Introduction
Breast cancer is one of the most common and
significant diseases affecting females. Breast tumors are comprised
of phenotypically diverse populations of breast cancer cells.
Current estimates indicate that one in eight females in American
who reach the age of 95 is likely to develop breast cancer
(1). The treatment of advanced breast
cancer is often unsuccessful and may cause disfigurement, making
early detection a high priority in the medical management of the
disease. The level of morbidity and high incidence of this disease
is notable. Within the next two decades the incidence of cancer is
expected to rise worldwide. It is noted that the management of
cancer is still not under control and new effective drugs are
lacking (2). Proteins associated with
the human epidermal growth-factor receptor kinase (ERBB or HER)
signaling network have proven to be useful targets for diagnostic
imaging with radioimmunoconjugates due to their overexpression in
various cancer phenotypes. In particular, the overexpression of the
HER2/neu (also known as ERBB2) was revealed to be associated with
increased tumor aggression, metastatic potential and poor prognosis
for disease-free survival in patients with breast, colorectal,
ovarian, lung, prostate and salivary gland tumors (3,4).
HER2/neu was revealed to be a key target for
anticancer drugs due to its intrinsic involvement in the
phosphatidylinositol-3-kinase-Akt/protein kinase B and the
mitogen-activated protein kinase (MAPK) pathways. These pathways
suppress apoptosis and promote tumor cell survival, gene
transcription, angiogenesis, cellular proliferation, migration,
mitosis and differentiation. There are three notable types of
anti-HER2/neu therapeutics; namely, monoclonal antibodies (mAbs)
directed against extracellular ligand-binding and dimerization
epitopes, tyrosine-kinase (TK) inhibitors and heat shock protein 90
(HSP90) inhibitors. Examples of each therapeutic type include
pertuzumab and trastuzumab (which block dimerization and suppress
signaling by binding to extracellular domains II and IV,
respectively), the HER2/neu TK inhibitor lapatinib, and HSP90
inhibitors including geldanamycin derivatives, SNX-5422,
NVP-AUY922, BIIB021 and PU-H71 (5–10). HSP90
is a molecular chaperone protein essential for the function of the
multiple growth and survival pathways required for the maintenance
and progression of cancer (11). In
breast cancer in particular, the overexpression of HSP90 is
associated with poor outcome and decreased survival (12,13).
Trastuzumab and related mAb fragments have been radiolabeled with a
wide range of radionuclides, and quantitative immune positron
emission tomography (PET) imaging has been employed to assess the
effect of Hsp90 inhibitors on the expression levels of HER2/neu
(14–18). The quantification of changes in
HER2/neu expression in response to HSP90 treatment has the
potential to facilitate patient-specific dose regimes.
During the last few years, the focus of drug
development has shifted to natural chemotherapeutic agents from
plants, which may be further modified to enhance their potential
and reduce their side effects. Natural products have been
particularly useful in cancer treatment, and if biologicals and
vaccines are discounted, then 75% of approved chemotherapeutic
agents are natural agents (19).
Erythrina is a genus of flowering plants in the pea family,
Fabaceae, with ~130 species reported. It is distributed worldwide
in tropical and subtropical regions in addition to certain more
temperate regions, and ~50% of its varieties have been studied. The
alkaloids isolated from various species of Erythrina
demonstrate hypotensive, anticonvulsant, hypnotic and analgesic
properties, among others (20).
Erythrina plant species are widely used in folk medicine to
treat health conditions including agitation, insomnia, anxiety and
inflammation (21,22). A previous study has revealed that an
alcoholic extract of the stem bark of E. suberosa induces
apoptosis in human promyelocytic leukemia HL-60 cells (23). The aim of the current study was to
investigate the anticancer potential of the flavonoid wighteone
isolated from the stem bark of E. suberosa, and to study
HSP90 expression and its correlation with wighteone metabolite
response in HER2-positive breast cancer cells.
