Introduction
The recent identification of cancer stem cells
(CSCs) or tumor-initiating cells (TICs) in multiple types of human
cancer provides a novel inroad to understanding tumorigenesis at
the cellular level (1). Recent
evidence supports the hypothesis that the TIC population is
responsible for tumor initiation and that TIC are defined by their
ability to self-renew, differentiate and initiate tumors upon
transplantation (2–8) In addition, it has been proposed that
current drugs used to target cancer are only capable of targeting
the differentiated cancer cells and available treatments have the
capability to shrink and de-bulk tumors but are unable to target
the TICs, the population responsible for tumor initiation.
Unfortunately, this inability of current anti-cancer treatments to
target TICs results in the re-establishment of the tumor by the
remaining and viable TIC population (9). Hence, the TIC hypothesis provides a
novel target for the treatment of cancer.
Prostate cancer is the third leading cause of
cancer-associated mortalities among men, behind colon and lung
cancer and leads all non-skin cancer malignancies. Prostate cancer
is initially treated with androgen deprivation therapy by either
surgical castration or medical castration with
gonadotropin-releasing hormone agonists (10). However, the response to androgen
deprivation therapy in the metastatic setting is transient and
tumors progress to castration-resistant prostate cancer, which is
marked by a gain-of-function in androgen receptor (AR) and AR
reactivation. According to the TIC hypothesis, AR would be
expressed in the prostate cancer stem cell since there would be
genetic selection for gain-of-function changes in AR, such as AR
gene amplification (11). TICs are
emerging as being important in prostate cancer metastasis and are
coming to the forefront as targets of therapy. The ability to
purify TICs and study mechanism(s) which may be utilized to target
TICs is very important in the development of future prostate cancer
treatment. Hurt et al (12)
showed that CD44+/CD24− cells purified from
the LNCaP cell line were more clonogenic, tumorigenic, and invasive
than the corresponding depleted cells. Duhagon et al
(13) demonstrated that TICs can be
enriched using a sphere formation assay resulting in the culture of
prostatospheres (PSs). Furthermore, Duhagon et al (13) provided a genomic profile of PSs that
coordinated with the genomic profile of the prostate
CD44+CD24− TIC population demonstrating that
PSs are representative of the TIC population. Klarmann et al
(14) demonstrated that the invasive
cells in the prostate LNCaP cell line are more tumorigenic in
NOD/SCID mice compared with noninvasive cells and have a genomic
profile similar to CD44+CD24− cells as well.
Hence, the CD44+/CD24− cells, the PSs and the
invasive cells in prostate cancer cell lines are all representative
of prostate TICs. These populations of cells express high levels of
stem cell-associated genes, including OCT3/4, BMI, β-catenin, and
smoothened (SMO) which is characteristic of TICs. Additionally,
TICs appear to be more resistant to conventional chemotherapies and
radiation, thereby, contributing to the development of metastatic
and resistant disease (9,15). Given these considerations, the present
study sought to investigate if prostate TICs can be targeted by
Traditional Chinese Medicines (TCM) to result in the prevention of
tumor initiation, progression and relapse.
Herbal therapies and products commonly used in TCM
are attracting increasing attention in the field of cancer. The
principles underlying TCM were established over thousands of years
based on clinical experience and practice. In China, the majority
of cancer patients use some form of Chinese medicine, including
prescription medications and non-prescription medications (16). On a global level, it has been reported
that more than half of all cancer patients now use some form of
complementary/alternative medicine, yet the majority of these
patients do not disclose this use to their physicians (17). There are numerous clinical reports
indicating that patients benefit from TCM treatment including Lin
et al (18), which observed
173 cases of non-small cell lung cancer (NSCLC) patients,
post-surgery, with two years of treatment with standard
chemoprevention alone or combined with TCM herbs: The result of
this study indicated that the relapse and distant metastasis rate
of patients in the TCM group was 45.09% and the control group was
50.6%. Yang et al (19)
evaluated the effectiveness of comprehensive TCM treatment in
reducing the relapse and metastasis of stage II and III colorectal
cancer based on conventional Western medicine (WM) therapy: In this
study, 222 patients were recruited and assigned to two groups based
on whether or not they were additionally treated with TCM
comprehensive therapy. The relapse/metastasis rate in the combined
group at 1-, 2-, 3-, 4-, and 5-years was 0 (0/98), 2.04% (2/98),
11.69% (9/77), 14.06% (9/64), and 21.28% (10/47), respectively
(18). In the group given WM, the
relapse/metastasis rates were 4.80% (5/104), 16.35% (17/104),
21.65% (21/97), 25.93% (21/81), and 38.18% (21/55), respectively,
for 1-, 2-, 3-, 4- and 5-years (19).
The median relapse/metastasis time was 26.5 months in the combined
group and 16.0 months in the WM group. These two studies provide a
strong foundation of evidence that TCM can prohibit the relapse and
metastasis of cancer. Additionally, it has been previously shown
that TCM therapy can also prevent tumorigenesis (20). Liang et al (21) demonstrated that the TCM Liuwei Dihuang
Wan, can prohibit progression of the precancerous disease of
esophageal cancer. In this specific study, 214 patients with
hyperplasia of esophageal epithelial cells were treated with Liu
wei Di Huang Wan and after 2 years, the cancerous changes in the
Liu wei Di Huang Wan treatment group was 1.4%, but in the placebo
group was 6.3% (22).
Unfortunately, the active ingredients in the
majority of TCM herbs and their mechanism(s) have not been
identified. However, it is clear that TCM is capable of preventing
tumorigenesis and both the relapse and metastasis of cancer
(23). Previous studies have
indicated that certain naturally occurring phytochemicals are
cytotoxic to TICs, such as parthenolide (PTL) derived from suayule,
can specifically target TICs in primary human acute myelogenous
leukemia (AML) (24). Additional
studies demonstrated that PTL has toxicity on both the side
population and mammospheres isolated from breast cancer which are
representative of TICs, and lastly, Kawasaki et al (25) demonstrated that PTL is cytotoxic to
prostate TICs. The phytochemical sulphoraphane, derived from
broccoli, can inhibit breast cancer TICs and down-regulate the
Wnt/beta-catenin self-renewal pathway (26). Gossypol, a bioactive phytochemical
produced by cotton plants, was effective at inhibiting prostate
tumor-initiating cell-driven tumor growth in a NOD/SCID xenograft
model (27).
Current evidence suggests that TICs are responsible
for tumorigenesis, relapse and metastasis (9) and can be targeted using naturally
occurring compounds, hence, the present study aimed to determine
whether components of TCM medicine, which are often phytochemicals,
could inhibit or eradicate prostate TICs as well. Cryptotanshinone
(CT), an active component of the Danshen root, is a popular TCM
herb used in the clinic to treat chronic hepatitis, coronary heart
disease (28,29) and cancers such as hepatic cancer
(30) and leukemia (31). The evidence indictes that CT has
biological effects ranging from anti-inflammatory, -bacterial,
-fibrotic, -oxidative, -mutagenic, and -platelet aggregation
activities (29,32–34). There
are a few reports on the antitumor effect of CT, which include
in vitro studies from Gong et al (35) that demonstrated that CT can inhibit
the growth of human prostate cancer cell lines in a dose-dependent
manner via cell cycle arrest and induction of apoptosis. In
addition, Park et al (36)
demonstrated that CT can suppress Bcl-2 expression and augment Fas
sensitivity in DU145 cells. Further work indicates that JNK and p38
MAPK act upstream of Bcl-2 in Fas-treated DU145 cells and that CT
can significantly block activation of these kinases (36). Shin et al (37) showed that CT is a potent anti-cancer
agent and has antitumor activity through the inhibition of STAT3.
