Introduction
Transcriptional coactivator with PDZ-binding motif
(TAZ) is a WW domain-containing transcriptional coactivator, which
interacts with a number of transcription factors, including Smad
and runt-related transcription factor (1,2). TAZ is a
component of the Hippo tumor suppressor signaling pathway that
serves a key role in the regulation of apoptosis, cell
proliferation and tumorigenesis (3,4). When the
Hippo pathway is activated, MstII and large tumor suppressor
kinase (LATS)1/2, human homologs of Drosophila Hippo and
LATS, phosphorylate TAZ and its paralog Yes-associated protein
(YAP), sequestering them to the cytoplasm (5). Conversely, the inactivation of the Hippo
pathway results in dephosphorylation of TAZ/YAP. Dephosphorylated
TAZ/YAP accumulate in the nucleus, and primarily function through
transcription factors to promote cell proliferation. YAP is a
moderately stable protein and is predominantly regulated by
nuclear-cytoplasmic shuttling. By contrast, TAZ is extremely
unstable with a half-life of <2 h, indicating that its
degradation is the primary method of TAZ inhibition (6). TAZ/YAP are activated at high frequencies
during tumorigenesis in various forms of cancer. YAP has been
detected in gastric, colon, esophageal, liver and non-small cell
lung cancer, and in lobular carcinoma of the breast (7–12), whilst
TAZ is expressed in the cell nuclei of breast, lung and colon
cancer (7,13,14).
Uterine cancer is the most common gynecological
malignancy, with an incidence rate of 12.9 cases per 100,000 women
and a mortality rate of 2.4 cases per 100,000 women. Endometrioid
adenocarcinoma is the most prevalent invasive malignancy of all
uterine cancers (15,16). Despite advances in the detection and
treatment of endometrioid adenocarcinoma, the patient prognosis
remains unfavorable. The clinical implications of numerous markers,
including CUB domain-containing protein 1 and aldehyde
dehydrogenase 1, have been previously investigated in uterine
endometrioid adenocarcinoma (17–19), but
few studies have analyzed the expression of Hippo pathway
components in association with this tumor. Recently, Tsujiura et
al (20) reported that YAP
modulates radiation sensitivity and oncogenic features in
endometrial cancer, including endometrioid and serous
adenocarcinoma. The knockdown of YAP expression in the HEC-1B cell
line increases sensitivity to radiation, and the nuclear expression
of YAP correlates with the poorly-differentiated form of
endometrioid adenocarcinoma. To date, no studies have investigated
TAZ expression in endometrioid adenocarcinoma. In the present
study, TAZ expression was immunohistochemically examined in 55
clinical samples of endometrioid adenocarcinoma, and the clinical
implications were evaluated.
Materials and methods
Patients and methods
A total of 55 samples were obtained from patients
who underwent a hysterectomy due to endometrioid adenocarcinoma at
Osaka University Hospital (Suita, Japan) between January 1999 and
January 2003. No prior chemotherapy/radiotherapy was administered
in any case. Each sample was examined, and the clinicopathological
findings are summarized in Table I.
Patient age ranged from 32–71 years (median, 56.1 years). Resected
specimens were macroscopically examined to determine the location
and size of the tumors. Histological specimens were fixed in 10%
formalin and routinely processed for paraffin-embedding. The
paraffin-embedded specimens were stored in a dark room in the
Department of Pathology, Osaka University Hospital, at room
temperature. The specimens were cut into 4-µm thick sections and
stained with hematoxylin and eosin, and then underwent an
immunoperoxidase procedure. Histological staging was determined
according to the International Federation of Obstetricians and
Gynecologists (FIGO) staging system (21). All patients were followed up with
laboratory examinations, including routine peripheral blood cell
counts at 1- to 6-month intervals, and X-ray, computed tomography
and pelvic examinations at 6- to 12-month intervals. The follow-up
period for survivors ranged from 7–176 months (median, 106 months).
