Introduction
Coronary artery fistulas (CAFs) are rare coronary
anomalies in which abnormal connections exist between a coronary
artery and a cardiac chamber or a major vessel (1,2). CAFs are
present in 0.002% of the global population and comprise 48.7% of
all congenital coronary anomalies. The fistula may be large
(>250 mm) and tends to enlarge over time (3). According to the American College of
Cardiology/American Heart Association guidelines (4), percutaneous or surgical closure is
strongly recommended for the treatment of a large fistula. Atrial
myxomas are the most prevalent type of primary heart tumor, and
present with nonspecific symptoms of right heart failure, syncope,
exertional dyspnea and pulmonary embolism (5,6). Cardiac
myxomas most frequently arise in the left atrium (85%), and rarely
in the right atrium (10%) or the ventricles (5%). Although this
tumor is histologically benign, it should be classified as a
potentially fatal tumor due to the potential for embolic
complications, and the standard treatment for cardiac myxoma is
surgical removal (7). The present
report describes the case of a patient with right CAF draining to
the myocardial void, which was misdiagnosed as right atrial myxoma
(RAM).
Case report
A 33-year-old man was admitted to The First
Affiliated Hospital of Zhengzhou University for intermittent chest
pain. The patient had a 13-year history of chest pain accompanied
by a sense of impending doom following activity, which was relieved
by rest. The duration, severity and frequency of the pain was
aggravated 3 days prior to hospitalization.