Notably, biological behaviors such as cell migration and invasion
are related to TICs, and there are reports indicating that CT has
the ability to block invasion of bovine aortic endothelial cells
induced by bovine fibroblast growth factor (bFGF) (38) and could function in inhibiting
leukocyte chemo tactic migration (39). Taken together, the present study
hypothesized that CT has the potential to target prostate TICs and
investigated if CT was capable of targeting TICs derived from the
LNCap prostate cell line. The results provide evidence that CT can
target TICs and selectively inhibit their proliferation.
Furthermore, the present study presents data that indicates CT
driven inhibition of TICs occurs by regulating the expression of
stemness genes that are associated with the self renewing ability
of TICs. Based on this data, CT, an important TCM compound, has a
potential effect in targeting TICs and it may provide an
alternative means of treatment and options in the investigation for
TIC targeting drugs.
Materials and methods
Cells and media
LNCaP cells were obtained from American Type Culture
Collection (Manassas, VA, USA). Cells were maintained in RPMI-1640
with 10% fetal bovine serum (FBS), 2 mM L-glutamine and penicillin
and streptomycin (all, Gibco®; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Assay of cell viability
Cells were plated at a density of 1,000 cells per
well in a 96-well plate and viability was measured using Cell-Titer
Glo assay (Promega Corporation, Madison, WI, USA). The Cell-Titer
Glo reagent was added to each well and equilibrated for 30 min
before measurements were taken. Luminescence was measured using an
Infinite M200 plate reader (Tecan Group, Ltd., Männedorf,
Switzerland).
Flow cytometric analysis and cell
sorting
LNCaP cells were detached with trypsin
(Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), washed once in FACs buffer [PBS containing 1–2% bovine serum
albumin (BSA; Gibco®; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA)], then stained with 5 µl of Invitrogen
anti-CD24-FITC (mouse monoclonal; catalog no., MHCD2401-4; Thermo
Fisher Scientific, Inc.) and 0.5 µl of antibody per 106
cells of anti-CD44-PE (mouse monoclonal; catalog no., 12-0441-82;
eBioscience, Inc., San Diego, CA, USA) and incubated at 4°C for 15
min. Following incubation, cells were washed twice with FACs
buffer. For flow cytometric sorting, cells were re-suspended in
FACs buffer at 20×106 cells/ml and separated on an Aria
cell sorter (BD Biosciences, San Jose, CA, USA). Live cells were
gated on the basis of forward and side scatter, and single cells
were gated on the basis of forward scatter and pulse width. Gates
were determined by analysis of unstained cells, isotype specific
stains, and single stains (WinMDI version 2.9; BD Biosciences). The
CD44+CD24- cells were not assessed for purity due to the low
numbers of cells obtained.
Apoptosis assay
LNCaP cells were treated with or without varying
concentrations of CT (0, 2.5, 5 and 10 µM). After 48 h, cells were
collected and a quantitative apoptotic death assay was performed
using Annexin V and propidium iodide (PI) staining. After
trypsinization, the cells were re-suspended in the binding buffer
with FITC-conjugated Annexin V and PI (BD Biosciences) for 20 min
at room temperature in the dark. All samples were then FACS
analyzed on an Aria cell sorter as described above to distinguish
early-apoptotic cells, late-apoptotic and necrotic cells.
Cell cycle analysis
In total, 1×104 cells were seeded and treated with
or without CT (catalog no., 10852-200806; National Institutes for
Food and Drug Control, Beijing, China) at different doses according
to the results of the cell viability assay (0, 2.5, 5 and 10 µM).
Following treatment, 1×106 cells were fixed in ice cold 70% ethanol
overnight. Following fixation, cells were centrifuged (5 min; 100 ×
g) and re-suspended in PBS containing 40 µg/ml propidium iodide and
100 µg/ml RNAse A (Sigma-Aldrich, St. Louis, MO, USA) and incubated
at 37°C for 1 h. Samples were then FACS analyzed on an Aria cell
sorter as described above and cell cycle analysis was
performed.
Wound healing
The cells were seeded in a 12-well tissue culture
dish at a concentration of 1×105 cells/well and
maintained in RPMI-1640. After the cells reached 80–90% confluency,
the tip of a micropipette was used to wound the cells, creating a
linear and cross-stripe scrape 2 mm apart. The cells were washed
with PBS to remove floating cellular debris and re-fed for an
additional 24 h with or without CT treatment (1.25, 2.50 and 5.00
µM). Images of wound closure or cell migration were captured when
the scrape wound was introduced and 24 h after wounding, using an
IX70 inverted microscope equipped with a digital camera (Olympus
Corporation, Tokyo, Japan).
Soft agar colony assay
Cells were seeded at a concentration of 1,000 cells
and suspended in RPMI-1640+10% FBS containing 0.6% agarose
(Sigma-Aldrich) and overlaid onto a 12-well plate containing a
solidified bottom layer of RPMI-1640+10% FBS plus agarose. Once the
top layer solidified, 200 µl of medium was placed on top to keep
the plates moist. The plates were incubated for 2 weeks until
colonies were visible. The plates were stained with 5 mM MTT
(Sigma-Aldrich) at 37°C for 1 h, then counted and imaged by using
GelCount™ automatic plate scanner (Oxford Optronics Ltd., Abingdon,
UK) and GelCount Version 0.025.1 software (Oxford Optronics).
Sphere formation assay
In order to obtain prostatospheres from LNCaP cells,
the exponentially growing cultures were dissociated to single cells
by standard trypsinization, washed three times with PBS and plated
in stem cell medium [SCM; Dulbecco's Modified Eagle Medium F12
(Gibco®; Thermo Fisher Scientific, Inc.), 10 ng/ml bFGF
(Sigma-Aldrich), 20 ng/ml endothelial growth factor
(Sigma-Aldrich), 5 mg/ml insulin (Sigma-Aldrich), and 0.4% BSA]
supplemented with 1% KO serum replacement (Invitrogen; Thermo
Fisher Scientific, Inc.) at a density of 1,000 cells/ml in tissue
culture treated flasks. After approximately 7 days, spheres were
counted and analyzed using a GelCount™ automatic plate scanner
(Oxford Optronics) and GelCount Version 0.025.1 software (Oxford
Optronics).
Gene expression analysis
Total RNA isolation was performed using TRIzol
reagent (Gibco®; Thermo Fisher Scientific, Inc.) on
untreated LNcap cells, CT-treated LNcap cells, untreated CSCs cells
that were enriched from LNcap cells and CT-treated CSCs cells that
were enriched from LNcap cells. cDNA was synthesized with
Invitrogen SuperScript III First-Strand Synthesis System for RT-PCR
(Thermo Fisher Scientific, Inc.) using random hexamers and
following the manufacturer's instructions. Analysis of gene
expression by reverse transcription quantitative-polymerase chain
reaction (RT-qPCR) was performed using TaqMan™ Gene Expression
assays (Nanog assay ID, Hs04399610_g1; SOX-2 assay ID,
Hs00367969_m1; OCT4 assay ID, Hs00999632_g1; BMI1 assay ID,
Hs00995536_m1; MMP9 assay ID, Hs00234579_m1; NFκB1 assay ID,
Hs00765730_m1; β-catenin assay ID, Hs00355049_m1; CXCR4 assay ID,
Hs00607978_s1; β-actin assay ID, Hs01060665_g1; Thermo Fisher
Scientific, Inc.) in a StepOne Real-Time PCR machine (Thermo Fisher
Scientific, Inc.). The cycling conditions were as follows: 10 min
at 95°C; and 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Relative mRNA expression levels were calculated as
2−∆∆Cq and were normalized against β-actin (40). The experiment was repeated three
times.