The present study was approved by the Ethical Review Board of the
Graduate School of Medicine, Osaka University (Suita, Japan).
 | Table I.Summary of characteristics of 55
patients with endometrioid adenocarcinoma. |
Table I.
Summary of characteristics of 55
patients with endometrioid adenocarcinoma.
| Characteristics | Patients, n |
|---|
| Tumor |
|
| T1 | 40 |
| T2 | 5 |
| T3 | 10 |
| Stage |
|
| I | 37 |
| II | 3 |
| III | 11 |
| IV | 4 |
| Tumor histological
grade |
|
| 1 | 25 |
| 2 | 20 |
| 3 | 10 |
| Lymph node
metastasis |
|
|
Negative | 43 |
|
Positive | 12 |
| Recurrence |
|
|
Negative | 44 |
|
Positive | 11 |
| Prognosis |
|
| Alive
with no recurrence | 44 |
| Alive
with recurrence | 3 |
| Succumbed
to disease | 8 |
Immunohistochemistry for TAZ
Subsequent to deparaffinization with xylene and
rehydration with graded alcohol treatment, sections were heated to
121°C in the Pascal Pressurized Heating Chamber (Dako, Glostrup,
Denmark). After cooling, the sections were washed in
phosphate-buffered saline, blocked with blocking solution (cat no.
X0909; Dako) and incubated with the primary rabbit anti-TAZ
polyclonal antibody (dilution 1:500 cat no. ab110239; Abcam,
Cambridge, UK). Next, the sections were treated with the ChemMate™