Materials and methods
Reagents and cell lines
The breast cancer cell line MCF-7 was provided by
the Breast Cancer Research Department of the Inner Mongolia
Autonomous Region People's Hospital, Hohhot, China. Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum (FBS) (Gibco®; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 1% glutamine (Sigma-Aldrich,
St. Louis, MO, USA), 100 IU/ml penicillin (Sigma-Aldrich) and 100
mg/l streptomycin (Sigma-Aldrich). The MTT kit was purchased from
Sigma-Aldrich. Annexin V-fluorescein isothiocyanate (FITC) and the
propidium iodide (PI) Annexin V-EGFP apoptosis detection kit were
purchased from KGI Chemical Corporations (Nanjing, China),
monoclonal mouse anti-rat HSP90 antibody (cat. no. ab13492;
1:1,000) from Abcam (Cambridge, UK) and monoclonal mouse anti-rat
GAPDH antibody (cat. no. sc-32233; 1:1,000) from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Wighteone was obtained as in
previous studies (2).
3-(4,5-dimethylthiazolyl)-2,5-iphenyltetrazolium bromide (MTT)
assay
Cell viability was determined using the MTT assay
(Sigma-Aldrich). Firstly, cells were divided into four groups:
group A: cells were treated with phosphate-buffered saline (PBS);
group B: cells were treated with 0.5 mM/l wighteone; group C: cells
were treated with 5.0 mM/l wighteone; group D: cells were treated
with 10.0 mM/l wighteone. Cells from groups A, B, C and D were
plated in 96-well plates (2×104 cells/well) and cultured
overnight in DMEM with 10% FBS. Following culture for 12, 24 and 48
h, the cells were treated with 0.5 mg/ml MTT for 4 h and lysed with
dimethyl sulfoxide (Sigma-Aldrich). Absorbance rates were measured
at 560 nm using a microplate reader (iMark™Microplate Absorbance
Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Annexin V-FITC/PI analysis
Apoptosis detection was conducted using the Annexin
V-FITC/PI apoptosis detection kit, according to the manufacturer's
instructions. Briefly, all four groups of cells were harvested by
trypsinization, washed in PBS and stained with Annexin V-FITC
conjugate and PI. Cells were then analyzed by flow cytometry (BD
FACSCalibur™, BD Biosciences, San Jose, NJ, USA) using BD CellQuest
acquisition and analysis software.
Western blot analysis
The total volume of all formulations was 10 µl. The
cells were diverted into a 1.5 ml Eppendorf tube (Eppendorf,
Hamburg, Germany) which contained radioimmunoprecipitation assay
buffer schizolysis liquid with protease inhibitor (Sigma-Aldrich).
After being put on ice for 5–10 min, the mixture was agitated to
make it fully dissolve, and put on ice for 30 min. Then the mixture
was centrifuged (100 × g) at 4°C for 20 min, and the top clear
liquid was collected and electrophoresed.
Statistical analysis
The data are presented as the mean ± standard
deviation from at least three independent experiments. One way
analysis of variance was used and all data analyses were performed
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significance
difference.