Western blot analysis
Total protein was isolated from LNCaP cells using
RIPA lysis buffer (Thermo Fisher Scientific Inc.) and quantified
using the BCA protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). A total of 20 µg of protein extract was loaded
per lane into a 4–20% Tris-glycine gel (Invitrogen; Thermo Fisher
Scientific Inc.), transferred to a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA), blocked in 5% BSA and
incubated with the following primary polyclonal rabbit antibodies
from Abcam (Cambridge, UK): Anti-Nanog (catalog no., ab80892;
dilution, 1:1,000); anti-actin (catalog no., ab194952; dilution,
1:10,000); anti-CXCR4 (catalog no., ab93478; dilution, 1:1,000);
anti-β-catenin (catalog no., ab6302; dilution, 1:5,000); anti-SOX2
(catalog no., ab97959; dilution, 1:1,000); and anti-OCT4 (catalog
no., ab125949; dilution, 1:1,000). All secondary antibodies
obtained from LI-COR (IRDye® goat anti-rabbit IgG;
catalog no., 926-32221; LI-COR Biosciences, Lincoln, NE, USA).
Blots were scanned using the LI-COR Odyssey IR Imaging System.
Statistical analysis
Unless specified, all experiments were performed in
triplicate and were repeated at least twice. Data is expressed as
mean values, standard error of the mean or standard deviation and
were analyzed by analysis of variance. SPSS version 10.0 (SPSS
Inc., Chicago, IL, USA) was used for analysis. The level of
significance was set at P<0.05.
Results
The anti-proliferative effects of CT
on LNCaP cells
To determine whether CT inhibited cellular
proliferation of the LNCaP line, cells were treated with increasing
concentrations of CT as indicated and cell viability was assessed.
Fig. 1A demonstrates that CT
inhibited cellular proliferation at 0.39 to 50 µM doses for 48 h.
Fig. 1B demonstrates that CT
inhibition of LNCaP proliferation is time-dependent as a time
period of 6–96 h was tested at various doses as indicated. The
IC50 of CT is 5 µM and is most effective after 48 h. The
anti-proliferative effect of CT on LNCaP cells was a dose and
time-dependent. Based on these results, the subsequent experiments
were performed with a dose of 5 µM and the time period the cells
are treated with CT varies based on the experiment performed and is
indicated.
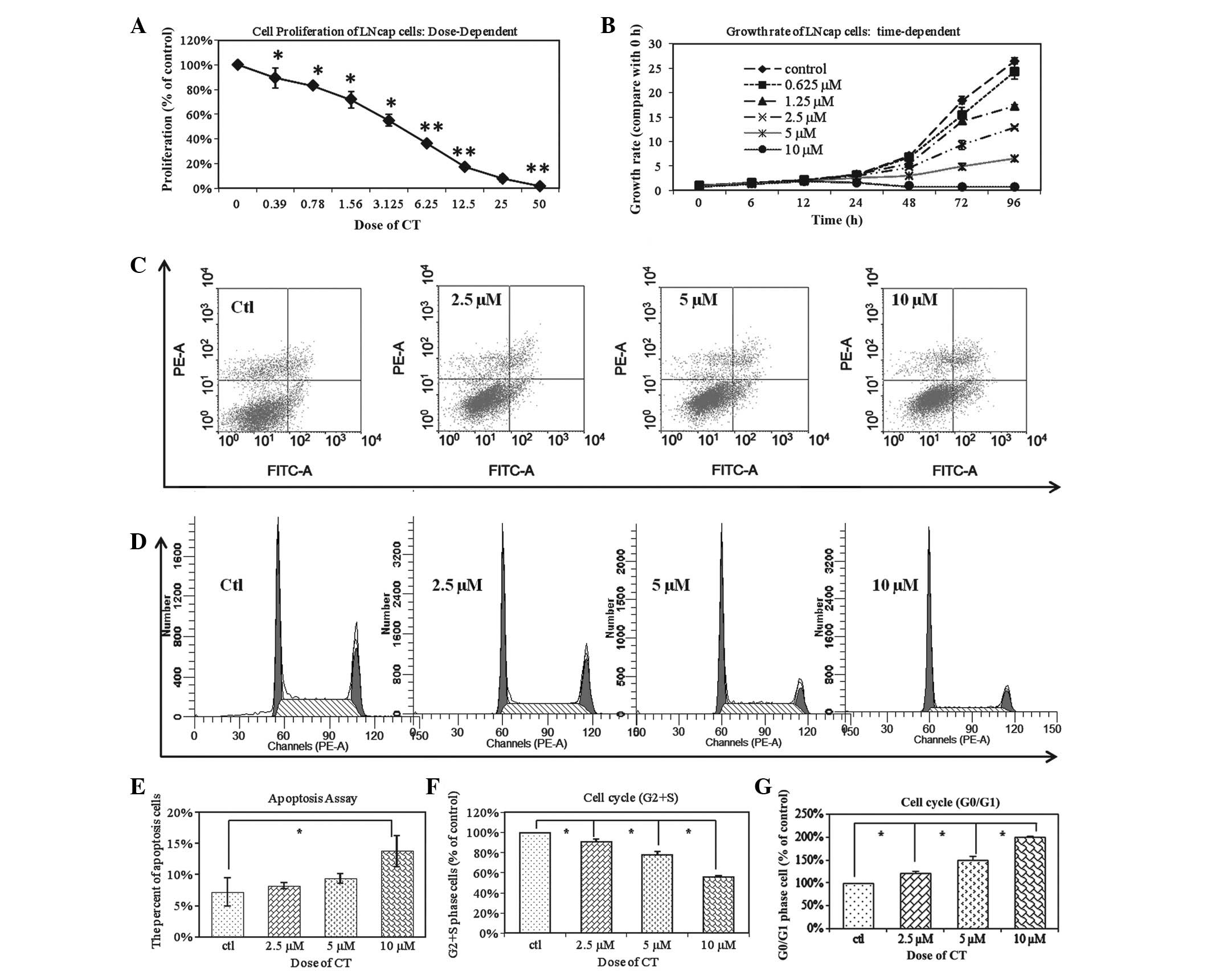 | Figure 1.CT changes the biological behaviors
of total LNCaP cells. (A) LNCaP cells were treated with an
increasing dose of CT ranging from 0.39 to 50 µM for 48 h. CT
inhibited the proliferation of total LNCaP cells in a
dose-dependent manner (*P<0.05; **P<0.01. (B) LNCaP cells
were observed over 6–96 h and treated with 0–10µM CT as indicated.
The IC50 of CT is 5 µM; inhibition was time-dependent
and greatest after 8 h. (C) LNCap cells were treated with
increasing doses of CT (2.5 µM, 5 µM and 10 µM). FACS analysis
demonstrated that CT induced the cell apoptosis of Total LNCaP cell
in a dose-dependent manner. (D) FACS analysis demonstrated that
treatment with CT results in LNCaP cell cycle arrest, in a
dose-dependent manner. (E) Statistical analysis of apoptosis
demonstrates there is a significant difference between the control
group and the 10 µM CT treated groups (*P<0.01). (F) The
relative percentages of CT treated LNCaP cells in the S and G2/S
phases of the cell cycle. The percentage of CT treated cells
distributed at the G2/S phase was significantly reduced in a
dose-dependent manner from 69.73 to 63.5, 54.25 to 39.05% after 48
h of treatment with 2.5, 5 and 10 µM CT, respectively (*P<0.01).