Envision™ Detection kit (Dako) that contains a polymerized
secondary antibody to increase detection sensitivity for the
primary antibody. 3,3′-Diaminobenzidine (Dako) was used as a
chromogen. As the negative control, staining was carried out in the
absence of the primary antibody. Sections were counterstained with
hematoxylin and observed by microscopy (BX50; Olympus, Tokyo,
Japan).
Evaluation of immunohistochemical
staining
TAZ staining was scored independently by two
pathologists who examined the samples in a blinded manner with
respect to the clinical information of the subjects. The intensity
of the signal was divided into 4 grades as follows: None, 0; weak,
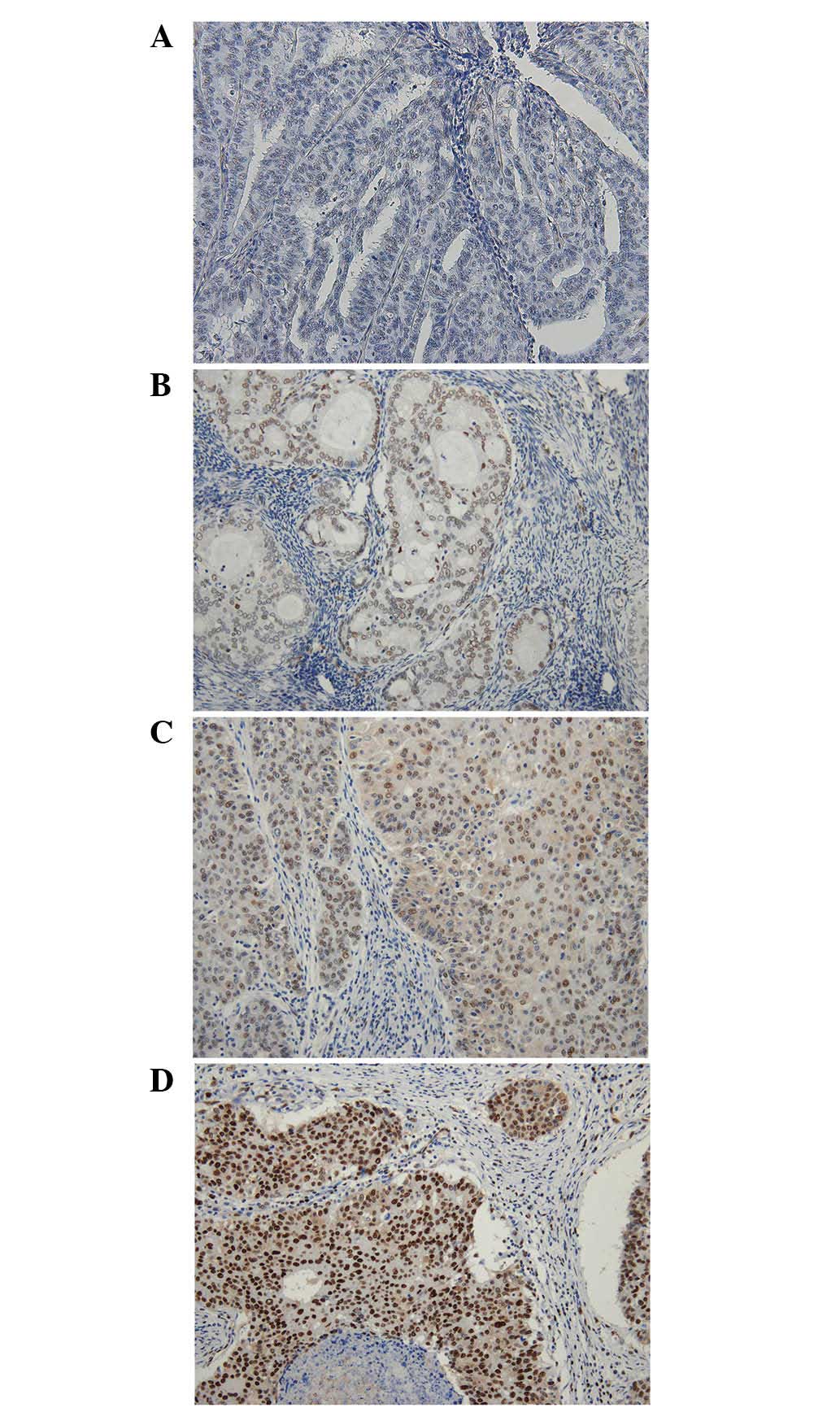
1; moderate, 2; and strong, 3 (Fig.
1). The area percentage of each grade was determined. The sum
of multiplying the area percentage of each grade by the signal
intensity was termed the TAZ histological score, where (% of 0 × 0)
+ (% of 1 × 1) + (% of 2 × 2) + (% of 3 × 3). The minimum score was
0 and the maximum was 300. Cases with a TAZ histological score of
<100 were categorized as TAZ-low and cases with a score of
>100 were categorized as TAZ-high.
Statistical analysis
