symptoms are not
typical at the early stage of this disease, resulting in
misdiagnosis. Cutaneous involvement, including macroglossia,
purpura, petechiae and ecchymoses, are occasionally observed as
elements among a constellation of signs, but rarely as the initial
manifestation of systemic amyloidosis, particularly the
myeloma-associated form (5,7). The current study presents a case of skin
purpura with mucosal bleeding and hematuria as the first symptom,
which was later diagnosed as MM.
Case report
A 42-year-old male initially presented on December
15, 2013, with skin purpura and recurrent episodes of hematuria
that had persisted for 6 months. The patient was diagnosed with
Henoch-Schönlein purpura (HSP) nephritis at a local hospital and
received prednisone (30 mg per day) for 3 months. Subsequent to
treatment, the symptoms of purpura and hematuria persisted, so the
patient was admitted to the Second Xiangya Hospital (Changsha,
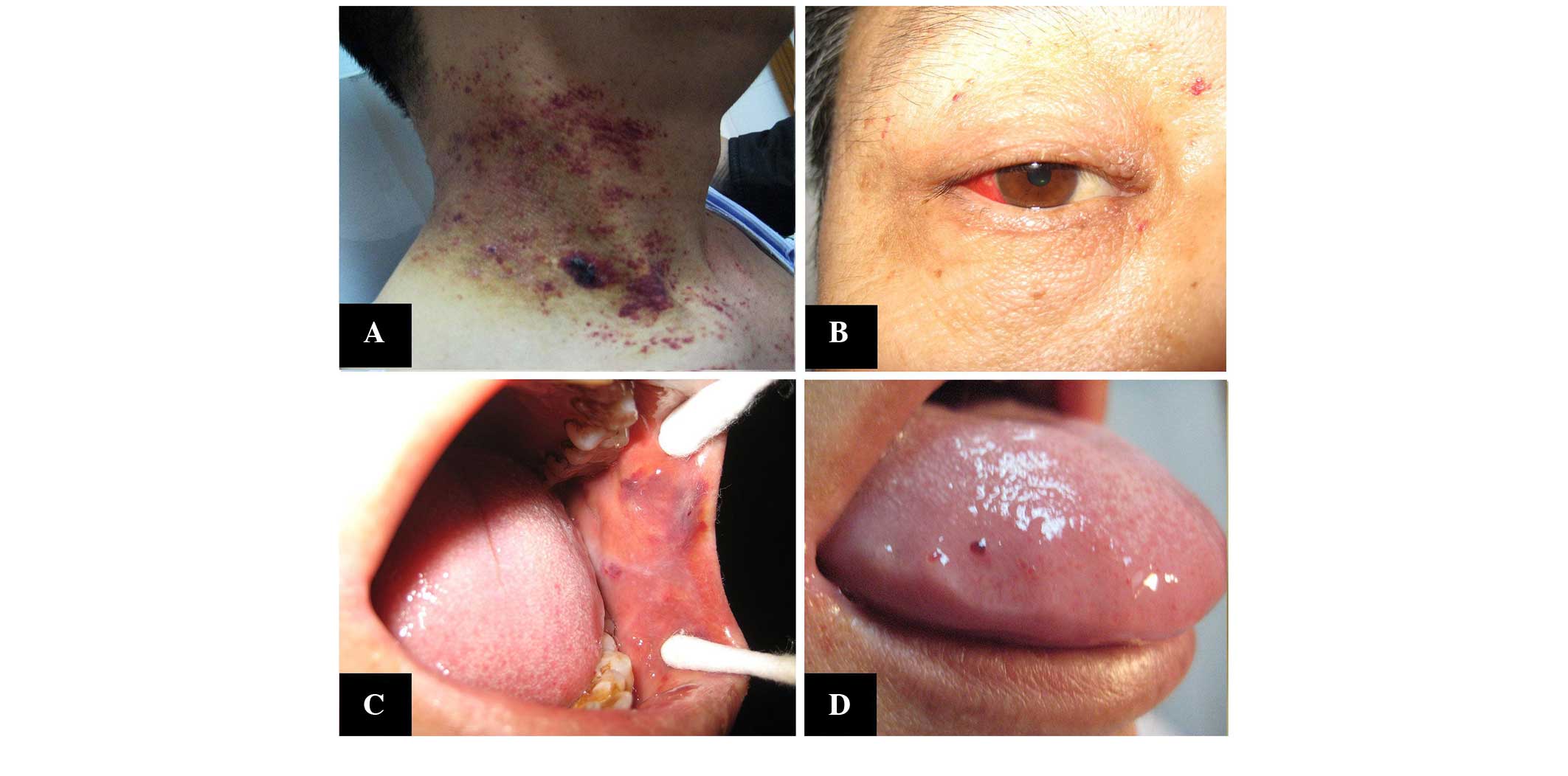
Hunan, China) on March 4, 2014, presenting with purpura, papules
petechiae and spontaneous ecchymosis, which was located scattered
around the neck, chest and limbs, accompanied by a small amount of
bleeding in the conjunctival and oral mucosa, and a swollen tongue
(Fig. 1), No itching, pain or oozing
of the skin purpura was noted. In addition, the patient reported no
dizziness, fatigue, bone pain, joint pain, hair loss, abdominal
pain or fever. Blood tests to determine red blood cell (RBC), white
blood cell, hemoglobin and platelet counts were normal, and renal
function and the serum calcium level were also normal. Urine
analysis showed an abnormally high RBC count of 29,279.9/µl (normal
range, 0–25/µl) and a negative result for protein in the urine. The
coagulation tests (prothrombin time, activated partial
thromboplastin time and fibrinogen level) were within normal