Results
Wighteone inhibits MCF-7 cell
proliferation
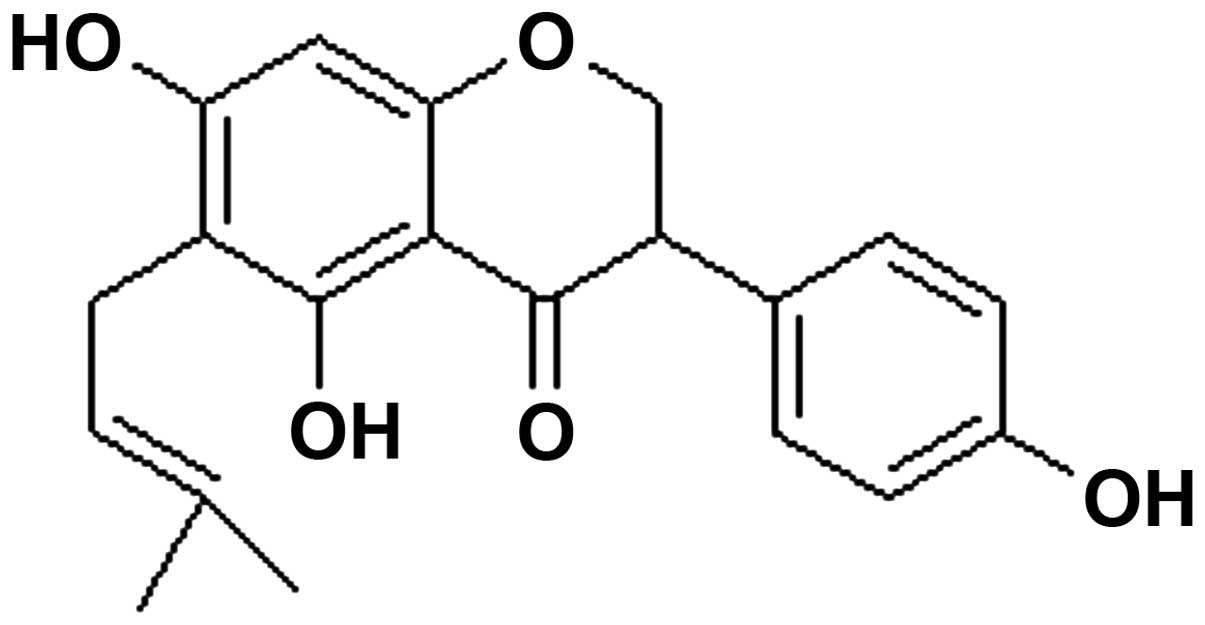
The structure of wighteone is shown in Fig. 1. The results obtained from the MTT
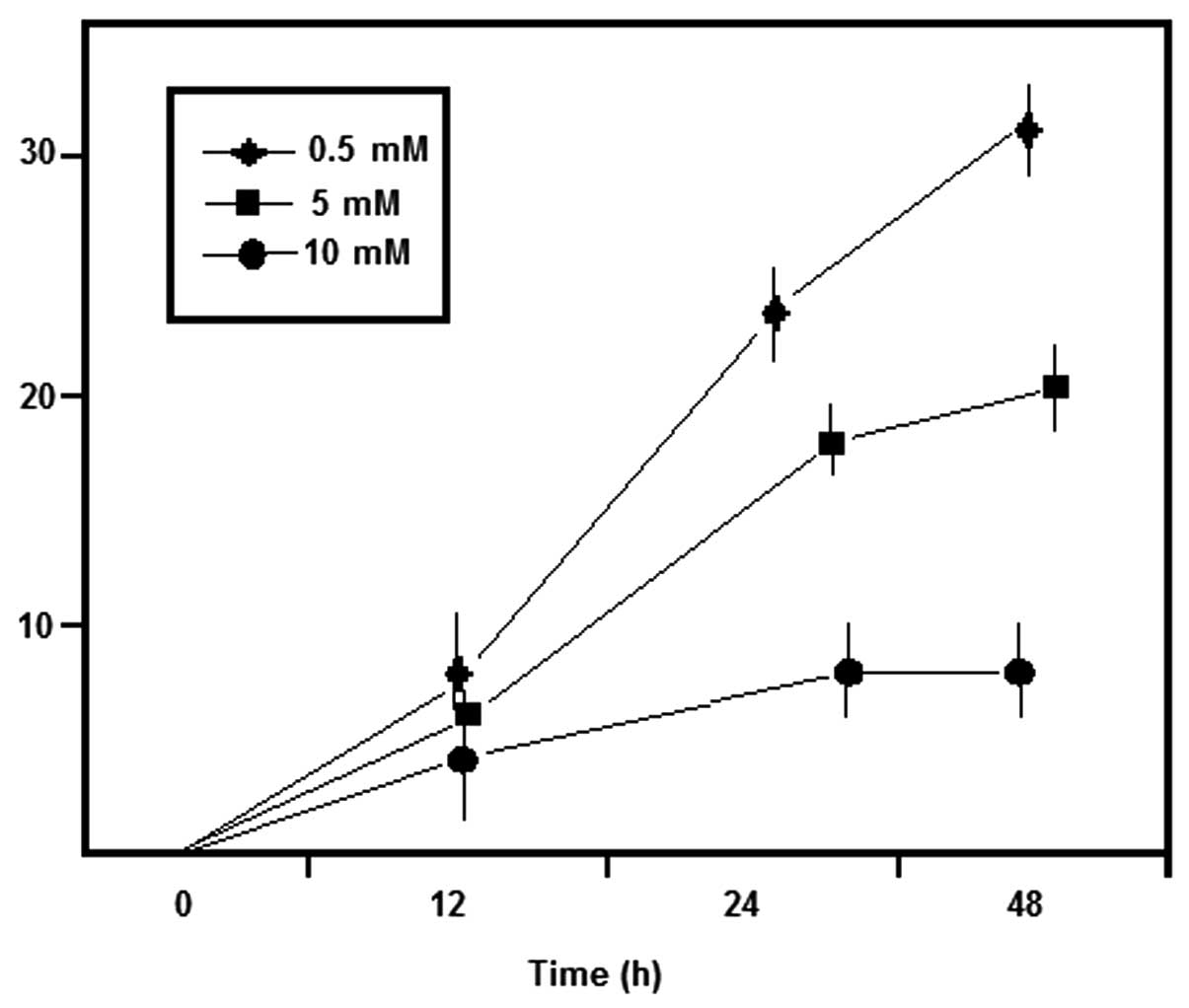
assay revealed that treatment with wighteone significantly
inhibited cancer cell proliferation compared with the control PBS
group (P=0.03). The results are shown in Table I and Figs.
2 and 3, Moreover, when
increasing the concentrations of wighteone, the proliferation rates
were gradually decreased.