Upon physical examination, the patient's heart rate
was regular at 50 bpm, blood pressure was 112/70 mmHg and body
temperature was 36.8°C. The lips and fingernails were neither
cyanotic nor clubbed. No thrills were detected in the precordial
region. Heart and lung sounds were normal, with no dry or moist
rales and no arrhythmia or cardiac murmurs.
All routine laboratory data were within normal
limits. A chest X-ray (Multix Fusion; Siemens AG, Munich, Germany)
indicated cardiomegaly, predominantly on the right side. In
addition, electrocardiography (ECG; 6951E; Nihon Kohden, Tokyo,
Japan) demonstrated right atrial enlargement, ST-segment depression
on the right precordial ECG and sinus bradycardia, with a heart
rate of 51 bpm. Echocardiography (Vivid 3; GE Vingmed Ultrasound,
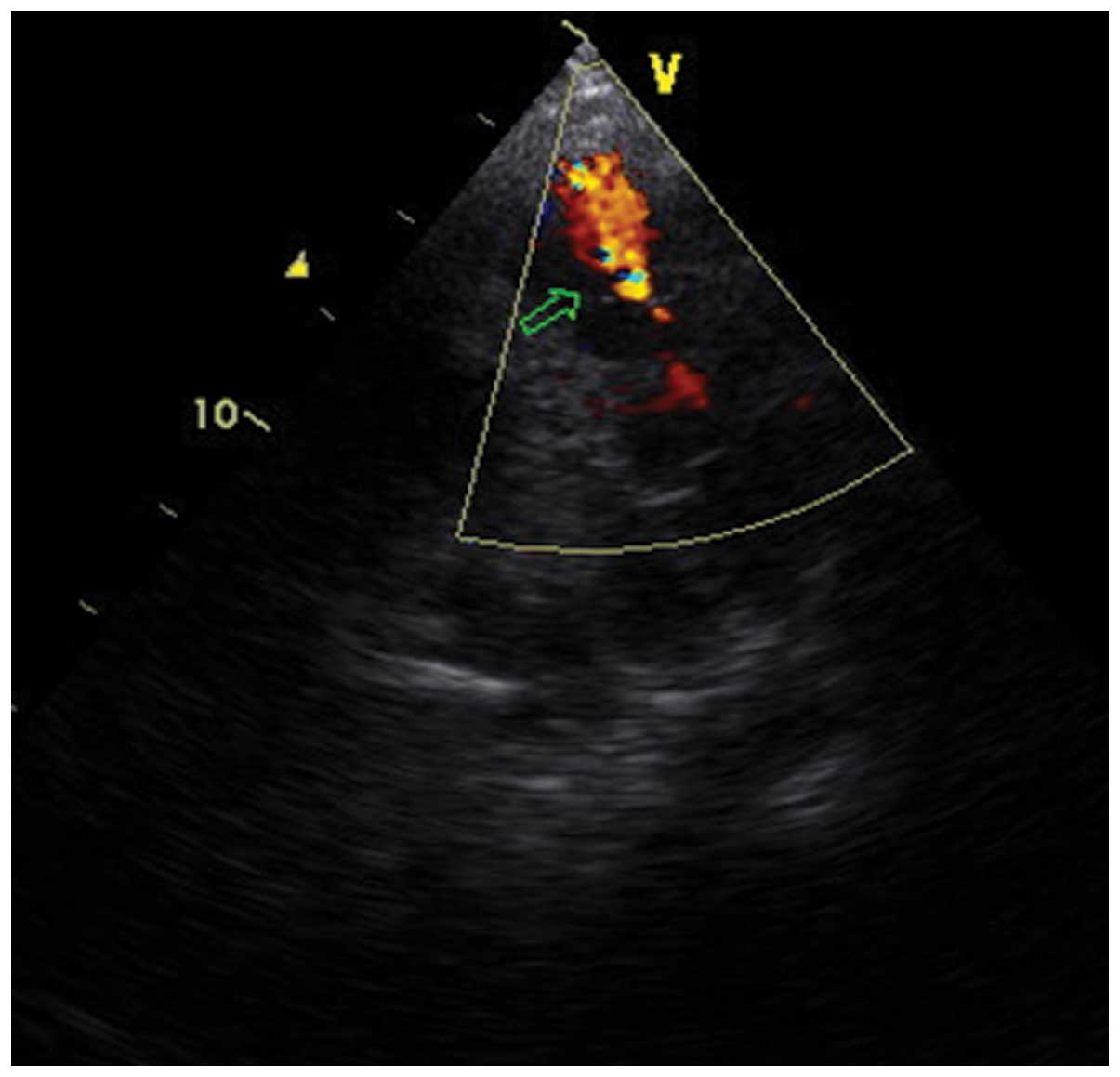
Horten, Norway) revealed an occupying mass in the right atrium
producing a partial dynamic tricuspid obstruction (Fig. 1). Further complete catheterization was
not conducted due to the risk of causing pulmonary embolization.
The condition was diagnosed as RAM, which typically causes partial
right ventricular inflow tract obstruction, based on the presence
of the occupying mass in the right atrium on echocardiography
(8).
Following preoperative preparation, the patient was
taken for cardiopulmonary bypass surgery (CPB). The patient's
hemodynamics, blood oxygen saturation, ECG, body temperature and
urine volume were monitored. The heart was approached through
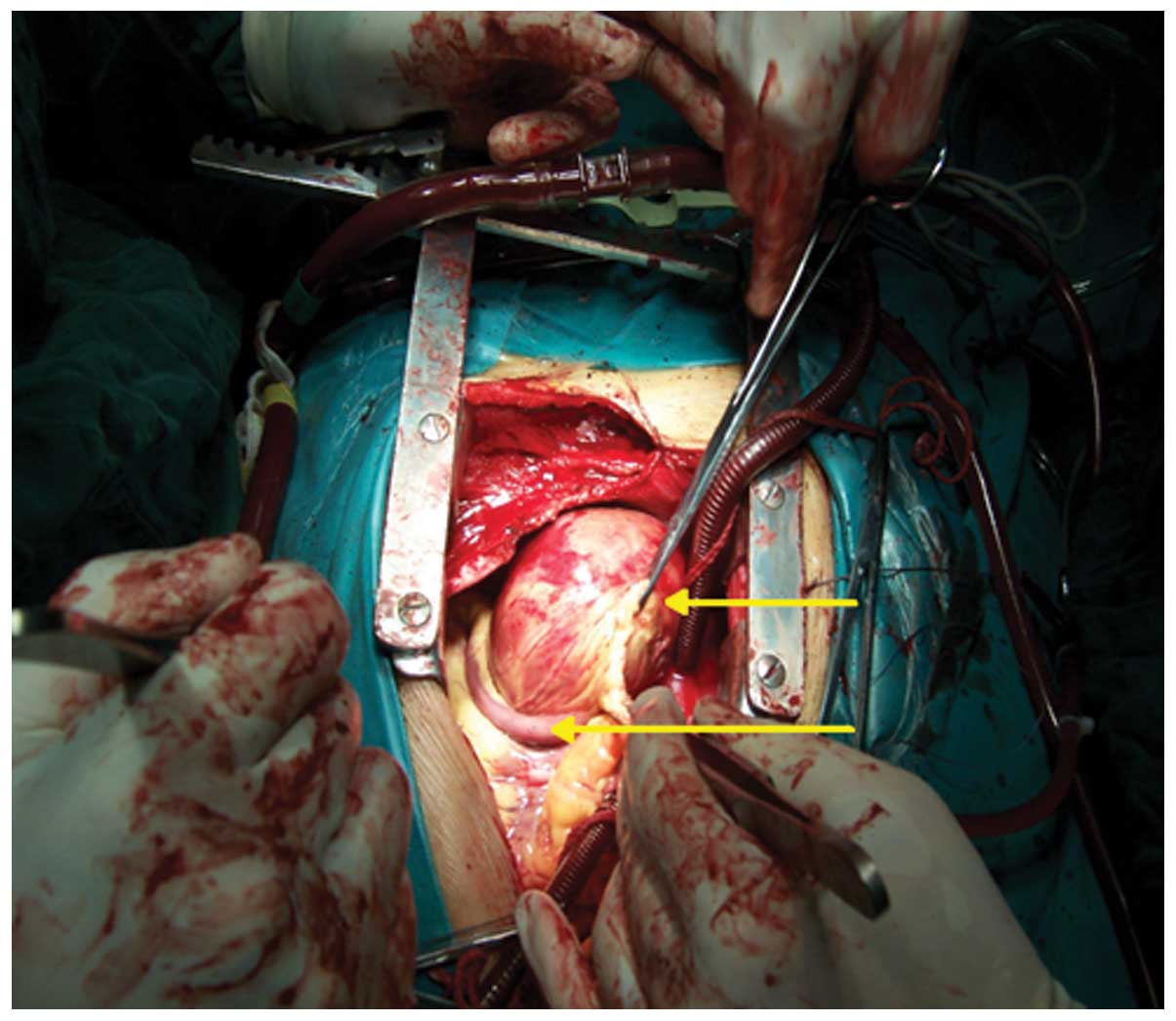
median sternotomy and CPB was performed. During surgery, the right