limits. The serum albumin level was 30.9 g/l (reference range,
40–55 g/l), and tests for aspartate aminotransferase, alanine
aminotransferase, total bilirubin and direct bilirubin for liver
function were normal. Tests for human immunodeficiency virus,
hepatitis B and hepatitis C were negative, but the erythrocyte
sedimentation rate was 46 mm/h (reference range, 0–15 mm/h). Serum
immunoglobulin (Ig) tests showed a high level of IgG at 21.60 g/l
(reference range, 7.23–16.8 g/l), but a decreased level of IgA at
0.24 g/l (reference range, 0.69–3.82 g/l) and IgM at 0.16 g/l
(reference range, 0.63–2.77 g/l). C3 and C4 were normal, while the
level of serum κ light chain was 1.69 g/l (reference range,
5.98–13.29 g/l) and the level of λ light chain was 20.40 g/l
(reference range, 2.80–6.65 g/l); the ratio of κ/λ was 0.08
(reference range, 1.47–2.95), and urine light chain analysis
revealed a type of Bence-Jones proteinuria with a monoclonal
increase in λ chain level (2.57 g; reference range, 0–0.02 g/l) and
a normal level of κ chain. The κ/λ ratio was 0.02 (reference range,
1.47–2.95). In addition, serum protein electrophoresis was
performed with the following results: β2-microglobulin, 3.45 mg/l
(reference range, 1–3 mg/l); α1 microglobulin, 2.03% (reference
range, 2.8–5.6%); α2 microglobulin, 6.74% (reference range,
8.4–14.2%); and γ globulin, 31.3% (reference range, 11.5–25.7%).
Other tests, including those for autoantibodies, such as anti-Sm
anti-SSA, anti-SSB and anti-Jo-1 antibodies, were normal, as
detected by an enzyme-linked immunosorbent assay kit (catalog no.
250402002-b; Changzhou Kelai Clinical Labratory, Changzhou,
Jiangsu, China) following the manufacturer's protocols. Factor VIII
activity was normal. Echocardiography and X-rays of the chest,
skull and pelvis were normal; bone-marrow biopsy revealed increased
plasma cells accounting for 16% of the marrow cellularity.