 | Table I.Inhibition of MCF-7 cell proliferation
following treatment with wighteone. |
Table I.
Inhibition of MCF-7 cell proliferation
following treatment with wighteone.
|
|
| Inhibition rate of
MCF-7 cancer cells (mean ± SD) |
|---|
|
|
|
|
|---|
| Group | Wighteone, mM/l | 12 h | 24 h | 48 h |
|---|
| A | Control | 0 | 0 | 0 |
| B | 0.5 | 4.90±0.27 |
5.62±0.12 |
6.44±0.07 |
| C | 2.0 | 6.34±0.24 | 15.18±0.28 | 21.36±0.12 |
| D | 8.0 | 7.73±0.33 | 21.16±0.11 | 29.17±0.02 |
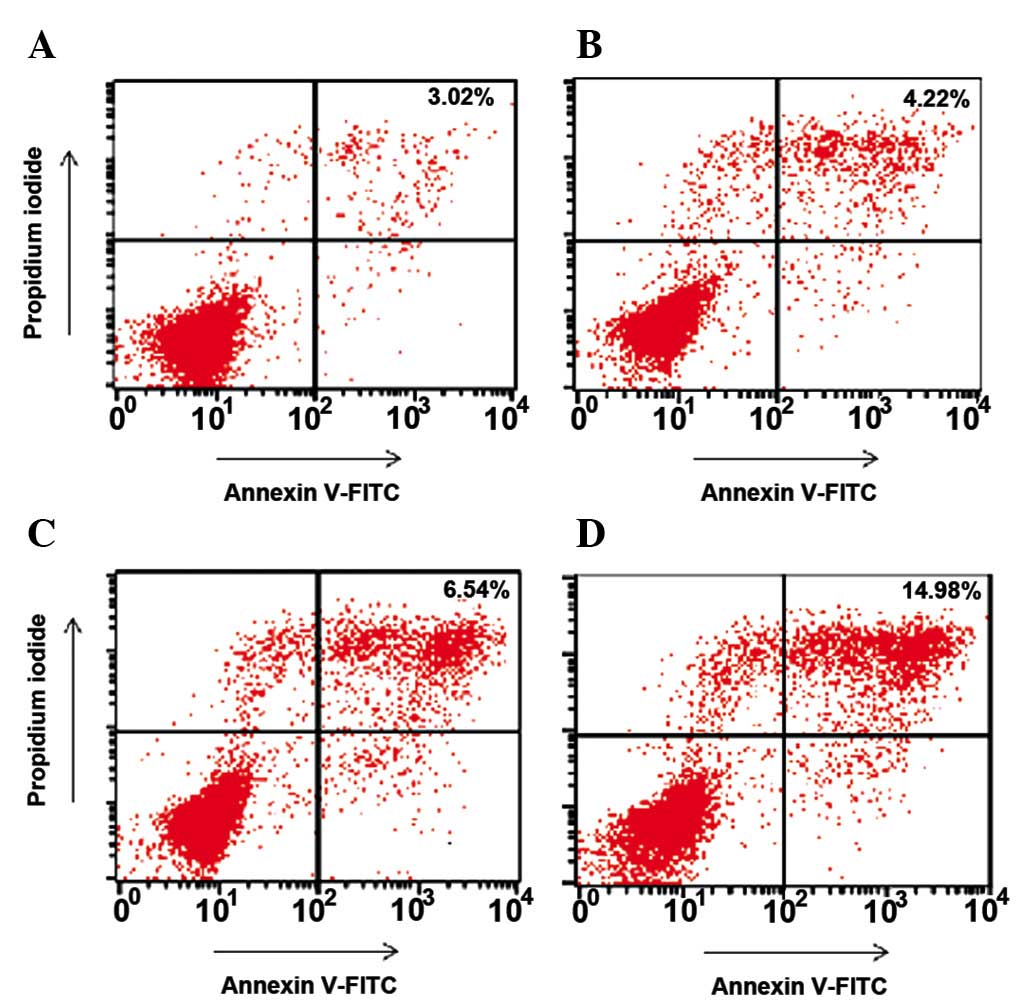
Apoptosis