Statistical analyses were performed using JMP Pro
10.0.2 software (SAS Institute Inc., Cary, NC, USA). The
χ2 test was used to analyze the association between TAZ
expression and clinicopathological factors in patients with
endometrioid adenocarcinoma. Overall survival (OS) was measured
from the time between diagnosis and mortality, and disease-free
survival (DFS) was measured as the time between diagnosis and
disease recurrence. Kaplan-Meier survival analysis was used to
calculate the OS and DFS rates, and differences in survival curves
were evaluated with the log-rank test. Cox's proportional hazards
regression model with a stepwise manner was used to analyze the
independent prognostic factors. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical analysis
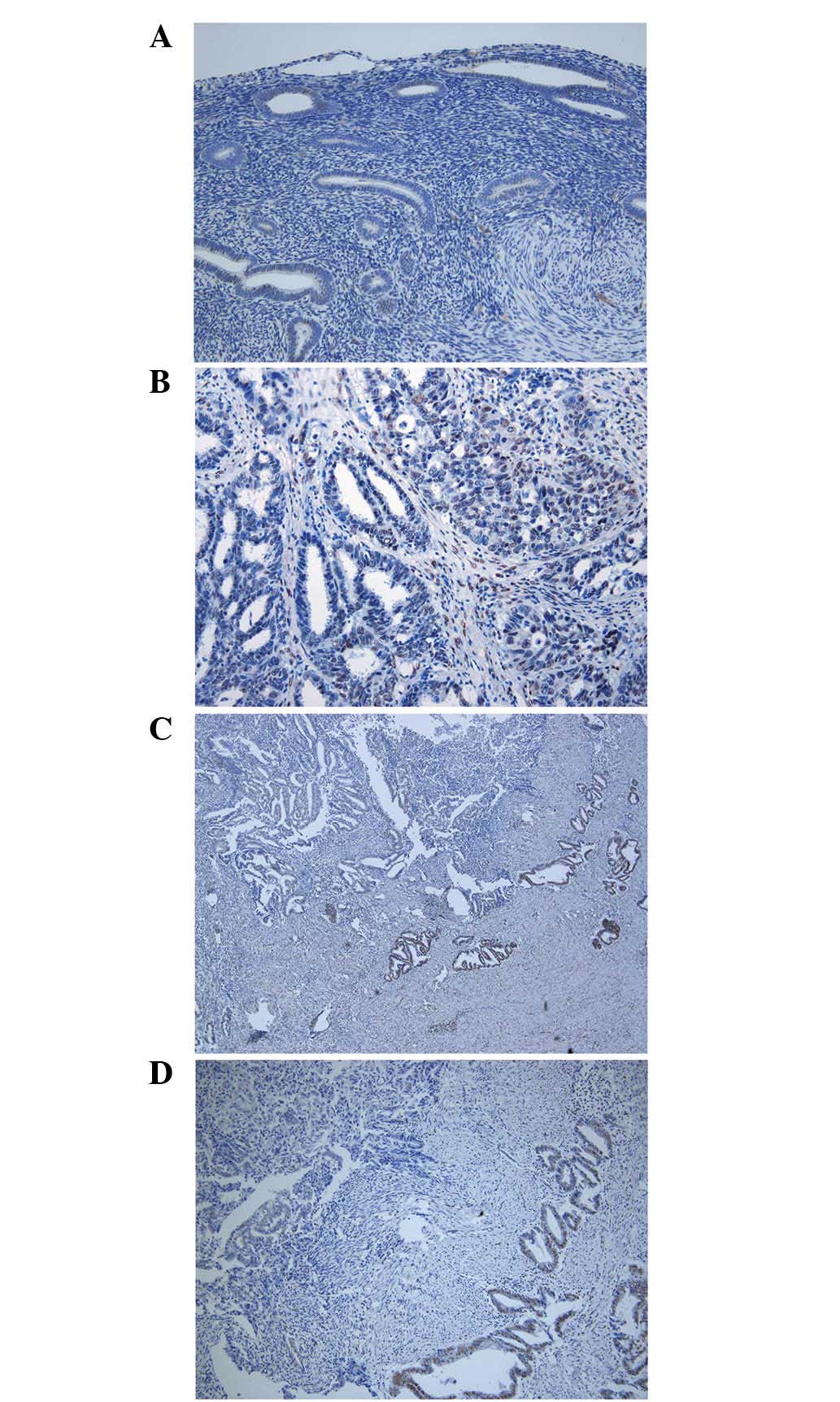
Limited signals representing TAZ expression were
detected in non-cancerous endometrial tissues (Fig. 2A), whereas cancerous endometrial
tissues expressed TAZ at varied intensities. TAZ was primarily
detected in the cell nuclei. Generally, cells with a solid
structure expressed high levels of TAZ when compared with cells
with a glandular structure (Fig. 2B).
Portions invading into the myometrium expressed high levels of TAZ
(Fig. 2C and D).