Examination of skin biopsy specimens demonstrated vascular
endothelial cell proliferation in the small blood vessels,
hemorrhage and a large overflow of red blood cells. In addition,
positive staining with Congo red showing apple-green birefringence
was observed under polarized light microscopy (Fig. 2). Furthermore, positive
immunohistochemistry showed a positive reaction for λ light chain,
and T cell markers (CD3, CD4, CD5, CD8 and CD43) were observed, as
detected using rabbit polyclonal CD3 antibody (1:100 dilution;
catalog no. ab5690), rabbit monoclonal CD4 antibody (1:500
dilution; catalog no. ab133616), rabbit monoclonal CD5 antibody
(1:250 dilution; catalog no. ab75877), rabbit polyclonal CD8
antibody with (1:200 dilution; catalog no. ab4055) and mouse
monoclonal CD43 antibody (1:20 dilution; catalog no. ab89691) (all
Abcam, Cambridge, UK), respectively. The specimens were negative
for B cell marker proteins. Based on the history of plasmacytoma,
the skin biopsy and the current presentation, a workup for MM
[lipoarabinomannan (LAM)-IgG merge-free light
chain-type]-associated skin light chain amyloidosis was initiated.
Since the MM was accompanied by amyloidosis, indicating the disease
was active (1), the patient was
referred to an oncologist who began treatment with a VDT regimen as
follows: In the induction of remission therapy, 1.3
mg/m2 intravenous bortezomib on days 1, 4, 8 and 11; 20
mg daily intravenous dexamethasone on days 1, 2, 4, 5, 8, 9, 11 and
12, every 4 weeks for 4 cycles; and 100 mg thalidomide (daily). In
addition, in post-remission therapy, 100 mg thalidomide (daily) was
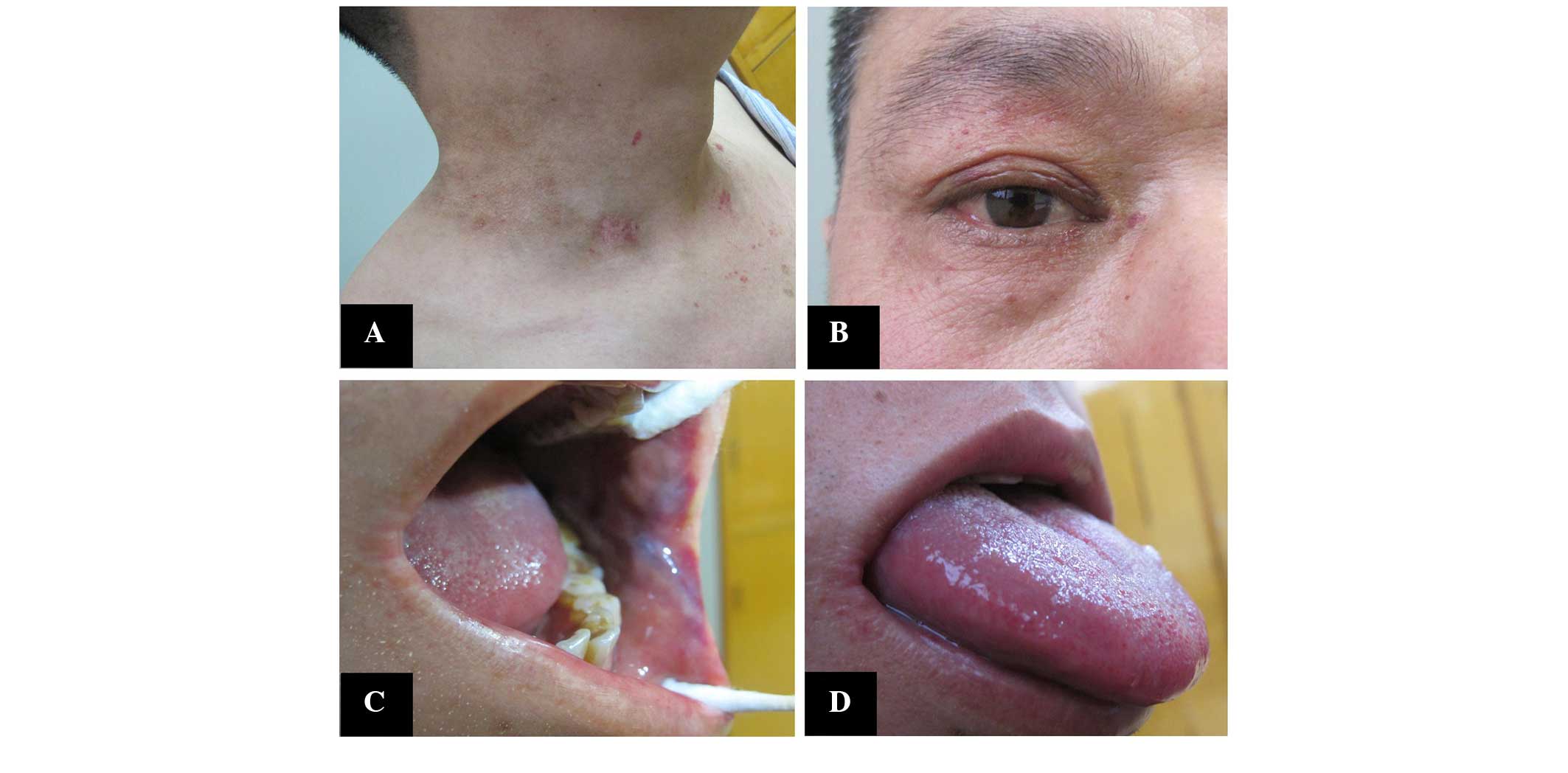
prescribed for 1 year. Following six courses of chemotherapy, the
skin lesions markedly improved (Fig.
3). In January 2015, follow-up examination showed that the
serum IgG level was 17.82 and the serum λ light chain level was
