Introduction
Adrenal cortical carcinoma (ACC) is a rare malignant
tumor known to have a poor prognosis. The incidence rate for ACC
has been reported to be ~0.5–2 million individuals/year (1–3). It has a
bimodal age distribution that peaks in children in the first decade
of life and adults in the fourth to fifth decades of life, although
the majority of patients with ACC are adults. ACC has a poor
overall survival rate of 16–37% despite treatment (4). The majority of adrenal tumors are
sporadic and unilateral, but 2–6% are bilateral and are associated
with Li-Fraumeni syndrome, multiple endocrine neoplasia type I,
Beckwith-Wiedemann syndrome and the Carney complex, primarily in
children (4). Tumor-associated
symptoms of ACC may be secondary to local or systemic disease
burden and/or hypersecretion of adrenal hormones, which has been
observed in 50–79% of adults and 90% of children with ACC (2). ACCs are classified into functional and
non-functional tumors. It has been reported that 1/3 of patients
with ACC also present with tumor thrombi that extend into the
inferior vena cava (IVC), 51% of which also extend into the right
atrium. The standard treatment for ACC is complete surgical removal
of the primary tumor, which may require the resection of adjacent
organs, such as the kidneys and spleen, and any caval thrombus that
are present, as well as en bloc resection of local lymph
nodes (5). Mitotane has been
administered for patients with metastatic disease, however, despite
the response rates exhibited, no improvement in survival time was
identified following treatment (6,7). The
present study reports a case of functional ACC in the right
adrenal, inferior to the right lobe of the liver with tumor
thrombus extension into the IVC and right atrium.
Case report
A 33-year-old male patient was admitted to The First
Affiliated Hospital, College of Medicine of Zhejiang University
(Hangzhou, China) on May 13, 2013 presenting with symptoms of
gynecomastia for 3 months and abdominal distention for 1 month.
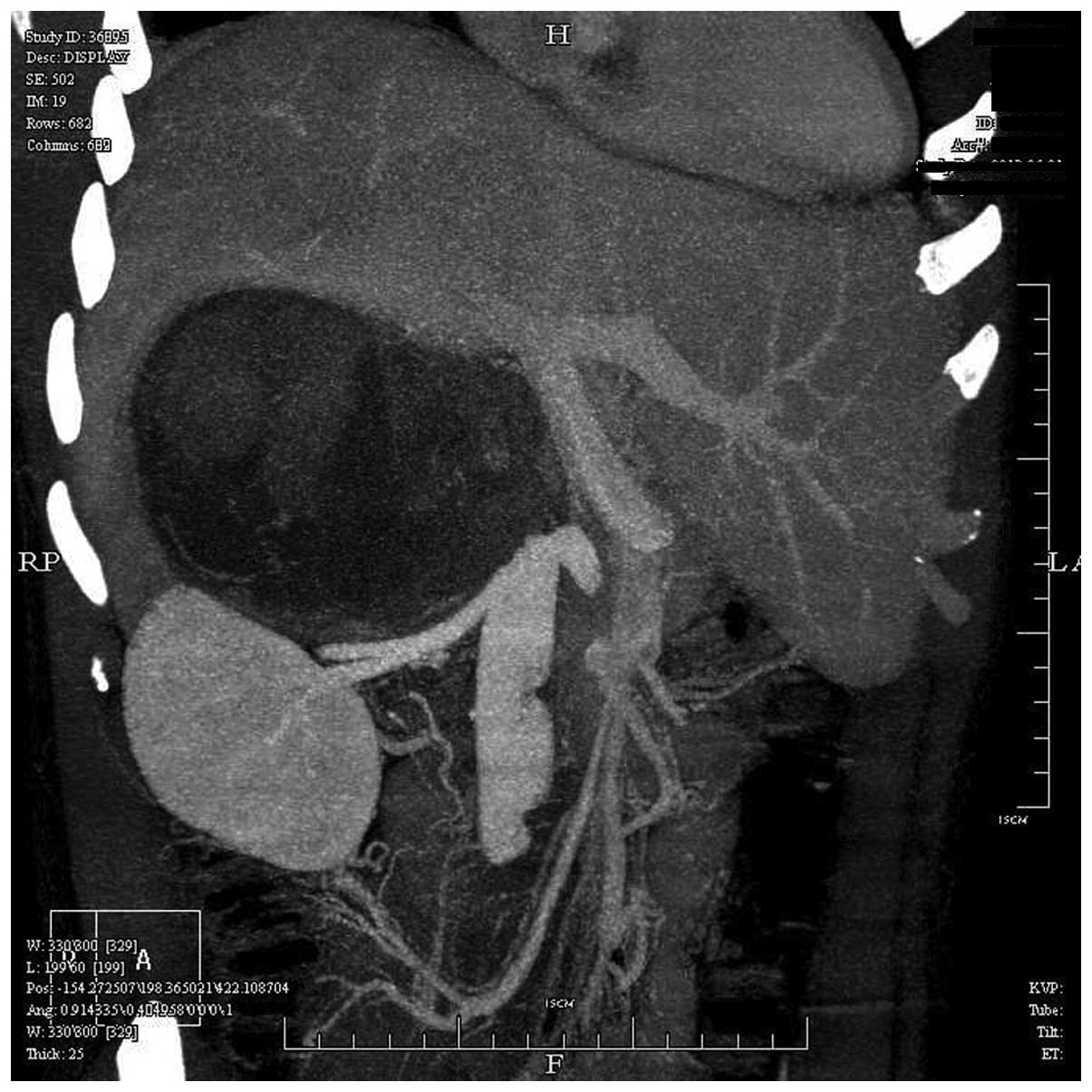
Abdominal computed tomography angiography (Brilliance iCT; Philips
Healthcare, DA Best, The Netherlands) revealed a large mass 18×15
cm in size in the right adrenal gland, located below the right lobe
of the liver, with tumor thrombus extension into the IVC. The left
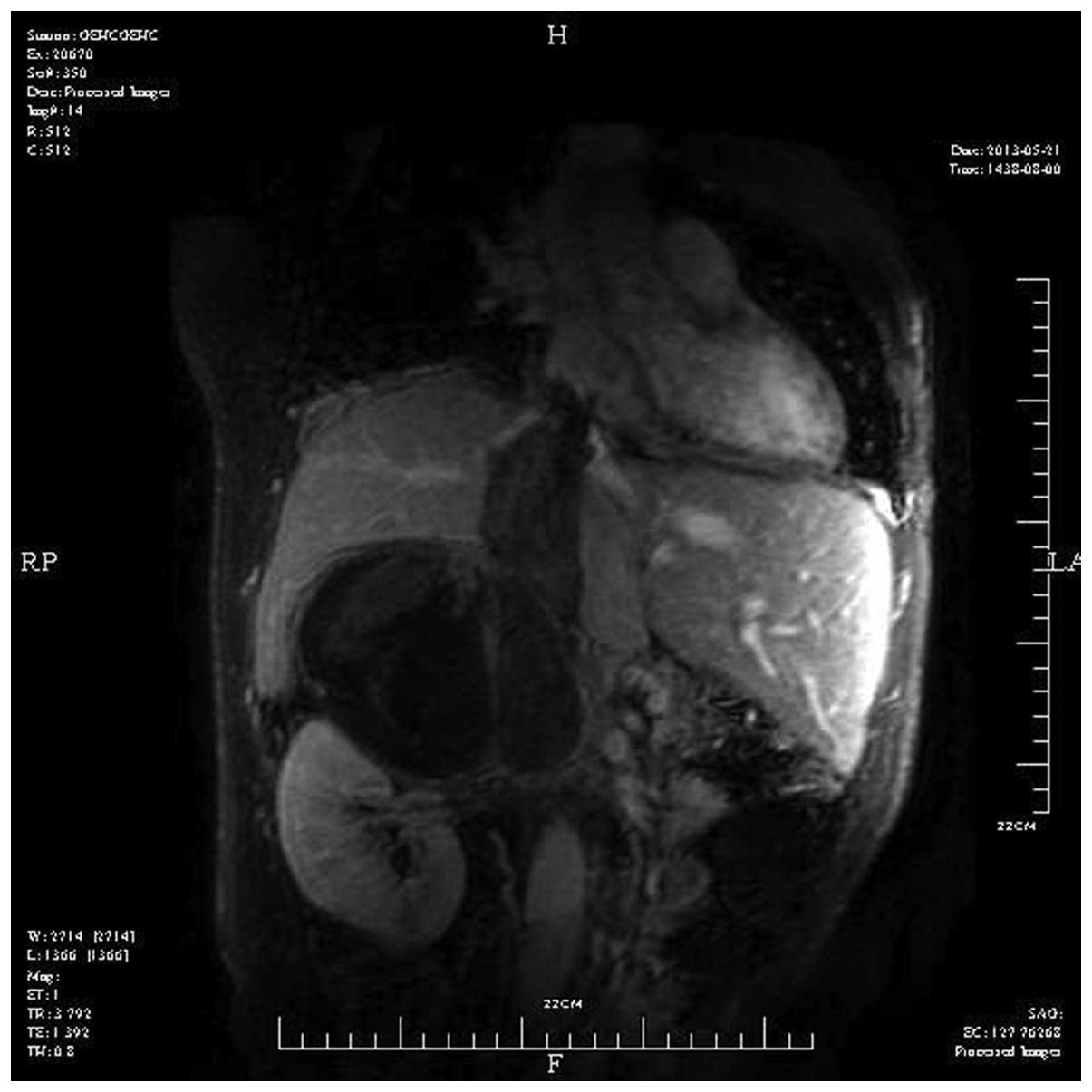
renal vein had been pushed down by the mass (Fig. 1). Magnetic resonance imaging scan
(Signa HDx 3.0T; GE Healthcare Life Sciences, Chalfont, UK) of the
IVC revealed a large mass in the right adrenal gland with tumor
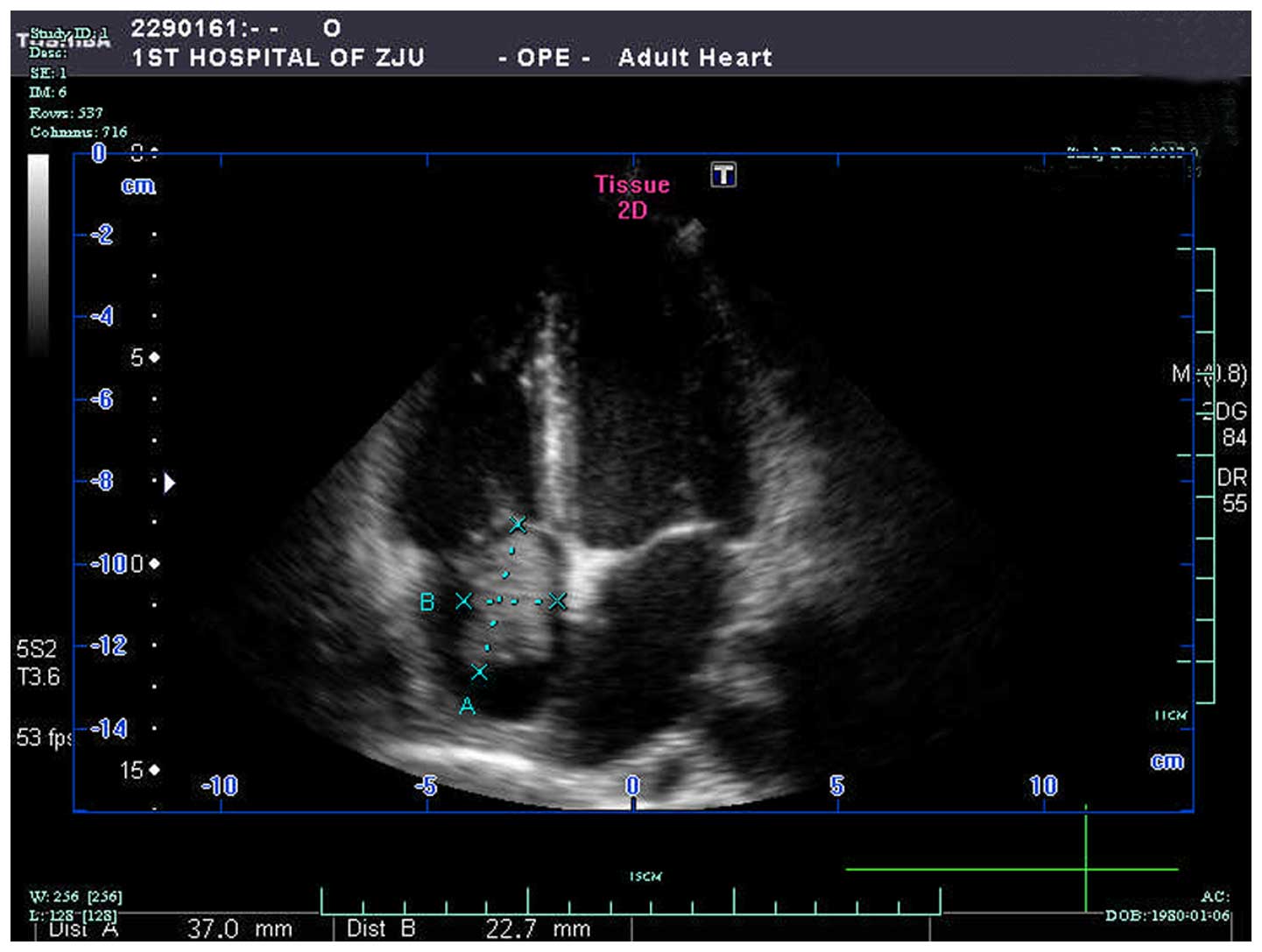