(G) A total of 36.55%, 45.75%, and 60.95% of LNCaP cells were
distributed at the G0/G1 phase after 48 h of treatment with 2.5, 5
and 10 µM CT, respectively. This was in comparison to the 30.27% of
untreated cells in G0/G1 phase (*P<0.01; **P<0.05). FACS,
fluorescent activated cell sorting; CT, cryptotanshinone. |
To further determine whether the inhibition in
proliferation by CT is due to an induction in apoptosis, LNCaP
cells were treated with CT and analyzed apoptosis using FACS
analysis. Fig. 1C demonstrates that
CT has an apoptotic effect on LNCaP cells and this effect is dose
dependent. However, there was a significant difference among the
control group and the 10 µM CT treated groups (Fig. 1E). Based on this observation, the
anti-proliferative effects were analyzed of CT was due to
perturbation of the cell cycle. LNCaP cells were treated with
different concentrations of CT: The number of CT treated LNCaP
cells distributed at the G2/S phase after 48 h of treatment with
2.5, 5 and 10 µM CT was also significantly decreased from 69.73 to
63.51%, 54.25%, and 39.05%, respectively in a dose dependent manner
(Fig. 1F; P<0.01). A total of
36.55, 45.75 and 60.95% of the cells were distributed at the G0/G1
phase after 48 h of treatment with 2.5, 5 and 10 µM CT,
respectively; this was in comparison to the 30.27% of untreated
cells in G0/G1 (Fig. 1G; P<0.01).
This data indicates that CT inhibits cellular proliferation by
inducing G0/G1 phase arrest in cancer cells.
CT affects biological behaviors of
total LNCaP cells
To determine if CT could affect specific biological
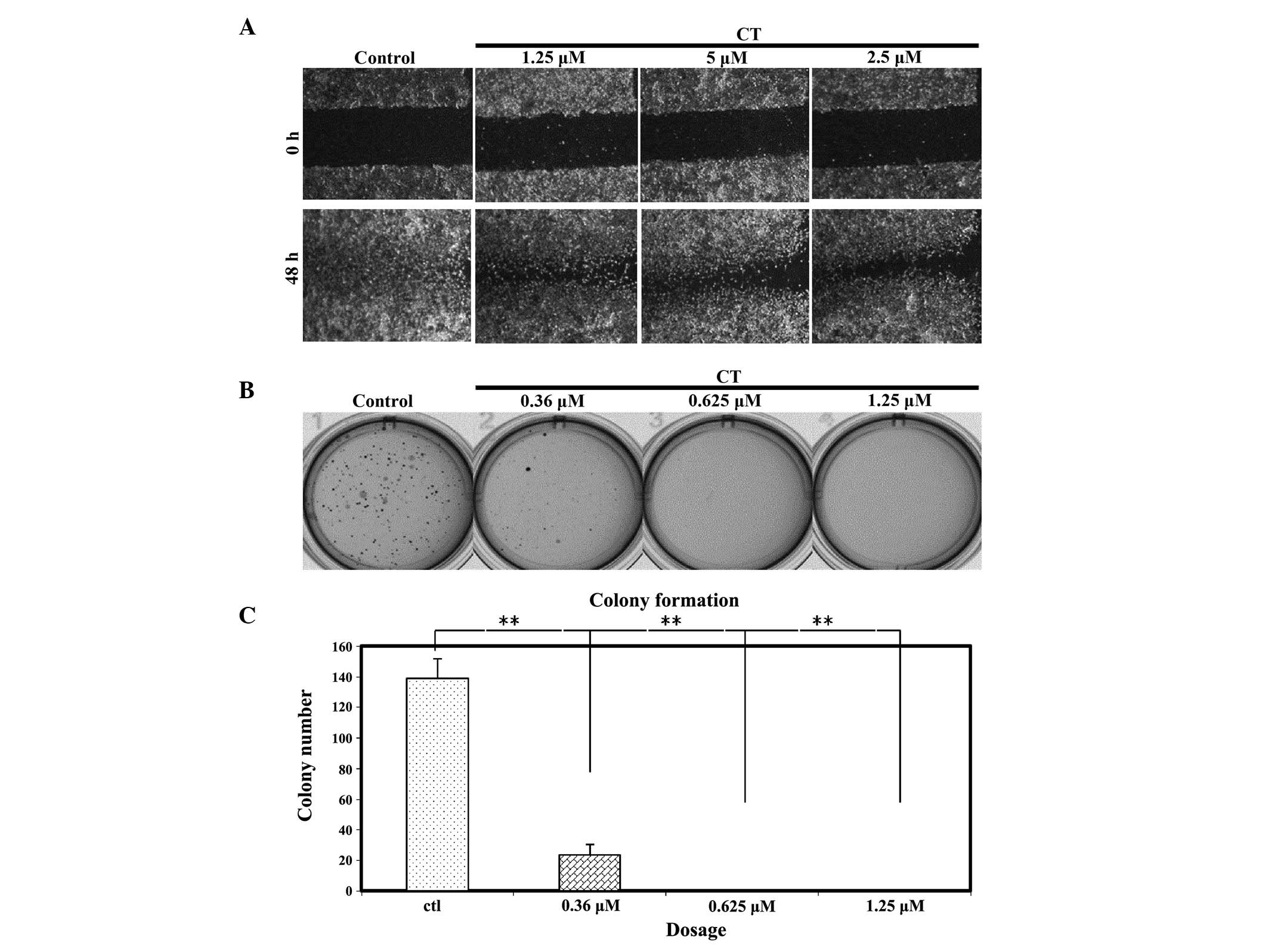
behaviors of LNCap cells such as the ability to migrate, a wound
healing assay was performed on LNCap cells treated with CT
(Fig. 2A). After making a wound,
LNCap cells were treated with various concentrations of CT, as
indicated, and then the wound was inspected microscopically over
time as the cells migrated to fill the damaged area. For the
untreated cells, migration occurred and the wound was healed in 48
h; however, in cells treated with CT, cell migration was partially
inhibited. The data suggests that the level of migration was
affected in a dose dependent manner.
An additional characteristic of cancer cells that
affects their biological behavior is the ability to form colonies
in anchorage independent conditions. The present study investigated
whether CT has the ability to affect colony formation. Fig. 2B and C demonstrates that the colony
formation efficiency of LNCaP cells was significantly inhibited by
CT at very low doses (0.36, 0.625 and 1.25 µM). Notably, at the
IC50 concentration of 5 µM, colony formation did not
occur (data not shown); therefore, a serial dilution of CT was
performed and it was observed that colonies do not form following
CT treatment when the dose is as low as 0.625 µM.
CT affects the biological behaviors of
LNCaP TICs
Based on the above data, CT has the ability to
inhibit cellular proliferation of LNCaP cells in a time and dose
dependent manner; reduced the percentage of cells which are in the
proliferative phase of the cell cycle; prolonged and partially
inhibited the ability to migrate after cell wound healing; induced
apoptosis of LNCaP cells and lastly, inhibited colony formation in
soft agar. As previously stated, there are various phenotypes
associated with prostate TICs such as cell migration and soft agar
colony formation (13,14). Hence, we sought to investigate if CT
has the ability to specifically regulate the biological behaviors
of LNCaP TICs.