atrium was opened; however, no occupying mass was found in the
right atrium. Unexpectedly, a giant cyst was located in the
anterior wall of right ventricle, the posterior part of which was
attached to the anterior tricuspid annulus (Fig. 2). In order to acquire a pathological
diagnosis of the mass, a quick frozen pathological section was
rapidly analyzed, and the pathological result indicated the
presence of myocardium, fibrous tissue and thrombus.
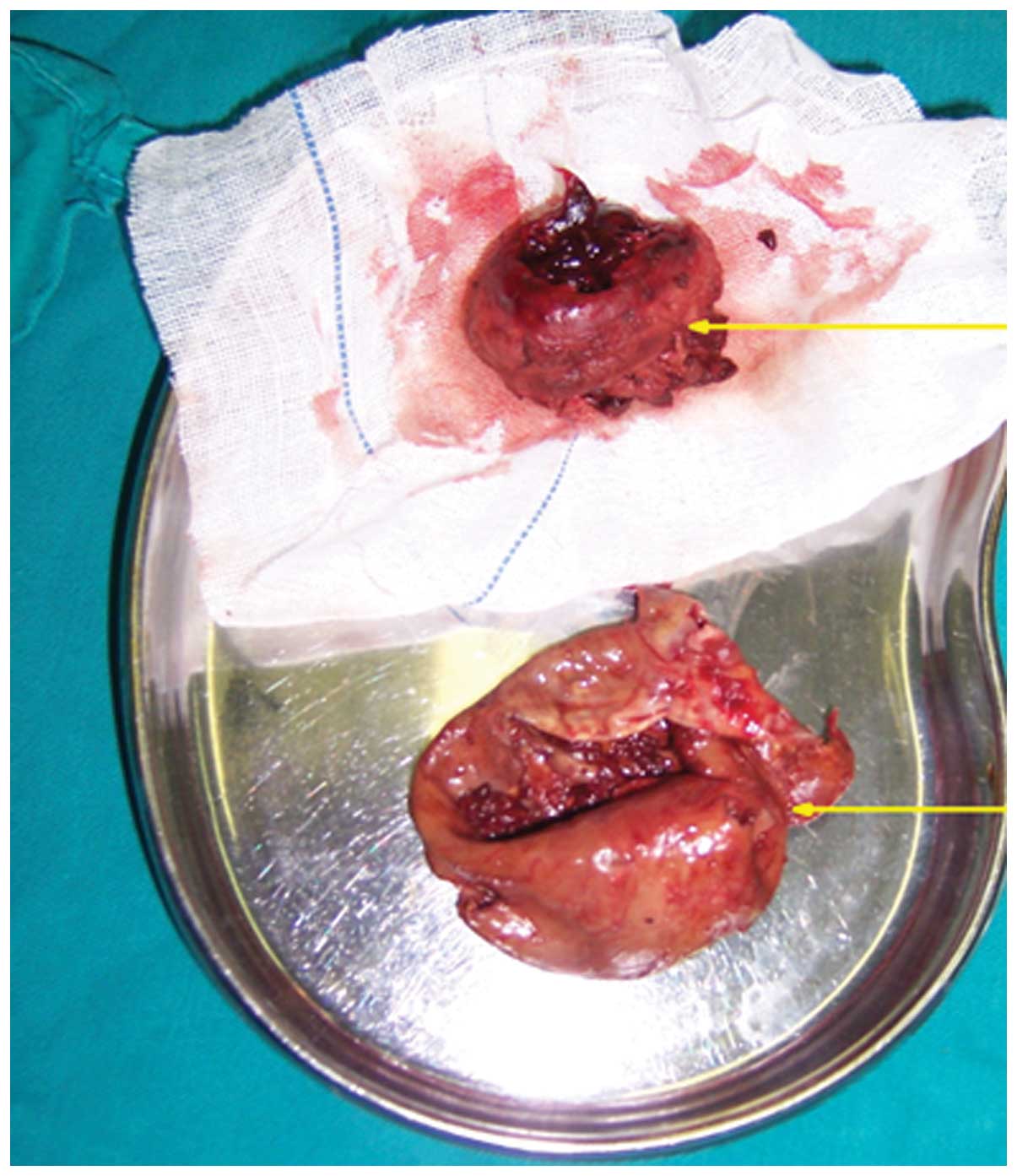
The hemorrhagic cyst was removed intact, and was
found to be filled with old thrombus and dark-gray necrotic tissues
(Fig. 3). An abnormal vascular
fistula of ~8 mm in diameter was discovered at the bottom of the
cyst. To clarify the fistulous communication, blood cardioplegic
solution was injected into the right coronary sinus and was
observed to overflow from the vascular fistula. Therefore, the
patient was diagnosed with right CAF draining to the myocardial
void, and neither the right atria or the right ventricle. The
fistula was sutured directly with a double-ended needle using an
interrupted mattress suture technique. No palpable thrill was
detected subsequently. No changes in baseline ECG, blood pressure,
pulse rate or blood oxygen saturation were observed during this
period. The patient was brought to the cardiac intensive care unit
and treated with diuresis and nitroglycerin for blood vessel
expansion. Ten days after the operation, the patient was discharged
from the hospital. Re-examination one month later revealed no
residual fistula flow.