The experimental studies revealed that the apoptosis
rates in groups A, B, C and D were 3.02, 4.22, 6.54 and 14.98%,
respectively. However, only the apoptotic rate in group D differed
significantly compared with that in group A (P=0.003). The
apoptotic rate in group D also demonstrated a significant
difference compared with that in group B (P=0.008; Fig. 4).
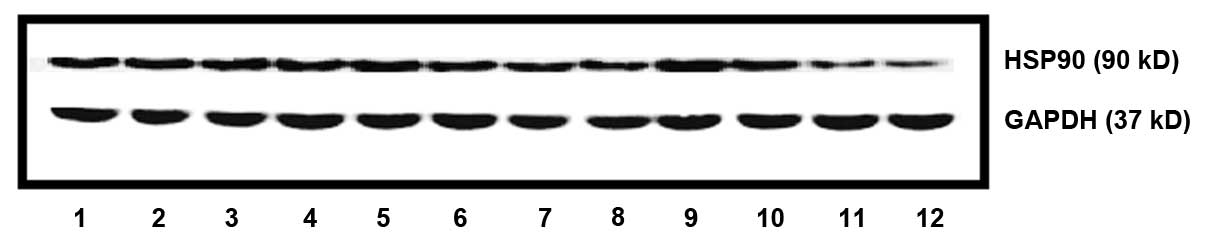
HSP90 protein expression
HSP90 protein expression of MCF-7 cells following
treatment with wighteone is shown in Fig.