thrombus extension into both the IVC and the right atrium (Fig. 2). Echocardiography (IE33; Philips
Healthcare) also identified the tumor thrombus in the right atrium
(Fig. 3). Chest X-ray showed no
metastasis.
The hormone levels of the patient were also
measured. The serum aldosterone values were as follows: Upright
position, 634.02 ng/dl (normal values, 70.00–300.00 ng/dl) and
supine position, 434.90 ng/dl (normal values, 30.00–160.00 ng/dl).
Angiotensin II values were as follows: Upright position, 86.10
(normal values, 50.00–120.00) and supine position, 85.62 (normal
values, 25.00–60.00). Plasma renin activity (PRA) values were as
follows: Upright position, 15.36 (normal values, 0.10–6.56) and
supine position, 0.91 (normal values, 0.15–2.33). Testosterone
levels were 26.20 ng/dl (normal values in men, 262.00–1,593.00
ng/dl). Estradiol levels were 208.00 pg/ml (normal values,
0.00–56.00 pg/ml). The levels of follicle stimulating hormone (FSH)
were 0.20 mIU/ml (normal values, 0.70–11.10 mIU/ml), while the
levels of luteinizing hormone (LH) were 1.40 mIU/ml (normal values,
0.80–7.60 mIU/ml). The levels of prolactin were 19.30 ng/ml (normal
values, 2.50–17.00 ng/ml), while the levels of progesterone were
1.18 ng/ml (normal values, 0.27–0.90 ng/ml). The levels of all
other adrenalcortical hormones were normal.