8.82 g/l, which were almost normal levels.
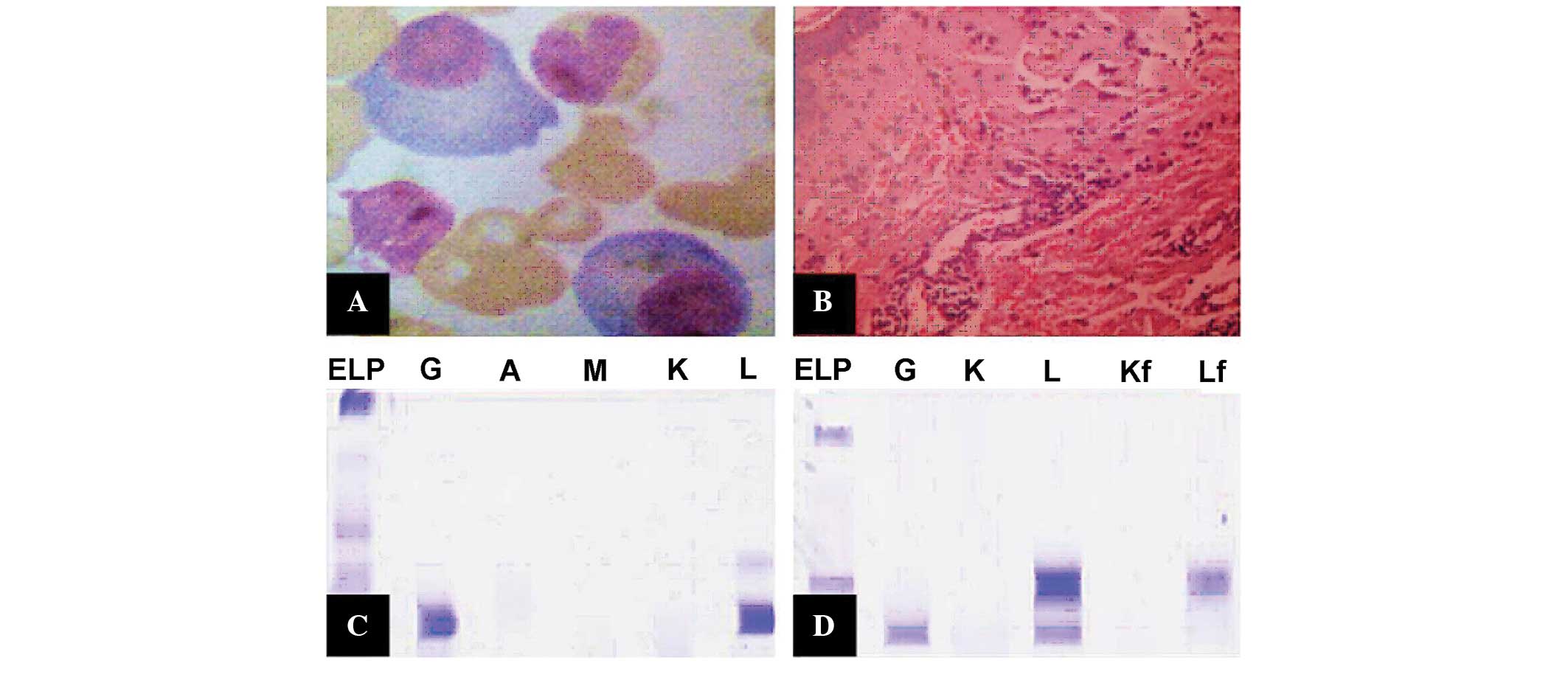 | Figure 2.Laboratory test results. (A)
Wright's-stained bone marrow aspirate smear showing the abnormal
proliferation of plasma cells (×1,000 magnification). (B)
Histopathological examination of the skin showing vascular
endothelial cell proliferation and hemorrhage (hematoxylin and
eosin stain; ×200 magnification). Immunofixation electrophoresis of
the (C) serum and (D) urine. ELP, positive immunofixation
electrophoresis; Ig, immunoglobulin; G, IgG; A, IgA; M, IgM; K, κ
light chain; L, λ light chain; Kf, free κ light chain; Lf, free λ
light chain. |
Discussion
In the present case, the patient presented with skin
purpura and hematuria, without proteinuria or clear bone
destruction and anemia, which can be misdiagnosed as HSP nephritis.
However, following treatment with prednisone for 3 months, the
purpura and hematuria did not improve. HSP is a small-vessel
vasculitis that affects the skin, joints, gut and kidneys. The
feature of a rash in HSP presents as palpable purpuric vasculitis
on the lower limbs spreading over the extensor surfaces to the
buttocks and occasionally to the upper limbs, while skin lesions
often occur on the lips, periorbital regions and eyelids of MM
patients, accompanied with macroglossia (8–10). Thus,
the location of the purpura and skin biopsy is critical in
differentiating between the diagnosis of MM-associated
AL-amyloidosis and HSP. It is believed that skin purpura and
lesions can also appear in autoimmune diseases and tumors,
particularly in malignancies of the blood, such as MM. Therefore,
further tests for serum immunological changes were performed in the
present study, together with a skin biopsy. Increased serum IgG
levels, as detected by serum Ig tests, increased λ chain levels, as
detected by serum and urine light chain analysis, and a urine Bence