The post-operative pathological findings revealed a
hemorrhagic cyst measuring 10×8×8 mm3 and weighing 98 g
(Fig. 3). Resected tissues were
frozen, cut into 5-µm sections and fixed in acetone (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for histological
analysis. Hematoxylin-eosin (Wuhan Boster Biological Technology,
Ltd.) staining identified that the outer layer of the sample was
tissue, not a blood clot; myocardium, fibrous tissue and thrombus
were also observed. For further detection, horseradish-peroxidase
(HRP) immunohistochemical staining was performed. Fresh tissue was
frozen in liquid nitrogen, embedded in optimal cutting temperature
compound (Tissue-Tek O.C.T compound; Sakura Finetek U.S.A., Inc.,
Torrance, CA, USA) and then cut into cryostat sections (4–8 µm
thick). Prior to staining, the slide was warmed to room
temperature, fixed in ice-cold acetone and air dried. After
rinsing, the sections were incubated with normal serum (Wuhan
Boster Biological Technology, Ltd.) followed by primary goat
polyclonal fibroblast-specific protein-1 (FSP-1) antibody (diluted
1:100 with antibody dilution buffer; cat no. sc-49158; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) for 1 h at room temperature.
After rinsing with phosphate-buffered saline (PBS; Wuhan Boster
Biological Technology, Ltd.), the sections were incubated with
peroxidase blocking solution [Tiangen Biotech (Beijing) Co., Ltd.,
Beijing, China) followed by mouse anti-rabbit monoclonal IgG
secondary antibody (cat no. M0809-1; 1:500; Hangzhou Hua'an Medical
& Health Instruments Co., Ltd., Hangzhou, China) for half 1 h.