5. HSP90 expression was significantly lower (P=0.03) in groups
B, C and D compared with that in group A. Moreover, the HSP90
expression at 48 h was significantly lower compared with that at 24
h (P=0.04).
Discussion
The use of plants as medicine dates back thousands
of years, and they have become an essential source of the
mainstream pharmacopoeia. Nature is an attractive source of new
therapeutic candidate compounds as a vast chemical diversity has
been observed in diverse plant species, animals, marine organisms
and microorganisms. The advantage of botanical compounds is their
ability to support the treatment of all phases of cancer. These
compounds regularly interact with several targets simultaneously
and often act synergistically. The Erythrina plant species
is widely used in folk medicine to treat various health problems
including agitation, insomnia, and anxiolytic and inflammatory
processes. Recently, the focus of drug development has shifted to
the natural chemotherapeutic agents from plants, which may further
be modified to enhance their potential and reduce their side
effects. The identification of drugs from medicinal plants has
played a significant role in the treatment of cancer; in fact, the
majority of new clinical applications of plant secondary
metabolites and derivatives over the last half century have been
towards combating cancer. This forms the basis of the present
study, in which our crucial aim was to develop a holistic molecular
approach in consideration of the fact that several genes are
mutated in cancer cells, which protects them from self-demise.
The predictive role of HSP90 tumor expression as a
biomarker of activity of specific inhibitors remains unclear,
although its overexpression is considered to confer a poor
prognostic outcome in various tumors, including lung cancer, breast
cancer and leukemia (24,25). With particular regard to breast
cancer, the independent poor prognostic role of HSP90
overexpression in multivariate modeling was previously demonstrated
in a large series of over 600 patients with a follow-up period of
more than 10 years, together with large tumor size, nodal
positivity, lower progestin receptor level and high HER2 level
(26).
All the experimental results revealed that with
increasing concentrations of wighteone the inhibition of
proliferation of MCF-7 cells gradually increased. The apoptotic
rate in the wighteone-treated groups was higher compared with the
control group (P<0.05). From this, it is clear that wighteone
induces MCF-7 cell apoptosis in vitro and restrains cell
proliferation. The activation of the AKT and MAPK pathways and the
high expression of HSP90 protein are common in HER2-positive breast
cancer (27) and there is a positive
correlation between HSP90 and breast cancer pathological stage,
local recurrence and distant metastasis, all of which result in
poor patient prognosis (28). Our
study reveals that wighteone blocks the expression of HSP90 protein
in MCF-7 cells, and that the inhibitory effect is enhanced with
increasing drug concentrations. Wighteone is able to control
proliferation and promote apoptosis in cancer cells, and it is
possible that wighteone decreases the expression of HSP90 protein,
which downregulates the key downstream molecular activation of the
AKT and MAPK pathways.
In conclusion, the present study confirmed that
wighteone significantly inhibits proliferation and promotes
apoptosis in HER2-positive breast cancer cells, and that this may
be associated with the inhibition of HSP90 protein expression in
cancer cells.
Acknowledgements
This study was supported by The Inner Mongolia
Autonomous Region People's Hospital (grant no. 20110936) and the
Natural Science Foundation of Inner Mongolia (grant no.
2014MS0801). The authors kindly acknowledge and thank these
institutions for their support throughout this study.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 1994. American Cancer Society. Atlanta, GA:
131994.
|
|
2
|
Kumar S, Pathania AS, Saxena AK, et al:
The anticancer potential of flavonoids isolated from the stem bark
of Erythrina suberosa through induction of apoptosis and
inhibition of STAT signaling pathway in human leukemia HL-60 cells.
Chem Biol Interact. 205:128–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baselga J and Swain SM: Novel anticancer
targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer.
9:463–475. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beliakoff J and Whitesell L: Hsp90: an
emerging target for breast cancer therapy. Anticancer Drugs.