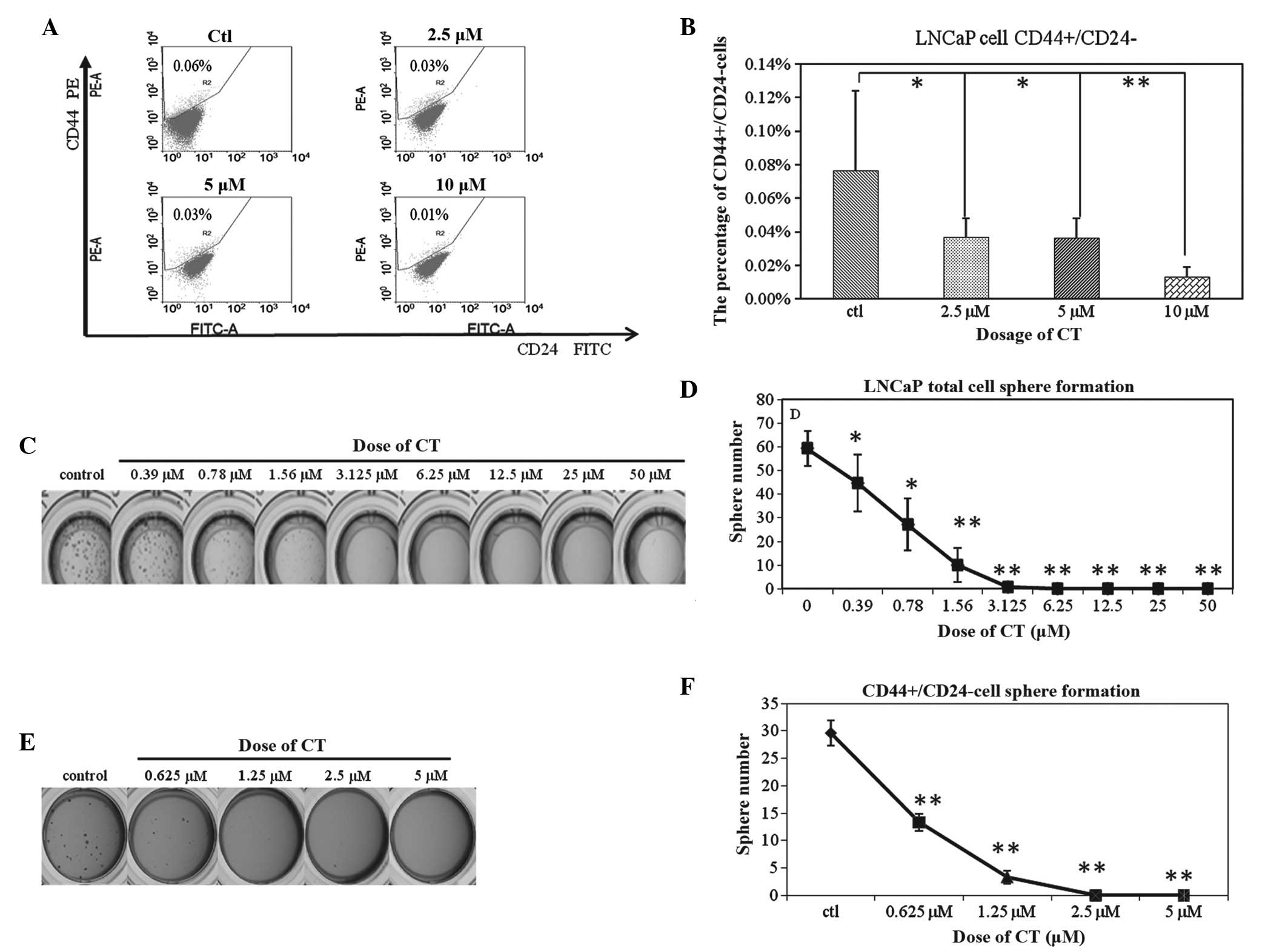
Our laboratory has previously shown that the
CD44+CD24− cell population is the TIC
population in the LNCaP cell line and the present study sought to
investigate if 48 h CT treatment would alter the percentage of
CD44+CD24− cells (14). A dose-dependent decrease of this
subpopulation was observed (Fig. 3A);
0.08±0.05%, 0.04±0.01%, 0.04±0.01% and 0.01±0.01% of the total
cells reflect the CD44+/CD24− population
after 48 h of treatment with 0, 2.5, 5 and 10 µM CT, respectively.
However, these results were not statistically significant (Fig. 3B). Based on these observations, this
decrease may be a result of CT functioning as an inhibitor of TIC
viability, TIC proliferation or as an inducer of differentiation,
resulting in an overall reduction of the TIC population.
Previously our laboratory has shown that the sphere
formation assay can enrich for TICs of LNCaP cells (13), hence, the total cells were cultured in
highly defined stem cell media as previously described and cultured
with or without CT. The results demonstrate that CT inhibit sphere
formation of LNCaP cells at a dose of 1.5 µM (Fig. 3C and D), a dose previously shown to
have little effect on total LNCap viability (less than 30% Fig. 1A). Based on these observations, it was
hypothesized that CT could specifically inhibit the proliferation
or self-renewing ability of TICs. To confirm whether CT could
indeed inhibit TIC self-renewal, the LNCaP CD44+CD24- population
was isolated by FACs sorting and cultured in SCM with or without
CT. As shown in Fig. 3E and F, the
ability to form spheres was decreased from 29.99±2.3 in the control
group to 13.3±1.52 in the 0.625 µM CT group, 3.33±1.15 in the 1.25
µM CT group, and 0 in the 2.5 µM and 5 µM CT group. Based on these
data, evidence is provided that CT inhibits the sphere forming
ability of CD44+CD24− cells. This evidence
supports the hypothesis that CT can target the self-renewing
ability of TICs.