After rinsing three times with PBS, the sections were incubated
with streptavidin-HRP [Tiangen Biotech (Beijing) Co., Ltd.] in PBS
for 30 min, rinsed again, incubated in 3,3′-diaminobenzidine
solution (Wuhan Boster Biological Technology, Ltd.), and then
rinsed in PBS and distilled water. The sections were subsequently
dehydrated through 95% ethanol for 2 min, 100% ethanol for 3 min,
and then cleared in xylene (Wuhan Boster Biological Technology,
Ltd.) for 5 min. Finally, coverslips were mounted with mounting
medium (Wuhan Boster Biological Technology, Ltd.). Staining was
visualized using a microscope (ECLIPSE E400; Nikon Corporation,
Tokyo, Japan).
Discussion
CAF is a rare heart disease. The incidence rate of
CAF is estimated to be 1 in 50,000 live births, and it is detected
in ~0.2% of the adult population (9).
The majority of these fistulas are congenital, originating from the
right coronary artery, and >50% drain into a right-sided cardiac
chamber (10). CAF may present with
heart murmur, congestive heart failure or angina (11,12).
Endocarditis may also occur in an untreated coronary fistula
(13). Although there are many
reports of congenital right CAF, a fistula draining to the
myocardial void and forming a hemorrhagic cyst appears to be an
extremely rare phenomenon (14,15).
Systolic and diastolic murmurs are common clinical
manifestations of CAF (16,17). However, in the present case, no
cardiac murmurs were noted on physical examination. ECG as a chief
adjuvant diagnostic examination revealed an occupying lesion in the
right atrium that was producing a partial dynamic tricuspid
obstruction. In addition, the chief complaint was the intermittent
chest pain accompanied by a sense of impending doom following
activity. Unfortunately, this symptom was misinterpreted due to the
RAM causing tricuspid valve obstruction. On the basis of these
findings, the case was misdiagnosed as RAM with obstruction in
right ventricular effluent tract.
The surgery was conducted as quickly as possible, in
anticipation of removing the myxoma and avoiding the possibility of
a dangerous pulmonary embolization. Unexpectedly, the occupying
mass was found not to be RAM, but a hemorrhagic cyst.
In retrospect, it is interesting to speculate about
what led to the incorrect diagnosis. Firstly, the right CAF drained
to the myocardial void in the current case, and not to the right
atria nor the right ventricle. A hemorrhagic cyst was formed, which
caused the obstruction of the CAF; subsequently, a characteristic
murmur was not present. Secondly, as the large cyst slanted towards
the right atrium and had no blood flow, it was easily misdiagnosed
as RAM based on the echocardiogram. Thirdly, the reason for the
chest pain was coronary steal syndrome in the early stage of
illness, and the myocardial injury caused by the hemorrhagic cyst
in the advanced stages of the disease. Unfortunately, an error was
made during interpretation of this. Finally, not enough attention
was paid to the ST-segment depression detected by the right
precordial leads on ECG. All the aforementioned evidence suggests
that patients may present with certain symptoms of right
ventricular myocardial ischemia, which may lead to
misdiagnosis.
Although echocardiography, due to its quickness,
effectiveness and non-invasive nature, has been used as major
screening tool to determine the presence, absence or status of
heart disease, misdiagnosis occurs frequently due to the suboptimal
acoustic window and the operator-dependent evaluation, particularly
with anomalies in the coronary artery (10). The selective coronary arteriography
examination remains the gold standard for the diagnosis,
particularly for patients who have atypical angina, and is
essential to plan surgical or percutaneous intervention (3). However, angiography may not always be
possible for all individuals, as it is invasive and expensive
(18). Furthermore, in this case, the
echocardiogram indicated an occupying mass in the right atrium
initially, and angiography may be dangerous due to the risk of
rupturing the mass and causing pulmonary embolization (19). If further examinations had been made
by magnetic resonance imaging or multi-slice computed tomography
angiography, it may have been possible to make a differential and
accurate diagnosis (1,10).
In summary, the current study describes the case of
a right CAF misdiagnosed as RAM. To the best of our knowledge, this
is the first report of a right CAF draining to the myocardial void.
To avoid misdiagnosis, thorough examination of patients is
required, particularly coronary angiography. The present case
additionally demonstrates how to perform CAF cardiac surgical
treatment. Although there is little evidence proving that this
surgical approach is more reasonable than percutaneous or surgical
closure, the present study provides a potential alternative
surgical method for the treatment of CAF.