15:651–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solit DB and Chiosis G: Development and
application of Hsp90 inhibitors. Drug Discov Today. 13:38–43. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Citri A, Kochupurakkal BS and Yarden Y:
The Achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone
machine and potential for pharmacological intervention. Cell Cycle.
3:51–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taldone T, Sun W and Chiosis G: Discovery
and development of heat shock protein 90 inhibitors. Bioorg Med
Chem. 17:2225–2235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pick E, Kluger Y, Giltnane JM, et al: High
HSP90 expression is associated with decreased survival in breast
cancer. Cancer Res. 67:2932–2937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song CH, Park SY, Eom KY, et al: Potential
prognostic value of heat-shock protein 90 in the presence of
phosphatidylinositol-3-kinase overexpression or loss of PTEN, in
invasive breast cancers. Breast Cancer Res. 12:R202010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith-Jones PM, Solit D, Afroze F, Rosen N
and Larson SM: Early tumor response to Hsp90 therapy using HER2
PET: comparison with 18F-FDG PET. J Nucl Med. 47:793–796.
2006.PubMed/NCBI
|
|
15
|
Costantini DL, Bateman K, McLarty K, et
al: Trastuzumab-resistant breast cancer cells remain sensitive to
the Auger electron-emitting radiotherapeutic agent
111In-NLS-trastuzumab and are radiosensitized by methotrexate. J
Nucl Med. 49:1498–1505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orlova A, Wållberg H, Stone-Elander S and
Tolmachev V: On the selection of a tracer for PET imaging of
HER2-expressing tumors: direct comparison of a 124I-labeled
affibody molecule and trastuzumab in murine xenograft model. J Nucl
Med. 50:417–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kramer-Marek G, Kiesewetter DO and Capala
J: Changes in HER2 expression in breast cancer xenografts after
therapy can be quantified using PET and (18)F-labeled affibody
molecules. J Nucl Med. 50:1131–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dijkers EC, Kosterink JG, Rademaker AP, et
al: Development and characterization of clinical-grade
89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med.
50:974–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hargreaves RT, Jonson RD, Millington DS,
et al: Alkaloids of American species of Erythrina. Lloydia.
37:569–580. 1974.PubMed/NCBI
|
|
21
|
Chopra RN, Nayar SL and Chopra IC:
Glossary of Indian Medicinal Plants. CSIR. New Delhi: 1956.
|
|
22
|
Garín-Aguilar ME, Luna JE, Soto-Hernández
M, et al: Effect of crude extracts of Erythrina americana
Mill. on aggressive behavior in rats. J Ethnopharmacol. 69:189–196.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Agrawal SK, Agrawal M, Sharma PR, et al:
Induction of apoptosis in human promyelocytic leukemia HL60 cells
by an extract from Erythrina suberosa stem bark. Nutr
Cancer. 63:802–813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gallegos Ruiz MI, Floor K, Roepman P,
Rodriguez JA, et al: Integration of gene dosage and gene expression
in non-small cell lung cancer, identification of HSP90 as potential
target. PLoS One. 3:e00017222008.PubMed/NCBI
|
|
25
|
Garcia-Carbonero R, Carnero A and Paz-Ares
L: Inhibition of HSP90 molecular chaperones: moving into the
clinic. Lancet Oncol. 14:e358–e369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pick E, Kluger Y, Giltnane JM, Moeder C,
Camp RL, Rimm DL and Kluger HM: High HSP90 expression is associated
with decreased survival in breast cancer. Cancer Res. 67:2932–2937.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ciocca DR, Clark GM, Tandon AK, Fuqua SA,
Welch WJ and McGuire WL: Heat shock protein hsp70 in patients with
axillary lymph node-negative breast cancer: prognostic
implications. J Natl Cancer Inst. 85:570–574. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim LS and Kim JH: Heat shock protein as
molecular target for breast cancer therapeutics. J Breast Cancer.
14:167–174. 2011. View Article : Google Scholar : PubMed/NCBI
|