The patient underwent a thoracoabdominal incision to
achieve the best exposure of the tumor. Radical right nephrectomy
and adrenalectomy were performed. The tumor was observed to be
located very close to the adjacent viscera, particularly to the
right kidney, IVC and right lobe of the liver, and the period of
time required for separating the tumor from the organs was
considerably lengthy. The right kidney was enveloped by the tumor,
and thus had to be removed; however, the right lobe of the liver
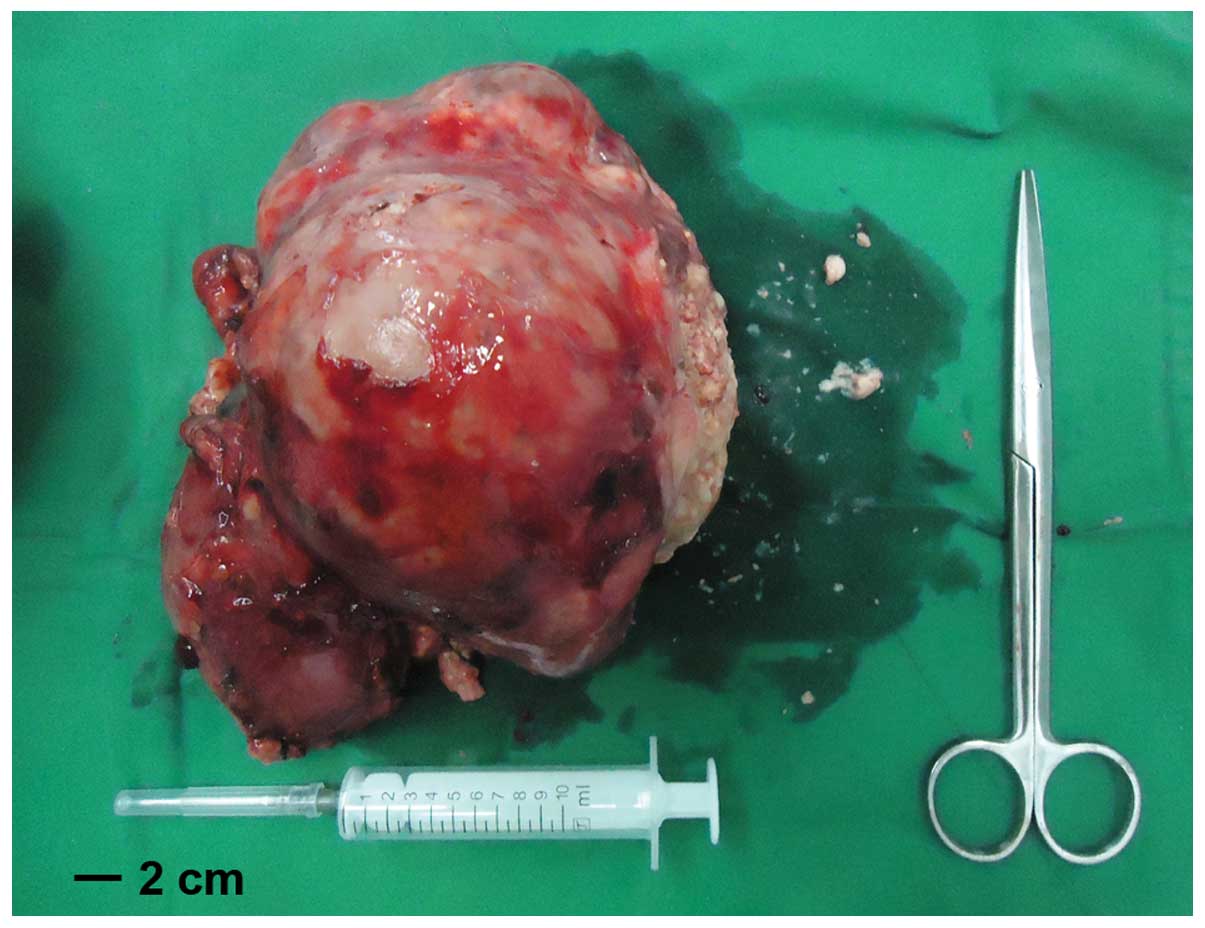
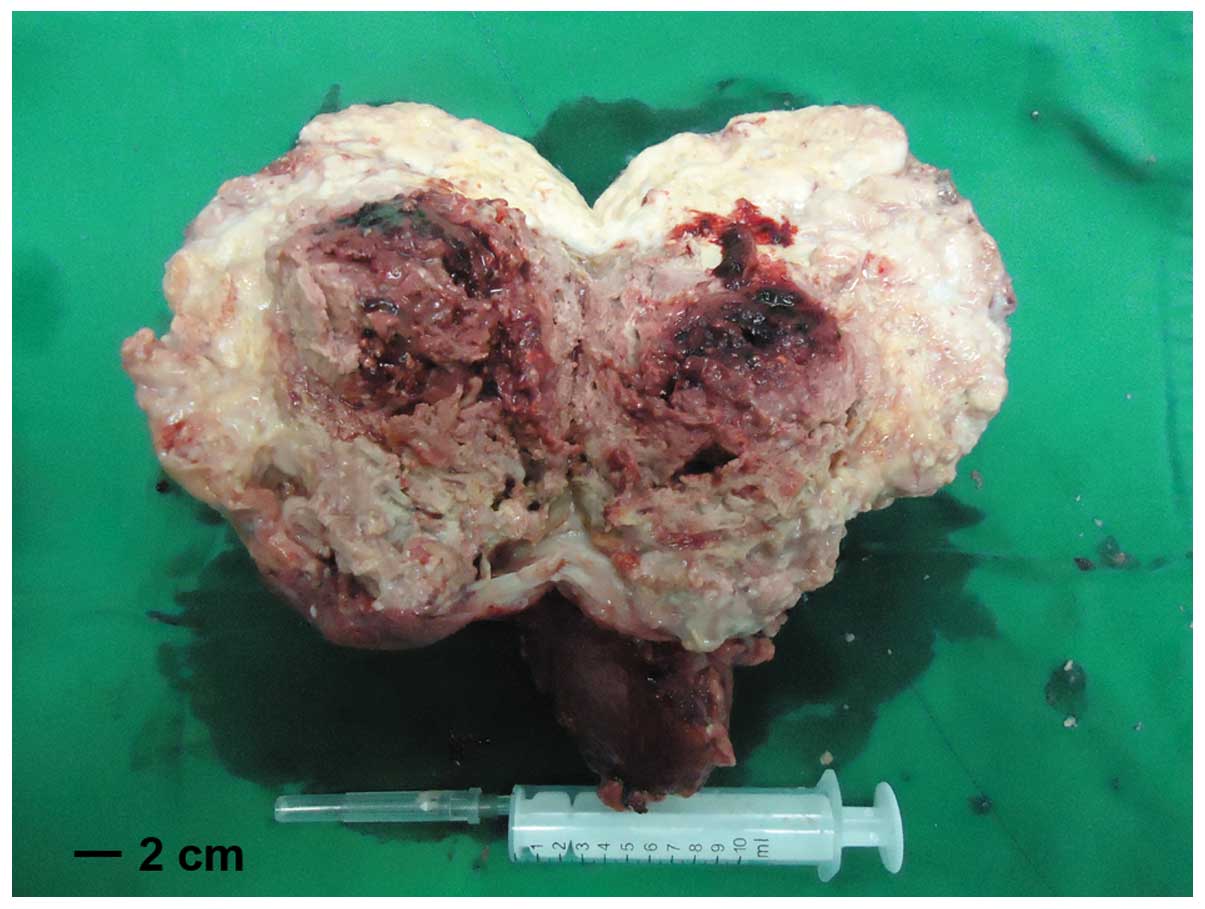
was successfully separated from the tumor. The tumor measured
~18×15 cm and weighted 1.5 kg, including the weight of the right
kidney (Fig. 4). Following
consultation with hepatobiliary and pancreatic, the patient was
subjected to vascular surgery. A resection of the right lobe of the
liver and IVC replacement were considered. However, the surgeons
(Department of Urology, The First Affiliated Hospital, Zhejiang
University) managed to separate the tumor from the viscera, thus
avoiding both of these procedures, although the estimated blood
loss was ~3,000 ml.