Association between TAZ expression and
clinicopathological features
The associations between TAZ expression level (TAZ
histological score) and clinicopathological features were evaluated
(Table II). Cases with low tumor
(T)-factor exhibited a lower TAZ histological score (T1 vs. T2 and
T3). Similarly, the histological score was significantly lower in
cases with low stage and low histological grade (stage I vs. II,
III and IV; grade 1 vs. 2 and 3), and without lymph node metastasis
and recurrence. Cases with a poor prognosis were associated with a
high TAZ histological score.
 | Table II.Association between TAZ expression
level and clinicopathological parameters. |
Table II.
Association between TAZ expression
level and clinicopathological parameters.
| Characteristics | TAZ histological
scorea |
|---|
| Tumor |
|
| T1 | 113±13 |
| T2 | 190±32b |
| T3 | 198±25b |
| Stage |
|
| I | 109±13 |
| II | 213±52b |
| III | 175±24b |
| IV | 211±36b |
| Tumor histological
grade |
|
| 1 | 82±13 |
| 2 | 176±14b |
| 3 | 187±31b |
| Lymph node
metastasis |
|
|
Negative | 120±13 |
|
Positive | 191±20b |
| Recurrence |
|
|
Negative | 110±11 |
|
Positive | 235±14b |
| Prognosis |
|
| Alive
with no recurrence | 112±11 |
| Alive
with recurrence | 250±21b |
| Succumbed
to disease | 230±18b |
Subsequently, the cases were divided into TAZ-high
and TAZ-low groups using a cut-off histological score of 100, as
this score was the most optimal for discriminating between disease
characteristics. A total of 23 cases were classified as TAZ-low and
32 were classified as TAZ-high. The association between TAZ
expression and clinicopathological features was re-evaluated
(Table III). TAZ-high cases were
significantly correlated with high T-factor (P=0.024), stage