CT regulates stemness genes associated
with LNCaP TICs
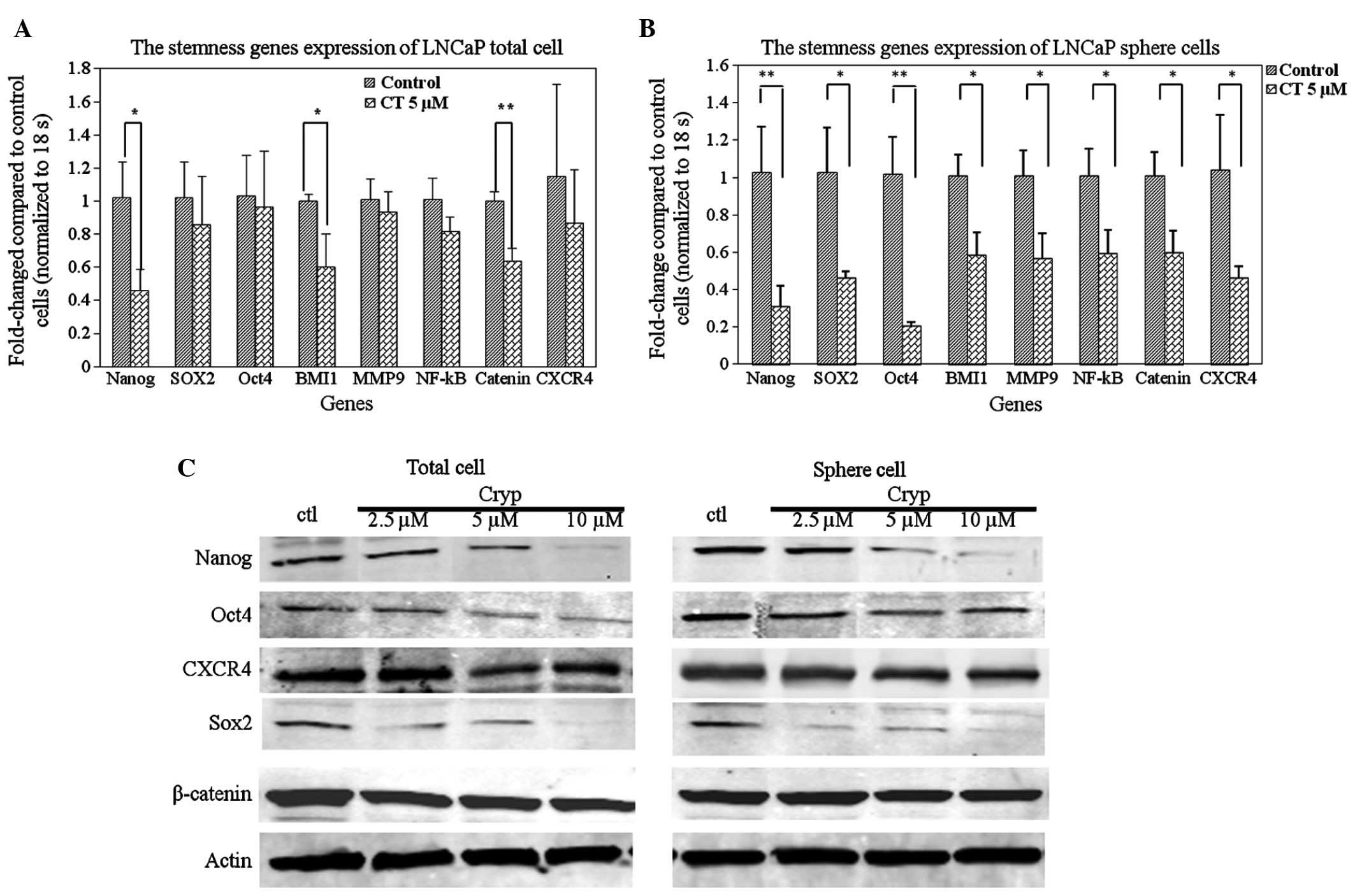
To further understand the possible molecular
mechanisms by which CT can regulate LNCaP TIC self-renewal, the
present study investigated whether CT can inhibit the expression of
TIC related genes implicated in self-renewal by RT-qPCR and western
blot analysis. Nanog, SOX2 and Oct3/4 genes are important
transcription factors orchestrating the self-renewal of stem cells
(41); therefore, these factors and
additional stem associated factors were selected. As shown in
Fig. 4A, it was demonstrated that CT
treatment reduced the expression levels of certain stemness genes
in total cells, specifically the expression of Nanog, BMI1 and
β-catenin: A statistically significant decrease of mRNA levels was
observed between the control and 5 µM CT treated groups (P<0.05,
P<0.05, P<0.01, respectively). To further investigate the
hypothesis that CT specifically affects TICs by decreasing the
expression of stemness genes, RT-qPCR was performed on LNCaP cell
spheres treated with or without CT. Fig.
4B demonstrates that treatment with CT resulted in a decrease
of expression of stemness genes in LNCaP spheres. The decrease in
expression was statistically significant in all the detected genes
(P<0.01). Lastly, western blot analyses confirmed the
observations in the RT-qPCR. CT treatment reduced Nanog, SOX2 and
OCT4 protein expression levels in a dose-dependent manner in total
(Fig. 4C, left) and sphere cells
(Fig. 4C, right).
The Wnt signaling pathway is important in
embryogenesis (42), and is also
essential for the self-renewal of stem cells by preventing cellular
differentiation. Our data shows that β-catenin expression is
down-regulated by CT (Fig. 4C) and
further confirms that CT affects important regulatory factors
involved in self-renewal of prostate TICs. The survival and
maintenance of stem cells requires a balance between the processes
of both self-renewal and differentiation. This is hypothesized to
be regulated by a niche or protective microenvironment that is
required to anchor the stem cells (43,44).
CXCR4, a G-protein coupled receptor, has recently been confirmed as
a TIC marker as well and is expressed at a significantly higher
level in this population compared to the non-TICs (45–48). To
determine if CT could affect CXCR4 expression, the expression
levels of CXCR4 were analyzed in LNCaP TICs at the mRNA and protein
level. The results indicated that CXCR4 is down-regulated at the
mRNA and protein levels in the total and TIC population (Fig. 4). Based on the ability of CT to
regulate stem associated genes and CXCR4, it is possible that CT
also regulates biological behaviors associated with the function of
the SDF1/CXCR4 axis such as migration and metastasis.
Discussion
Our previous work indicates that both sphere forming
and CD44+CD24− populations in LNCaP cells
reflect a population with a capacity to undergo self-renewal and
initiate tumor formation which are major properties of TICs
(12,13). An additional characteristic of TICs is
the upregulation and increased expression of stem associated
transcription factors such as Nanog, SOX2 and OCT4, which are found
to modulate embryonic stem cell self-renewal (41). The evidence supporting the
aggressiveness and ability of these cells to initiate tumors and
function in metastasis has led to the study of these TICs as an
important potential target for cancer therapy. The present study
provides several lines of evidence that CT has the ability to
inhibit the self-renewing ability of prostate TICs. The present
study demonstrated that CT treatment reduced the percentage of
CD44+CD24− prostate cancer cells (Fig. 3A) and reduced the number of LNCaP
derived prostatospheres (Fig. 3B and
C). Lastly, it was demonstrated that CT can regulate several
genes known to regulate LNCaP TICs, including SOX2, Nanog and OCT4
which are critical transcription factors in the regulation of both
ES and TIC cell properties (38)
(Fig. 4). Collectively, this data
indicates that CT can reduce and effect the TIC population.
As recent studies have shown, Nanog, SOX2 and OCT4
serve crucial roles in stem cell self-renewal. These coordinated
transcription factor networks involving OCT4, SOX2, and Nanog have
currently emerged as the master regulatory mechanisms of stem cell
self-renewal and differentiation (49). It has been shown that knockdown of
OCT4 can induce ES cells to differentiate into
trophectoderm-like cells (50) and
knockdown of SOX2 results in ES cell differentiation
(51,52). MicroRNAs responsible for the
regulation of OCT4, SOX2, and Nanog coding
regions are found to modulate embryonic stem cell differentiation
as well (41). Notably, miR-302 which
can target OCT4/SOX2/Nanog has been shown to have a role in
converting differentiated cells to induced pluripotent stem cells
(53,54). However, their dysfunction in cancer
may contribute to the maintenance of an undifferentiated
proliferative phenotype by preventing the expression of
differentiation genes and allowing the expression of genes
promoting stem cell renewal (55,56).