In order to extract the tumor thrombus, which had
extended into the IVC and right atrium, the right lobe of the liver
had to be mobilized. Therefore, extracorporeal circulation
(8) was performed by cardiothoracic
surgeons (Department of Urology, The First Affiliated Hospital,
Zhejiang University). The superior and inferior venae cavae, first
hepatic portal artery and vein, and left renal vein were clamped,
in order to reduce bleeding. Subsequently, the right atrium was
opened, and the tumor thrombus was successfully removed from the
IVC and right atrium under visual control, using extracorporeal
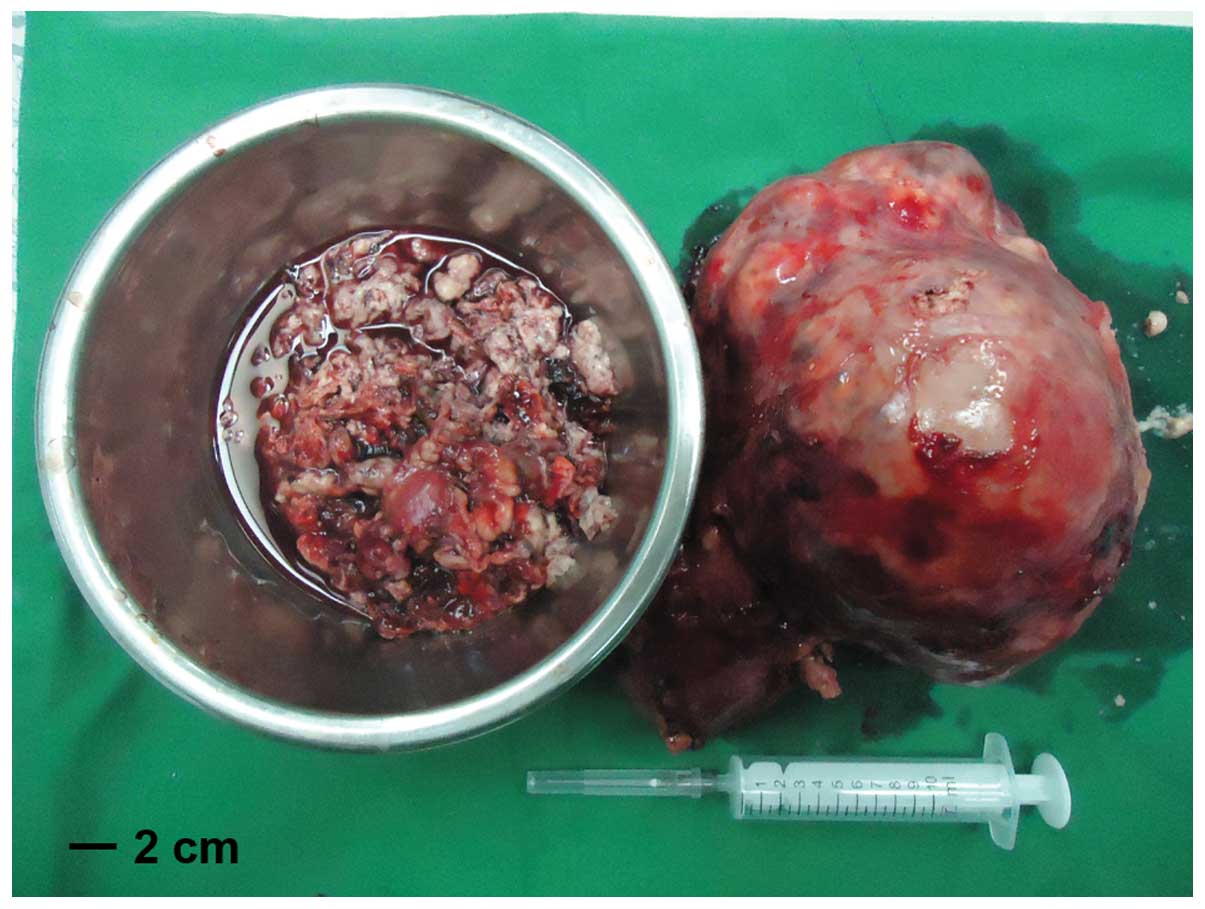
circulation assisted by cardiothoracic surgeons. The tumor thrombus
exhibited a gelatinous and friable appearance (Fig. 5 and 6).