Jones protein level of >1 g/24 h was accompanied by an abnormal
result for immunofixation electrophoresis, and positive staining
with Congo red showing apple-green birefringence in the skin biopsy
specimens. Thus the patient was diagnosed with MM-associated skin
amyloidosis with the initial symptom of skin purpura.
AL amyloidosis is almost always associated with a
plasma cell dyscrasia. In total, ~20% of amyloidosis is caused by
MM, however, only 5–15% of patients with MM develop amyloid light
chain deposits (11). A previous
study reported a case of MM preceded by vascular purpura with
cutaneous leukocytoclastic viscosity (12). It has also been reported that the
purpura, papules, spontaneous ecchymosis and petechiae are
occasionally the first and only sign of systemic amyloidosis
(13). However, there have been no
studies reporting MM (LAM-IgG light chain-type)-associated skin
amyloidosis with skin purpura as the initial symptom. The present
study discussed a case of MM-associated amyloidosis, in which the
patient first presented with skin purpura and hematuria, without
other systemic damage, and was thus misdiagnosed with purpura
nephritis in the early stage. Furthermore, MM-associated AL
amyloidosis also varies from other types of kidney disease,
including chronic nephritis, where patients present with hematuria,
proteinuria and anemia, which are usually observed in advanced
stages of chronic nephritis. However, hypertension can also often
be observed in chronic nephritis with impaired renal function. The
mechanisms that cause intracutaneous bleeding may include abnormal
M protein (14) and injury by
tumorocellular infiltration or amyloidosis to the fragile vascular
wall (15). Of course, these
presentations of skin purpura are not specific to systemic
amyloidosis associated with MM, but they may be an important aid to
the diagnosis and direct treatment of this disease in the
clinic.