Further evidence supporting the critical role of OCT4, SOX2, Nanog
and Lin28 in stem cells were seen when transfection of these
factors in 293 FT cells demonstrated an impaired ability to
differentiate and form immature ectodermal tumors after they were
transplanted into nude mice (57). In
a lung adenocarcinoma model, OCT4 and Nanog are highly expressed in
CD133+ but not in the CD133− population
(58) and in a glioblastoma model,
Nanog appears to be critical in the ability of undifferentiated
stem cells to undergo self-renewal and is the most differentially
expressed (59). The ability of CT to
inhibit OCT4, SOX2, Nanog by CT confirms that CT can target the
self-renewal ability of prostate TICs.
Another important gene in TIC regulation is
β-catenin and our data further indicates that CT can down-regulate
expression of β-catenin as well. The Wnt signaling pathway is
essential for the maintenance of the majority of tissue stem cell
compartments by preventing cellular differentiation such as seen in
intestinal and hematopoietic stem cells (60). β-catenin is a key protein in the
regulation of this pathway, therefore, inhibition of β-catenin by
CT further confirms that CT can target the self-renewal ability of
prostate TICs.
In order for stem cells to survive and maintain the
balance between self-renewal and differentiation, a niche or
protective microenvironment is required to anchor the stem cells
(43,44,61) CXCR4
is a G-protein coupled receptor that is expressed constitutively in
a wide variety of normal tissues, including lymphatic tissues,
thymus, brain, spleen, stomach, and small intestine (62). Within the microenvironment, both
stromal derived factor (SDF-1) and its receptor CXCR4 is essential
for the cell anchoring process (15,63). It is
well documented that disseminated prostate cancer cells express
CXCR4 and can home to sites where SDF is present such as the bone
and lymph nodes (63). Recently,
CXCR4 was detected on TICs and mRNA levels were found to be
significantly higher in this population in comparison to the
non-TICs (44–48). The upregulation of CXCR4 cell surface
expression corresponded to a significant increase in EMT in
response to SDF1-α in vitro (64,65). In
the present study, CT was shown to down-regulate CXCR4 expression
in total LNCaP cells and the TIC population; hence, it is possible
CT can further regulate TICs by regulating the CXCR4-SDF1 axis and
disrupting its ability to interact with the microenvironment.
However, further work needs to be performed to confirm this.
In conclusion, this is the first report
demonstrating a role for CT as a regulator and inhibitor of
prostate TICs. CT can specifically inhibit the self-renewal of TICs
by targeting key transcriptional regulators. The data demonstrating
that CT can regulate these factors indicates that CT has the
potential to function as a natural anti-cancer agent targeting
prostate TICs.
Acknowledgements
The authors acknowledge Ms Kathleen Noer, Ms Roberta
Matthai and Ms Guity Mohammadi for their expert technical
assistance in FACs separation and analysis of the cells lines. The
authors also thank Dr. Jeffery White, Dr. Libin Jia for support for
this project in the Office of Cancer Complementary and Alterative
Medicine, National Cancer Institute (Rockville, MD, USA). This work
has been funded in part with Federal funds from the National Cancer
Institute, National Institutes of Health, U.S. (contract no.,
HHSN261200800001E). This research was supported in part by the
National Natural Science Foundation of China (grant no., 81473467).
The content of this paper does not necessarily reflect the views or
policies of the Department of Health and Human Services, nor does
mention of trade names, commercial products, or organizations imply
endorsement by the US Government.
References
|
1
|
Harris MA, Yang H, Low BE, Mukherjee J,
Guha A, Bronson RT, Shultz LD, Israel MA and Yun K: Cancer stem
cells are enriched in the side population cells in a mouse model of
glioma. Cancer Res. 68:10051–10059. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haraguchi N, Inoue H, Tanaka F, Mimori K,
Utsunomiya T, Sasaki A and Mori M: Cancer stem cells in human
gastrointestinal cancers. Hum Cell. 19:24–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: An old idea-a paradigm shift. Cancer Res. 66:1883–1890;
discussion 1895–1896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharifi N, Gulley JL and Dahut WL:
Androgen deprivation therapy for prostate cancer. JAMA.
294:238–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharifi N, Hurt EM and Farrar WL: Androgen
receptor expression in prostate cancer stem cells: Is there a
conundrum? Cancer Chemother Pharmacol. 62:921–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurt EM, Kawasaki BT, Klarmann GJ, Thomas
SB and Farrar WL: CD44+ CD24(−) prostate cells are early cancer
progenitor/stem cells that provide a model for patients with poor
prognosis. Br J Cancer. 98:756–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duhagon MA, Hurt EM, Sotelo-Silveira JR,
Zhang X and Farrar WL: Genomic profiling of tumor initiating
prostatospheres. BMC Genomics. 11:3242010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klarmann GJ, Hurt EM, Mathews LA, Zhang X,
Duhagon MA, Mistree T, Thomas SB and Farrar WL: Invasive prostate
cancer cells are tumor initiating cells that have a stem cell-like
genomic signature. Clin Exp Metastasis. 26:433–446. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu S, Li N and Lv J: Investigation of the
use of Chinese medicine in patients with malignant conditions.
Zhong Guo Zhong Yi Yao Xin Xi Za Zhi. 17:1–3. 2010.(In
Chinese).
|
|
17
|
Smith M and Boon HS: Counseling cancer
patients about herbal medicine. Patient Educ Couns. 38:109–120.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HS and Zhang Y: Evidence-based medical
study of TCM on no small cell lung cancer. Shi Jie Ke Xue Ji
Shu-Zhong Yi Yao Xian Dai Hua Za Zhi. 10:121–125. 2008.(In
Chinese).
|
|
19
|
Yang YF, Ge JZ, Wu Y, Xu Y, Liang BY, Luo
L, Wu XW, Liu DQ, Zhang X, Song FX and Geng ZY: Cohort study on the
effect of a combined treatment of traditional Chinese medicine and
western medicine on the relapse and metastasis of 222 patients with
stage I and III colorectal cancer after radical operation. Chin J
Integr Med. 14:251–256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Yan S and Jiang T: Inhibitory
effect of liuwei dihuang decoction on induced mutation and
spontaneous tumor. Zhong Xi Yi Jie He Za Zhi. 10:433–435. 1990.(In
Chinese). PubMed/NCBI
|
|
21
|
Liang JT, Yan CY, Wang SF, Wu G and Wen
PG: Experimental cancer research Liuwei Dihuang Wan prevention.
Zhong Yi Za Zhi. 6:71–74. 1983.(In Chinese).
|
|
22
|
He XX, Yan C and Endi W: The analysis of
the cyto-diagnosis results of liuweidihuangwan treating esophagel
precancerous disease and gastric precancerous disease. Hebei Yiyao.
9:4–6. 1998.(In Chinese).
|
|
23
|
Zhang Y and Lin HS: Tumor stem cells may
be the final target of traditional Chinese medicine in preventing
cancer recurrence and metastasis. Zhongguo Zhong Xi Yi Jie He Za
Zhi. 29:461–463. 2009.(In Chinese). PubMed/NCBI
|
|
24
|
Guzman ML, Rossi RM, Karnischky L, Li X,
Peterson DR, Howard DS and Jordan CT: The sesquiterpene lactone
parthenolide induces apoptosis of human acute myelogenous leukemia
stem and progenitor cells. Blood. 105:4163–4169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawasaki BT, Hurt EM, Kalathur M, Duhagon
MA, Milner JA, Kim YS and Farrar WL: Effects of the sesquiterpene
lactone parthenolide on prostate tumor-initiating cells: An
integrated molecular profiling approach. Prostate. 69:827–837.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhang T, Korkaya H, Liu S, Lee HF,
Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS and Sun D:
Sulforaphane, a dietary component of broccoli/broccoli sprouts,
inhibits breast cancer stem cells. Clin Cancer Res. 16:2580–2590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volate SR, Kawasaki BT, Hurt EM, Milner
JA, Kim YS, White J and Farrar WL: Gossypol induces apoptosis by
activating p53 in prostate cancer cells and prostate
tumor-initiating cells. Mol Cancer Ther. 9:461–470. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stickel F, Brinkhaus B, Krähmer N, Seitz
HK, Hahn EG and Schuppan D: Antifibrotic properties of botanicals
in chronic liver disease. Hepatogastroenterology. 49:1102–1108.