(P=0.041) and histological grade (P=0.001), lymph node metastasis
(P=0.046), recurrence (P=0.002) and a poor prognosis (P=0.007). The
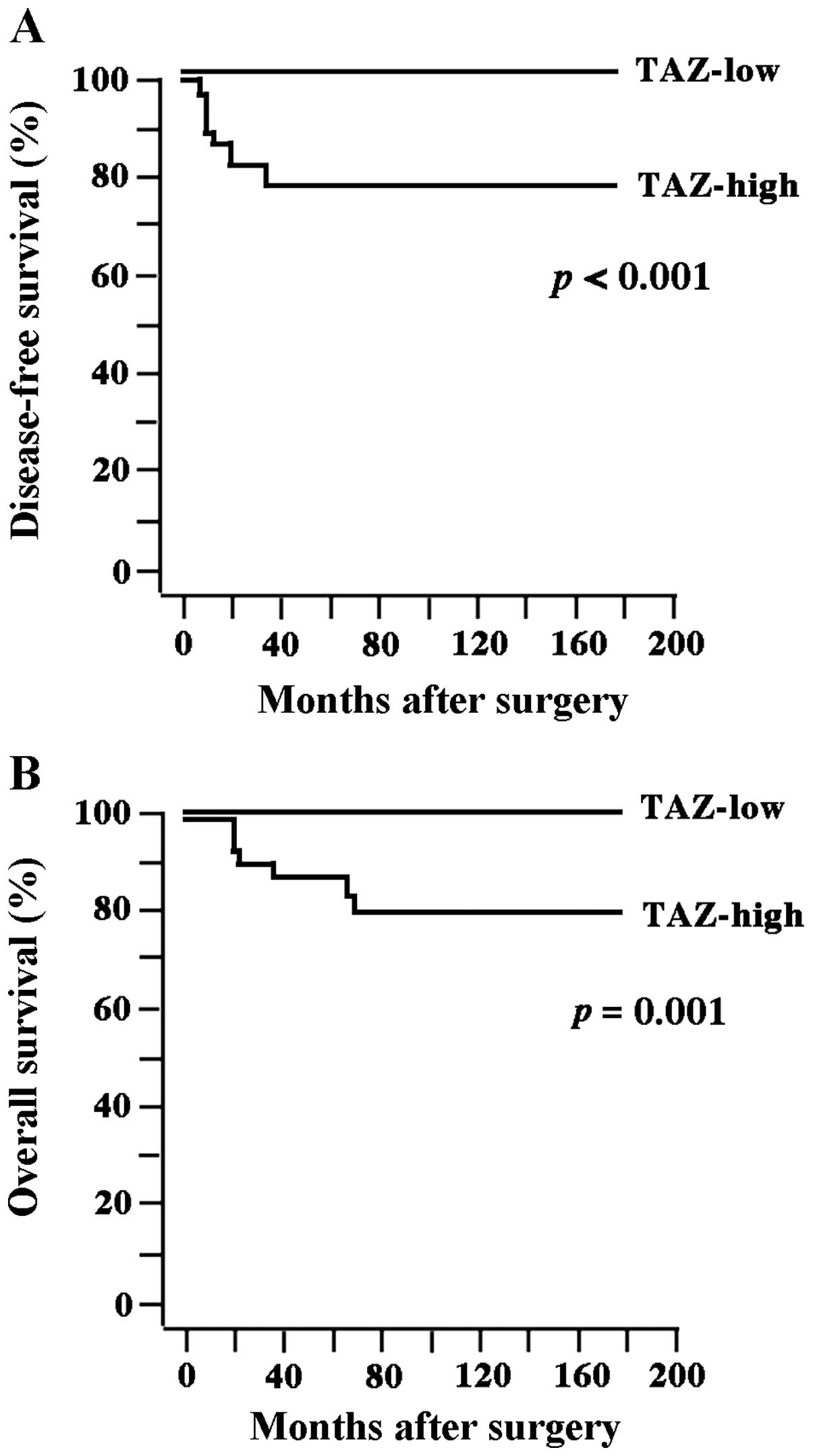
5-year DFS and OS rates were 80.0 and 85.5%, respectively. Tumors
recurred in 11 patients, and 8 of these patients succumbed to the
disease. There was a significant difference in DFS (P<0.001) and
OS rates (P=0.001) between the TAZ-high and TAZ-low groups
(Fig. 3).
 | Table III.Association between TAZ histological
score and clinicopathological parameters. |
Table III.
Association between TAZ histological
score and clinicopathological parameters.
|
| TAZ histological
score |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low | High | P-value |
|---|
| Tumor |
|
| 0.024 |
| T1 | 21 | 19 |
|
| T2 | 0 | 5 |
|
| T3 | 2 | 8 |
|
| Stage |
|
| 0.041 |
| I | 20 | 17 |
|
| II | 0 | 3 |
|
|
III | 3 | 8 |
|
| IV | 0 | 4 |
|
| Histological
grade |
|
| 0.001 |
| 1 | 17 | 8 |
|
| 2 | 3 | 17 |
|
| 3 | 3 | 7 |
|
| Lymph node
metastasis |
|
| 0.046 |
|
Negative | 21 | 22 |
|
|
Positive | 2 | 10 |
|
| Recurrence |
|
| 0.002 |
|
Negative | 23 | 21 |
|
|
Positive | 0 | 11 |
|
| Prognosis |
|
| 0.007 |
| Alive
with no recurrence | 23 | 21 |
|
| Alive
with recurrence | 0 | 3 |
|
|
Succumbed to disease | 0 | 8 |
|
Univariate analysis demonstrated that T-factor,
stage, lymph node metastasis and TAZ expression were significant
factors for OS and DFS rate (Table
IV). Multivariate analysis was subsequently performed on these
four factors. The results indicated that none of the factors
significantly affected OS or DFS rate (Table IV).
 | Table IV.Univariate and multivariate analyses
of prognostic factors for overall and disease-free survival. |
Table IV.