References
|
1
|
Yoshitake I, Hata M, Sezai A, Niino T,
Unosawa S, Shimura K, Kasamaki Y and Minami K: Cardiac angiosarcoma
with cardiac tamponade diagnosed as a ruptured aneurysm of the
sinus valsalva. Jpn J Clin Oncol. 39:612–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lebreiro A, Pinho T, Silva JC, Madureira
A, Macedo F, Ramos I and Maciel MJ: Percutaneous closure of a giant
coronary artery fistula draining into superior vena cava. Rev Port
Cardiol. 29:433–437. 2010.(In Portuguese). PubMed/NCBI
|
|
3
|
Ogden JA: Congenital anomalies of the
coronary arteries. Am J Cardiol. 25:474–479. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dehmer GJ, Blankenship JC, Cilingiroglu M,
Dwyer JG, Feldman DN, Gardner TJ, Grines CL and Singh M: Society
for Cardiovascular Angiography and Interventions; American College
of Cardiology; American Heart Association: SCAI/ACC/AHA expert
consensus document: 2014 update on percutaneous coronary
intervention without on-site surgical backup. Catheter Cardiovasc
Interv. 84:169–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azevedo O, Almeida J, Nolasco T, Medeiros
R, Casanova J, Bartosch C, Almeida J and Pinho P: Massive right
atrial myxoma presenting as syncope and exertional dyspnea: Case
report. Cardiovasc Ultrasound. 8:232010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HJ, Seol SH, Choi BJ, Park SH, Kim DK,
Kim U, Yang TH, Kim DK, Kim DI and Kim DS: A case of a right atrial
and inferior vena caval thrombus resembling a right atrial myxoma.
J Cardiovasc Ultrasound. 18:58–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Demir M, Akpinar O and Acarturk E: Atrial
myxoma: An unusual cause of myocardial infarction. Tex Heart Inst
J. 32:445–447. 2005.PubMed/NCBI
|
|
8
|
Berdajs DA, Mosbahi S, Charbonnier D,
Hullin R and von Segesser LK: Analysis of flow dynamics in right
ventricular outflow tract. J Surg Res. 197:50–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gürbüz A, Yetkin U, Tetik O, Kestelli M
and Yesil M: Right coronary artery fistula draining into the right
atrium and associated with mitral valve stenosis: A case report.
Heart Surg Forum. 10:E325–E328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gulati GS, Ramamurthy S and Sharma S:
Utility of multislice computed tomography in the diagnosis of a
right coronary artery fistula to the right atrium. J Postgrad Med.
53:191–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benlafqih C, Léobon B, Chabbert V and
Glock Y: Surgical exclusion of a symptomatic circumflex coronary to
right atrium fistula. Interact Cardiovasc Thorac Surg. 6:413–414.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gradaus F, Peters AJ, Schoebel FC, Gradaus
D, Leschke M and Strauer BE: Angina pectoris in ‘coronary steal
syndrome’ caused by a coronary fistula in the left ventricle. Dtsch
Med Wochenschr. 123:1030–1034. 1998.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rein AJ, Yatsiv I and Simcha A: An unusual
presentation of right coronary artery fistula. Br Heart J.
59:598–600. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Said SA: Current characteristics of
congenital coronary artery fistulas in adults: A decade of global
experience. World J Cardiol. 3:267–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura M, Matsuoka H, Kawakami H,
Komatsu J, Itou T, Higashino H, Kido T and Mochizuki T: Giant
congenital coronary artery fistula to left brachial vein clearly
detected by multidetector computed tomography. Circ J. 70:796–799.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okamura T, Nagashima M, Yamada Y,
Hiramatsu T and Yamazaki K: Effective long-term surgical management
of congenital coronary artery fistulas. Tohoku J Exp Med.
223:205–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ceresnak S, Gray RG, Altmann K, Chen JM,
Glickstein JS and Hellenbrand WE: Coronary artery fistulas: A
review of the literature and presentation of two cases of coronary
fistulas with drainage into the left atrium. Congenit Heart Dis.
2:208–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iglesias JF, Roguelov C, Kabir T, Vogt P
and Eeckhout E: Indications for urgent coronary angiography. Part
I: ST-segment elevation acute coronary syndromes. Rev Med Suisse.
5:1195–1196, 1198–1201. 2009.(In French). PubMed/NCBI
|
|
19
|
Kazuno K, Akasaka N, Kiyokawa K and
Sasajima T: Ruptured aneurysm of coronary artery-to-pulmonary
artery fistula. Asian Cardiovasc Thorac Ann. 20:324–326. 2012.
View Article : Google Scholar : PubMed/NCBI
|