The portal and renal vein were repeatedly opened and closed, in
order to irrigate the visible tumor thrombus and prevent residual
tumor thrombus and embolism. A segment wall of the IVC was excised
due to tumor thrombus invasion, and repaired using the pericardial
patch repair technique (9). The
procedure was uneventful. The patient returned to the ward after 1
day in the intensive care unit. The patient was administered
adjuvant treatment with 4 g mitotane daily 7 days following
surgery, and was discharged 12 days following surgery. No signs of
metastasis or recurrence were identified at the 1-month
follow-up.
The hormone levels of the patients were measured
again 7 days following surgery. The serum aldosterone levels were
as follows: Upright position, 327.11 ng/dl (normal values,
70.00–300.00 ng/dl) and supine position, 184.67 ng/dl (normal
values, 30.00–160.00 ng/dl). The angiotensin II levels were as
follows: Upright position, 85.71 ng/l (normal values, 50.00–120.00
ng/l) and supine position, 77.84 ng/l (normal values, 25.00–60.00
ng/l). PRA values were as follows: Upright position >24.00
ng/ml/h (normal values, 0.10–6.56 ng/ml/h) and supine position,
5.16 ng/ml/h (normal values, 0.15–2.33 ng/ml/h). Testosterone
levels were at 138.00 ng/dl (normal values in men, 262.00–1,593.00
ng/dl). Estradiol levels were 101.00 pg/ml (normal values,
0.00–56.00 pg/ml). The levels of FSH were 2.70 mIU/ml (normal
values, 0.70–11.10 mIU/ml) and those of LH were 11.20 mIU/ml
(0.80–7.60 mIU/ml). The levels of prolactin were 18.60 ng/ml
(normal values, 2.50–17.00 ng/ml), and those of progesterone were
1.32 ng/ml (normal values, 0.27–0.90 ng/ml). All other adrenal
cortical hormone levels were normal. The post-surgical levels of
aldosterone had decreased compared with the preoperative levels,
although they remained considerably high. PRA also remained at a
high level.
Resected specimens were formalin (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China)-fixed, paraffin
(Beijing Solarbio Science & Technology Co., Ltd.)-embedded and
cut into 3-µm sections. Histopathological staining with hematoxyli
n and eosin (Beijing Solarbio Science & Technology Co., Ltd.)
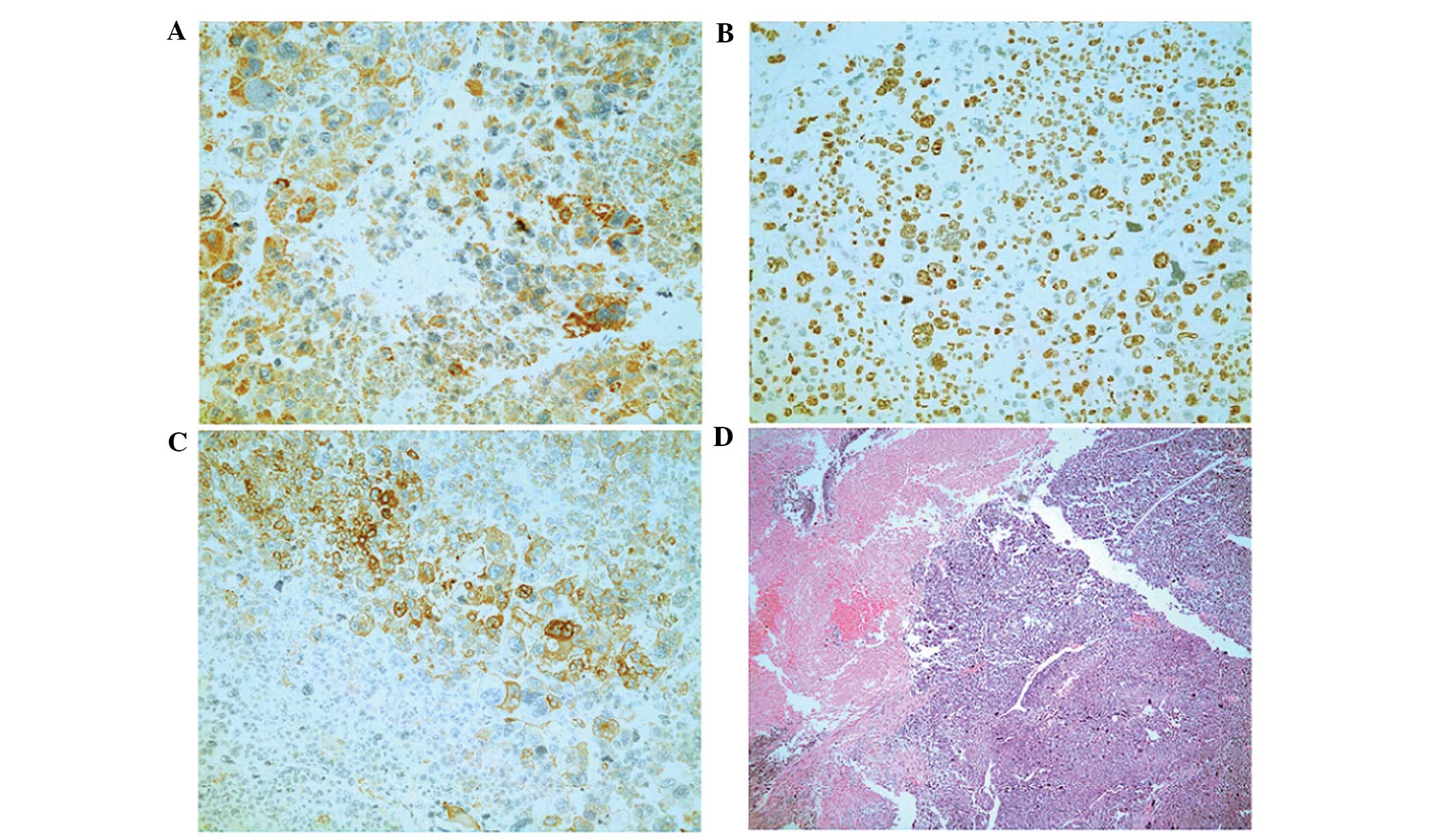
confirmed the diagnosis of adrenal cortical carcinoma with
associated tumor thrombus. Tissue sections were incubated with
primary antibodies overnight at 4°C. Immunohistochemical findings
included the following (Fig. 7):
Pan-cytokeratin+ (mouse monoclonal; catalog no.,
Kit-0009), synuclein+ (mouse monoclonal; catalog no.,
MAB-0538), S-100− (mouse monoclonal; catalog no.,
Kit-0007), melan-A+ (mouse monoclonal; catalog no.,
MAB-0275), hepatocyte marker− (mouse monoclonal; catalog
no., MAB-0249) and 70% Ki-67+ (mouse monoclonal; catalog
no., Kit-0005) (Fuzhou Maixin Biotech Co., Ltd.).