Univariate and multivariate analyses
of prognostic factors for overall and disease-free survival.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor | 1.66
(1.14–2.74) | 0.007 | 1.11
(0.61–2.87) | 0.77 | 1.86
(1.24–3.33) | 0.002 | 1.62
(0.84–6.56) | 0.176 |
| Stage | 3.81
(1.57–17.4) | 0.002 | 0.61
(0.02–7.77) | 0.72 | 4.00
(1.65–18.2) | 0.001 | 0.35
(0.01–6.79) | 0.504 |
| Histological
grade | 1.55
(0.53–4.57) | 0.412 | 0.77
(0.24–2.91) | 0.67 | 1.66
(0.57–4.78) | 0.339 | 0.91
(0.28–3.27) | 0.869 |
| Lymph node
metastasis | 3.07
(1.15–6.04) | 0.002 | 25.98
(0.73–148549.7) | 0.09 | 3.05
(1.14–6.03) | 0.002 | 18.98
(0.34–108330.1) | 0.188 |
| TAZ histological
index | 3.42
(1.22–12.9) | 0.018 | 2.85
(0.65–18.9) | 0.17 | 3.94
(1.37–15.1) | 0.009 | 3.00
(0.72–22.6) | 0.139 |
Discussion
As major downstream effectors of the Hippo signaling
pathway, TAZ and YAP are not only similar in terms of their
structures, but also with regard to their functions (1). Overexpression of YAP has been reported
to be associated with a poor prognosis in several types of human
cancer, including ovarian, hepatocellular and breast cancer, and
malignant mesothelioma (3,9,12,22,23).
Tsujiura et al (20) reported
that high levels of YAP expression in the cell nuclei of
endometrioid adenocarcinoma correlates with the
poorly-differentiated histological type and a poor prognosis. To
date, no studies have investigated TAZ expression in endometrioid
adenocarcinoma. In the present study, it was demonstrated that
patients with endometrioid adenocarcinoma and high TAZ expression
had a significantly shorter survival time, and that high TAZ
expression was associated with high clinical stage. In addition,
the expression level of TAZ was higher in grade 2 and grade 3
tumors than in grade 1 tumors, indicating that
poorly-differentiated histological type was correlated with TAZ
expression. These results were consistent with the functional
similarities between TAZ and YAP.
YAP/TAZ is primarily regulated by
nuclear-cytoplasmic shuttling. The activation of the Hippo pathway
results in phosphorylation of YAP/TAZ by LATS1/2, which sequesters
YAP/TAZ to the cytoplasm, where it is subsequently degraded
(5). YAP/TAZ nuclear accumulation is
a key determinant of their function, as YAP/TAZ is a transcription
factor that functions in the nucleus (1,2). In
endometrioid adenocarcinoma, YAP has been reported to be located in
the cytoplasm and nucleus (20). By
contrast, the present study demonstrated that TAZ was predominantly
located in the nucleus. The half-life of TAZ is known to be shorter
than that of YAP. Thus, when TAZ is sequestered to the cytoplasm it
may be easily degraded, which may possibly explain why limited TAZ
expression was detected in the cytoplasm of the endometrioid
adenocarcinoma tissues.
In conclusion, to the best of our knowledge, the
present study has demonstrated for the first time that TAZ
expression is correlated with a poor prognosis, and that it serves
as an independent prognostic factor for survival in patients with
uterine endometrioid adenocarcinoma. TAZ may become a future
clinical marker of a poor prognosis in this disease.
Acknowledgements
The authors would like to thank Ms. M. Sugano, Ms.
E. Maeno, Ms. T. Sawamura and Ms. Y. Tsuruta (Department of
Pathology, Osaka University) for providing technical assistance.
The present study was supported by grants from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (nos.
T264604700, T254604350 and T258602680).
References
|
1
|
Kanai F, Marignani PA, Sarbassova D, Yagi
R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC
and Yaffe MB: TAZ: A novel transcriptional co-activator regulated
by interactions with 14-3-3 and PDZ domain proteins. EMBO J.