References
|
1
|
Vela-Ojeda J, García-Ruiz Esparza MA,
Padilla-González Y, Sánchez-Cortes E, García-Chávez J,
Montiel-Cervantes L, Reyes-Maldonado E, Majluf-Cruz A and Mayani H:
Multiple myeloma-associated amyloidosis is an independent high-risk
prognostic factor. Ann Hematol. 88:59–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinner S, Witteles W, Witteles R, Lam A,
Arai S, Lafayette R, George TI, Schrier SL and Liedtke M: The
prognostic value of diagnosing concurrent multiple myeloma in
immunoglobulin light chain amyloidosis. Br J Haematol. 161:367–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Girnius S, Seldin DC, Skinner M, Finn KT,
Quillen K, Doros G and Sanchorawala V: Short and long-term outcome
of treatment with high-dose melphalan and stem cell transplantation
for multiple myeloma-associated AL amyloidosis. Ann Hematol.
89:579–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohashi T, Kikuchi N and Yamamoto T:
Unusual milia amyloidosis as initial signs of multiple
myeloma-associated systemic amyloidosis. Int J Dermatol.
52:981–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagano S, Mori M, Kato A, Ono Y, Aoki K,
Arima H, Takiuchi Y, Tabata S, Yanagita S, Matsushita A, et al:
Therapeutic effects of lenalidomide on hemorrhagic intestinal
myeloma-associated AL amyloidosis. Intern Med. 52:1101–1105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Tahan S, Jan F, Do D and Wu H: Nail
dystrophy as the initial sign of multiple myeloma-associated
systemic amyloidosis. J Cutan Pathol. Mar 8–2016.(Epub ahead of
print). View Article : Google Scholar
|
|
8
|
Vella FS, Simone B and Antonaci S:
Palmodigital purpura as the only skin abnormality in
myeloma-associated systemic amyloidosis. Br J Haematol.
120:9172003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oliver AJ: Multiple myeloma presenting
with amyloid purpura and macroglossia: A case report and literature
review. Compendium. 15(712): 714–716. 1994.
|
|
10
|
Yücel A, Akman A, Denli YG, Acar MA,
Karakas M, Hazar B, Gümürdülü D, Ergin M and Memisoglu HR: A case
of systemic amyloidosis associated with multiple myeloma presented
as macroglossia and purpura. J Eur Acad Dermatol Venereol.
18:378–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HJ, Chang SE, Lee MW, Choi JH and Moon
KC: Systemic amyloidosis associated with multiple myeloma
presenting as periorbital purpura. J Dermatol. 35:371–372. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peterlin P, Ponge T, Blin N, Moreau P,
Hamidou M and Agard C: Paraneoplastic cutaneous leukocytoclastic
vasculitis disclosing multiple myeloma: A case report. Clin
Lymphoma Myeloma Leuk. 11:373–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuñón J, Oliva-Encabo R and Cortés M:
Diagnosis of cardiac amyloidosis by skin lesions. Rev Esp Cardiol.
67:6662014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eby CS: Bleeding and thrombosis risk in
plasma cell dyscrasias. Hematology Am Soc Hematol Educ Program.
2007:158–164. 2007.
|
|
15
|
Lipsker D and Boeckler P: Cutaneous
manifestations of paraproteinemia and their mechanisms. Presse Med.
36:1135–1140. 2007.(In French). View Article : Google Scholar : PubMed/NCBI
|