2002.PubMed/NCBI
|
|
29
|
Zhou L, Chow M and Zuo Z: Improved quality
control method for Danshen products-consideration of both
hydrophilic and lipophilic active components. J Pharm Biomed Anal.
41:744–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng ZS, Rao RS, Ni YW, Tan YS and Gong
ZF: Hepatic artery blood medicine treatment of advanced liver
cancer efficacy. Zhong Xi Yi Jie He Za Zhi. 13:330–332. 1993.(In
Chinese).
|
|
31
|
Zhang X, Liu ZH, Liu Y, Zhang ZH, Wan CC,
Xia YJ, Jiang Z, Jin YJ, Wang YW and Lu GQ: The effect of compound
prescription salvia miltiorrhiza inoculation fluid (CPSMIF) in the
treatment of leukemia patients combined with acute tumor
dissolution synthesis (ATDS). Xian Dai Zhong Liu Yi Xue Za Zhi.
18:1204–1206. 2010.(In Chinese).
|
|
32
|
Kim SY, Moon TC, Chang HW, Son KH, Kang SS
and Kim HP: Effects of tanshinone I isolated from salvia
miltiorrhiza bunge on arachidonic acid metabolism and in vivo
inflammatory responses. Phytother Res. 16:616–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ng TB, Liu F and Wang ZT: Antioxidative
activity of natural products from plants. Life Sci. 66:709–723.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sung HJ, Choi SM, Yoon Y and An KS:
Tanshinone IIA, an ingredient of salvia miltiorrhiza BUNGE, induces
apoptosis in human leukemia cell lines through the activation of
caspase-3. Exp Mol Med. 31:174–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong Y, Li Y, Lu Y, Li L, Abdolmaleky H,
Blackburn GL and Zhou JR: Bioactive tanshinones in salvia
miltiorrhiza inhibit the growth of prostate cancer cells in vitro
and in mice. Int J Cancer. 129:1042–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park IJ, Kim MJ, Park OJ, Park MG, Choe W,
Kang I, Kim SS and Ha J: Cryptotanshinone sensitizes DU145 prostate
cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and
MAPK regulation. Cancer Lett. 298:88–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shin DS, Kim HN, Shin KD, Yoon YJ, Kim SJ,
Han DC and Kwon BM: Cryptotanshinone inhibits constitutive signal
transducer and activator of transcription 3 function through
blocking the dimerization in DU145 prostate cancer cells. Cancer
Res. 69:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hur JM, Shim JS, Jung HJ and Kwon HJ:
Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in
vitro. Exp Mol Med. 37:133–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Z, Zheng J and Xu W: Study on the
effect of ofloxacin and tanshinone IIA on human leukocyte
chemotactic migration in vitro. Zhongguo Yi Xue Ke Xue Yuan Xue
Bao. 19:232–235. 1997.(In Chinese). PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, OCT4 and SOX2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Funayama N, Fagotto F, McCrea P and
Gumbiner BM: Embryonic axis induction by the armadillo repeat
domain of beta-catenin: Evidence for intracellular signaling. J
Cell Biol. 128:959–968. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L and Neaves WB: Normal stem cells and
cancer stem cells: The niche matters. Cancer Res. 66:4553–4557.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avigdor A, Goichberg P, Shivtiel S, Dar A,
Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, et al:
CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of
human CD34+ stem/progenitor cells to bone marrow. Blood.
103:2981–2989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ehtesham M, Mapara KY, Stevenson CB and
Thompson RC: CXCR4 mediates the proliferation of glioblastoma
progenitor cells. Cancer Lett. 274:305–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Salmaggi A, Boiardi A, Gelati M, Russo A,
Calatozzolo C, Ciusani E, Sciacca FL, Ottolina A, Parati EA, La
Porta C, et al: Glioblastoma-derived tumorospheres identify a
population of tumor stem-like cells with angiogenic potential and
enhanced multidrug resistance phenotype. Glia. 54:850–860. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Soeda A, Park M, Lee D, Mintz A,
Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T,
Kassam AB, et al: Hypoxia promotes expansion of the CD133-positive
glioma stem cells through activation of HIF-1alpha. Oncogene.
28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kashyap V, Rezende NC, Scotland KB,
Shaffer SM, Persson JL, Gudas LJ and Mongan NP: Regulation of stem
cell pluripotency and differentiation involves a mutual regulatory
circuit of the NANOG, OCT4 and COX2 pluripotency transcription
factors with polycomb repressive complexes and stem cell microRNAS.
Stem Cells Dev. 18:1093–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chew JL, Loh YH, Zhang W, Chen X, Tam WL,
Yeap LS, Li P, Ang YS, Lim B, Robson P and Ng HH: Reciprocal
transcriptional regulation of Pou5f1 and SOX2 via the OCT4/SOX2
complex in embryonic stem cells. Mol Cell Biol. 25:6031–6046. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B,
Ng HH and Robson P: Transcriptional regulation of nanog by OCT4 and
SOX2. J Biol Chem. 280:24731–24737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu
DT, Chen DT and Ying SY: Mir-302 reprograms human skin cancer cells
into a pluripotent ES-cell-like state. RNA. 14:2115–2124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wilson KD, Venkatasubrahmanyam S, Jia F,
Sun N, Butte AJ and Wu JC: MicroRNA profiling of human-induced
pluripotent stem cells. Stem Cells Dev. 18:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-MicroRNAS with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A MicroRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Oka Y, Nakajima K, Nagao K, Miura K, Ishii
N and Kobayashi H: 293FT cells transduced with four transcription
factors (OCT4, SOX2, NANOG and LIN28) generate aberrant ES-like
cells. J Stem Cells Regen Med. 6:149–156. 2010.PubMed/NCBI
|
|
58
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of OCT4 and nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Field M, Alvarez A, Bushnev S and Sugaya
K: Embryonic stem cell markers distinguishing cancer stem cells
from normal human neuronal stem cell populations in malignant
glioma patients. Clin Neurosurg. 57:151–159. 2010.PubMed/NCBI
|
|
60
|
Kléber M and Sommer L: Wnt signaling and
the regulation of stem cell function. Curr Opin Cell Biol.
16:681–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Weidt C, Niggemann B, Kasenda B, Drell TL,
Zänker KS and Dittmar T: Stem cell migration: A quintessential
stepping stone to successful therapy. Curr Stem Cell Res Ther.
2:89–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cronin PA, Wang JH and Redmond HP: Hypoxia
increases the metastatic ability of breast cancer cells via
upregulation of CXCR4. BMC Cancer. 10:2252010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Onoue T, Uchida D, Begum NM, Tomizuka Y,
Yoshida H and Sato M: Epithelial-mesenchymal transition induced by
the stromal cell-derived factor-1/CXCR4 system in oral squamous
cell carcinoma cells. Int J Oncol. 29:1133–1138. 2006.PubMed/NCBI
|