19:6778–6791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varelas X, Sakuma R, Samavarchi-Tehrani P,
Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW and Wrana JL:
TAZ controls Smad nucleocytoplasmic shuttling and regulates human
embryonic stem-cell self-renewal. Nat Cell Biol. 10:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in
Drosophila and mammals. Cell. 130:1120–1133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow A, Hao Y and Yang X: Molecular
characterization of human homologs of yeast MOB1. Int J Cancer.
126:2079–2089. 2010.PubMed/NCBI
|
|
6
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang
A, Wen W and Zhu Q: Overexpression of YAP and TAZ is an independent
predictor of prognosis in colorectal cancer and related to the
proliferation and metastasis of colon cancer cells. PLoS One.
8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang W, Tong JH, Chan AW, Lee TL, Lung RW,
Leung PP, So KK, Wu K, Fan D, Yu J, et al: Yes-associated protein 1
exhibits oncogenic property in gastric cancer and its nuclear
accumulation associates with poor prognosis. Clin Cancer Res.
17:2130–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muramatsu T, Imoto I, Matsui T, Kozaki K,
Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T and Inazawa J: YAP
is a candidate oncogene for esophageal squamous cell carcinoma.
Carcinogenesis. 32:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Dong Q, Zhang Q, Li Z, Wang E and
Qiu X: Overexpression of yes-associated protein contributes to
progression and poor prognosis of non-small-cell lung cancer.
Cancer Sci. 101:1279–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Su L and Ou Q: Yes-associated
protein promotes tumour development in luminal epithelial derived
breast cancer. Eur J Cancer. 48:1227–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan SW, Lim CJ, Guo K, Ng CP, Lee I,
Hunziker W, Zeng Q and Hong W: A role for TAZ in migration,
invasion, and tumorigenesis of breast cancer cells. Cancer Res.
68:2592–2598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS
and Yang X: TAZ is a novel oncogene in non-small cell lung cancer.
Oncogene. 30:2181–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horn LC, Meinel A, Handzel R and Einenkel
J: Histopathology of endometrial hyperplasia and endometrial
carcinoma: An update. Ann Diagn Pathol. 11:297–311. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mamat S, Ikeda J, Enomoto T, Ueda Y,
Rahadiani N, Tian T, Wang Y, Qiu Y, Kimura T, Aozasa K and Morii E:
Prognostic significance of CUB domain containing protein expression
in endometrioid adenocarcinoma. Oncol Rep. 23:1221–1227.
2010.PubMed/NCBI
|
|
18
|
Rahadiani N, Ikeda J, Mamat S, Matsuzaki
S, Ueda Y, Umehara R, Tian T, Wang Y, Enomoto T, Kimura T, et al:
Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid
adenocarcinoma and its clinical implications. Cancer Sci.
102:903–908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Ikeda JI, Rahadiani N, Mamat S,
Ueda Y, Tian T, Enomoto T, Kimura T, Aozasa K and Morii E:
Prognostic significance of elongator protein 3 expression in
endometrioid adenocarcinoma. Oncol Lett. 3:25–29. 2012.PubMed/NCBI
|
|
20
|
Tsujiura M, Mazack V, Sudol M, Kaspar HG,
Nash J, Carey DJ and Gogoi R: Yes-associated protein (YAP)
modulates oncogenic features and radiation sensitivity in
endometrial cancer. PLoS One. 9:e1009742014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaino RJ: FIGO staging of endometrial
adenocarcinoma: A critical review and proposal. Int J Gynecol
Pathol. 28:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goulev Y, Fauny JD, Gonzalez-Marti B,
Flagiello D, Silber J and Zider A: SCALLOPED interacts with YORKIE,
the nuclear effector of the hippo tumor-suppressor pathway in
Drosophila. Curr Biol. 18:435–441. 2008. View Article : Google Scholar : PubMed/NCBI
|