The patient succumbed to the disease 18 months
subsequent to surgery due to extensive metastasis. Written informed
consent was obtained from the patient for the publication of the
present study.
Discussion
ACC is a rare type of malignant tumor associated
with a poor prognosis (1–3). The incidence rate for ACC has been
reported to be ~0.5–2/1,000,000/year (10,11). ACC
can be classified into functional and non-functional, based on
hormone levels (functional, abnormal hormone secretion;
non-functional, normal hormone secretion). Hypercortisolism is
considered to be the most common symptom of functional ACC, while
primary hyperaldosteronism secondary to ACC with symptoms of
hypertension and hypokalemia is rare (2,12). ACC
with abnormal levels of reproductive hormones is also rare
(2). Androgen-secreting ACCs can
induce hirsutism and virilization in women, while
estrogen-secreting adrenal tumors can cause gynecomastia and
testicular atrophy in men (13–15).
Tritos et al (16) reported
that ACC functional tumors are associated with worse survival than
non-functional tumors. By contrast, other studies did not identify
any association between tumor status and survival in patients with
ACC (7,17). In the present case, the tumor caused
the patient's endocrine system to malfunction. Due to its
irregularity, the endocrine status of a patient is may be difficult
to explain (2). In the present
patient, the aldosterone and estradiol levels increased, while the
testosterone levels decreased, which resulted in the presentation
of gynecomastia. The patient complained of bilateral breast pain
and swelling, without testicular atrophy or impotence. In addition,
the patient presented with abdominal distention, which was due to
the large size of the tumor.
ACC is an aggressive tumor, whose early symptoms are
not obvious and its prognosis is poor (2,9). The
median survival for untreated patients is 2.5 months (18). Based on the staging system established
by the European Network for the Study of Adrenal Tumors (ENS@T)
(19), the present patient was
diagnosed with stage III ACC. Tritos et al (16) analyzed 31 cases of ACC and observed
that the prognostic factors were associated with the time of
diagnosis, age, absence of metastatic disease, tumor stage and
whether or not complete surgical resection had been performed. By
contrast, the authors did not identify any association between
prognosis and gender or tumor thrombus extension into the IVC. The
median survival of patients with ACC may be prolonged to 17 months,
or even 26 months if the patient has undergone radical surgery
(9). There have been numerous reports
of cases where radical resection of the tumor was independently
associated with improved survival (17,20,21).
ACC with tumor thrombus extension into the IVC is
considered a rare occurrence (18).
The majority of patients with this condition present with large
tumors, and 51% of these tumors extend into the right atrium
(18). ACC with tumor thrombus
extension into the right atrium alone should not be considered a
contraindication against radical surgical therapy (15). Despite the increased risk associated
with the surgical procedure, clinicians should attempt a radical
resection or a maximal tumor load debulking (15). In general, extracting the tumor
thrombus under extracorporeal circulation is relatively safe, if
the patient has no other systemic disorders (8,9). This
method may reduce the duration of the surgery, minimize the blood
loss and prevent avoid embolism (9).
Preoperative planning and intraoperative cooperation among multiple
departments are imperative for this type of surgical procedure. In
the present case, the tumor was removed en bloc, and the
tumor thrombus was extracted under extracorporeal circulation. The
procedure was successful and uneventful. According to Chiche et
al (15), only in 3/15 cases were
vena cava resection or graft replacement required, whereas opposite
side renal vein resection and graft replacement was not required in
any of the cases included in that study. In the present case, if
the separation of the tumor from the viscera had been rendered
impossible, resection of the right lobe of the liver or graft
replacement of the IVC would have been required. In the end, the
tumor was successfully separated from the viscera and the patient
recovered well from the surgery.
The patient was administered adjuvant treatment 7
days following surgery, which consisted of 4 g mitotane daily, in
order to maintain plasma concentrations of the drug of 14–20 mg/l
(22). Adjuvant mitotane therapy for
ACC is controversial, since there is a limited number of randomized
controlled trials, due to the low incidence of the disease. Early
studies disagreed on whether mitotane was able to improve patient
survival (7,17,23). A
subsequent retrospective study (24)
demonstrated that mitotane can improve recurrence-free survival but
not overall survival in patients with ACC. In 2010, a panel of
international experts proposed adjuvant mitotane treatment for ACC
patients with potential residual disease or >10% Ki67 positivity
detected upon immunohistochemical examination (25). The present patient was administered
mitotane, since, according to the ENS@T staging system, the patient
exhibited stage III disease and 70% Ki67 positivity. No signs of
metastasis or recurrence were identified at the 1-month
follow-up.
Based on the available literature, as well as
personal experience, the present authors propose that extension of
the tumor thrombus into the right atrium alone should not be
considered a contraindication against radical surgical therapy in
patients with ACC. However, preoperative planning and
intraoperative cooperation among multiple departments for the
surgical procedure are imperative. In conclusion, despite the
association of ACC with poor prognosis, radical surgery-based
therapy may result in favorable outcomes for ACC patients.
References
|
1.
|
Aubert S, Wacrenier A, Leroy X, Devos P,
Carnaille B, Proye C, Wemeau JL, Lecomte-Houcke M and Leteurtre E:
Weiss system revisited: A clinicopathologic and immunohistochemical
study of 49 adrenocortical tumors. Am J Surg Pathol. 26:1612–1619.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Roman S: Adrenocortical carcinoma. Curr
Opin Oncol. 18:36–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fassnacht M and Allolio B: Clinical
management of adrenocortical carcinoma. Best Pract Res Clin
Endocrinol Metab. 23:273–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ng L and Libertino JM: Adrenocortical
carcinoma: Diagnosis, evaluation and treatment. J Urol. 169:5–11.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ekici S and Ciancio G: Surgical management
of large adrenal masses with or without thrombus extending into the
inferior vena cava. J Urol. 172:2340–2343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hoffman DL and Mattox VR: Treatment of
adrenal cortical carcinoma with o,p'-DDD. Med Clin North Am.
56:999–1012. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Luton JP, Cerdas S, Billaud L, Thomas G,
Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y and
Blondeau P: Clinical features of adrenocortical carcinoma,
prognostic factors, and the effect of mitotane therapy. N Engl J
Med. 322:1195–1201. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nardi P, Pellegrino A, Pugliese M, Bovio
E, Chiariello L and Ruvolo G: Cardiac surgery with extracorporeal
circulation and concomitant malignancy: Early and long-term
results. J Cardiovasc Med (Hagerstown). 17:152–159. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Bertherat J, Coste J and Bertagna X:
Adjuvant mitotane in adrenocortical carcinoma. N Engl J Med.
357:1256–1257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kerkhofs TM, Verhoeven RH, Van der Zwan
JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV and
Haak HR: Adrenocortical carcinoma: A population-based study on
incidence and survival in the Netherlands since 1993. Eur J Cancer.
49:2579–2586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kebebew E, Reiff E, Duh QY, Clark OH and
McMillan A: Extent of disease at presentation and outcome for
adrenocortical carcinoma: Have we made progress? World J Surg.
30:872–878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Puvaneswary M and Thong K: Primary
hyperaldosteronism: Due to adrenal cortical carcinoma. Australas
Radiol. 37:88–89. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Coonrod DV and Rizkallah TH: Virilizing
adrenal carcinoma in a woman of reproductive age: A case
presentation and literature review. Am J Obstet Gynecol.
172:1912–1915. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lanigan D, Choa RG and Evans J: A
feminizing adrenocortical carcinoma presenting with gynaecomastia.
Postgrad Med J. 69:481–483. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chiche L, Dousset B, Kieffer E and Chapuis
Y: Adrenocortical carcinoma extending into the inferior vena cava:
Presentation of a 15-patient series and review of the literature.
Surgery. 139:15–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tritos NA, Cushing GW, Heatley G and
Libertino JA: Clinical features and prognostic factors associated
with adrenocortical carcinoma: Lahey Clinic Medical Center
experience. Am Surg. 66:73–79. 2000.PubMed/NCBI
|
|
17.
|
Bodie B, Novick AC, Pontes JE, Straffon
RA, Montie JE, Babiak T, Sheeler L and Schumacher P: The Cleveland
Clinic experience with adrenal cortical carcinoma. J Urol.
141:257–260. 1989.PubMed/NCBI
|
|
18.
|
Hedican SP and Marshall FF: Adrenocortical
carcinoma with intracaval extension. J Urol. 158:2056–2061. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lebastchi AH, Kunstman JW and Carling T:
Adrenocortical carcinoma: Current therapeutic state-of-the-art. J
Oncol. 2012:2347262012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Allolio B, Hahner S, Weismann D and
Fassnacht M: Management of adrenocortical carcinoma. Clin
Endocrinol (Oxf). 60:273–287. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dackiw AP, Lee JE, Gagel RF and Evans DB:
Adrenal cortical carcinoma. World J Surg. 25:914–926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ochi T, Tanji N, Shimamoto K, Ikeda T,
Toshino A and Yokoyama M: Application of cardiopulmonary bypass for
resection of renal cell carcinoma and adrenocortical carcinoma
extending into the right atrium. Int J Urol. 13:202–205. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Haak HR, Hermans J, van de Velde CJ,
Lentjes EG, Goslings BM, Fleuren GJ and Krans HM: Optimal treatment
of adrenocortical carcinoma with mitotane: Results in a consecutive
series of 96 patients. Br J Cancer. 69:947–951. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Terzolo M, Angeli A, Fassnacht M, Daffara
F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P,
Grossrubatscher E, et al: Adjuvant mitotane treatment for
adrenocortical carcinoma. N Engl J Med. 356:2372–2380. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Berruti A, Fassnacht M, Baudin E, Hammer
G, Haak H, Leboulleux S, Skogseid B, Allolio B and Terzolo M:
Adjuvant therapy in patients with adrenocortical carcinoma: A
position of an international panel. J Clin Oncol. 28:e401–e402.
2010. View Article : Google Scholar : PubMed/NCBI
|