Introduction
Ependymomas may occur in any part of the ventricular
system, but they mainly develop in the posterior fossa (1–3). The
occurrence of ependymomas is more common in pediatric patients than
in adult patients, accounting for 8–13% of all intracranial tumors
in children and up to 4% of brain tumors in adults. Approximately
one-half of ependymomas are found in children aged <3 years
(1–13). In children, ependymomas usually occur
in the fourth ventricle and display a series of symptoms and signs,
including increased intracranial pressure, ataxia, vertigo, chronic
cerebellar tonsillar hernia and brainstem dysfunction (1,14). Despite
the fact that infratentorial tumors exhibit a lower biological
activity than supratentorial tumors (9,15–17), tumors of the posterior fossa carry a
higher surgical risk, due to the technical difficulties in
performing a complete excision, which may lead to treatment failure
(9,15).
Ependymomas of the fourth ventricle are usually
approached with a posterior median craniotomy/craniectomy, followed
by splitting of the vermis; however, this trans-vermian approach
could easily injure the vermis, cerebellar dentate and globose
nuclei, which may result in tremor, ataxia, atonia and cerebellar
mutism, thereby affecting the prognosis (13,14,18–25).
The establishment of the novel trans-cerebellomedullary fissure
(CMF) approach, which provides sufficient access to the fourth
ventricle without sacrificing the inferior vermis, has been
associated with favorable outcomes (13,14,18–20,22–32).
The trans-CMF approach is gaining popularity among the available
surgical approaches that are used for the complete removal of
fourth ventricular tumors excessively extending laterally into the
cerebellomedullary cistern (13,14,18,19,23,27,31,32).
In children, ependymomas of the fourth ventricle usually originate
from the ependymal layer and are well-circumscribed (1,2). Also,
ependymomas in the present study usually exhibted a cleavage plane
between the tumor tissue and brainstem during the surgery,
particularly in low-grade disease. Therefore, sufficient exposure
and complete removal of the tumor may improve the prognosis to a
great extent. The present study reports 26 consecutive cases of
ependymomas of the fourth ventricle in children who received
posterior fossa craniotomy via the trans-CMF approach for the
excision of the tumors, and presents a review of the surgical
procedures and findings.
Patients and methods
Patients
Between March 2006 and September 2010, 26 patients
with ependymomas of the fourth ventricle underwent surgery at the
Department of Neurosurgery of The First Affiliated Hospital of
China Medical University (Shenyang, China). The clinical records of
these cases were retrospectively reviewed, and the demographics and
major clinical manifestations of the patients are listed in
Table I. The patient population was
composed of 15 male and 11 female pediatric patients (male:female
ratio, 1.36:1) aged 2.5–14.0 years (mean age, 8.7 years). The
clinical course of the patients lasted between 2 weeks and 3 years
(median time period, 7.5 months). The most common symptoms included
headache, dizziness, nausea and/or vomiting, cranial nerve
deficits, brainstem dysfunction and hydrocephalus (Table I). A total of 3 patients exhibited
obvious signs of hydrocephalus, including supratentorial ventricle
enlargement, papilledema, urinary incontinence, unsteady gait and
drowsiness. Of these 3 patients, 2 had received preliminary
ventriculoperitoneal shunting in local hospitals in the month prior
to surgery. The procedures in the present study were performed
according to the ethical standards of the Declaration of Helsinki
(33), and written informed consent
was obtained from the legal guardian of each patient.
 | Table I.Summary of patient characteristics
from the 26 reviewed cases of ependymoma of the fourth
ventricle. |
Table I.
Summary of patient characteristics
from the 26 reviewed cases of ependymoma of the fourth
ventricle.
| Characteristic | Value |
|---|
| Patients, n | 26 |
| Gender, n |
|
|
Male | 15 |
|
Female | 11 |
| Disease course |
|
|
Range | 2 weeks-3
years |
|
Average | 7.5 months |
| Age, years |
|
|
Range | 2.5–14.0 |
|
Mean | 8.7 |
| Clinical features,
n (%) |
|
|
Headache and/or dizziness | 15 (57.7) |
| Nausea
and/or vomiting | 19 (73.1) |
| Cranial
nerve deficits | 11 (42.3) |
|
Abducent
paralysis | 7
(26.9) |
|
Dysphagia and/or
hoarseness | 5
(19.2) |
|
Tinnitus and/or
deafness | 2 (7.7) |
|
Trigeminal
paralysis | 1 (3.8) |
| Ataxia, nystagmus
and/or gait disturbance | 13 (50.0) |
| Motor and sensory
dysfunction | 18 (69.2) |
| Simple vertigo | 4
(15.4) |
| Bruns sign | 3
(11.5) |
| Papilledema | 7
(26.9) |
| Consciousness
disorder | 1 (3.8) |
Neuroimaging studies
Contrast-enhanced magnetic resonance imaging (MRI;
Signa HDxt 3.0T; GE Healthcare, Chalfont, UK) and/or computed
tomography (CT; Brilliance 64-slice CT; Philips Medical Systems,
Cleveland, OH, USA) scans were evaluated preoperatively in all
cases. Imaging was used to evaluate the tumor size, anatomical
boundaries, association with and compression of the brainstem, as
well as extent of tumor invasion. The maximum tumor diameter range
was 2.7–8.1 cm, with a mean diameter of 4.1 cm. Supratentorial
ventricular enlargement was present in 12 cases (46.2%), and
cerebellar tonsillar hernia and/or syringomyelia in 7 cases
(26.9%). Remarkable brainstem compression and displacement were
observed in 14 cases (53.8%). All ependymomas were observed to
originate from the fourth ventricle, with slight and heterogeneous
enhancement and occasional cystic degeneration, calcification
and/or tumor apoplexy. Tumor invasion into the cisterna magna was
observed in 7 cases (26.9%), and corpora quadrigemina involvement
in 1 case (3.8%).
Surgical procedures
Following admission, all patients were routinely
treated with 20% mannitol solution (China Otsuka Pharmaceutical
Co., Ltd, Tianjin, China) to decrease intracranial pressure. To
sufficiently relieve intracranial hypertension in the 12 patients
with supratentorial ventricular enlargement, 3 patients with
hydrocephalus received frontal ventriculostomy 2 or 3 days prior to
tumor removal, and the other 9 patients underwent occipital
ventriculostomy intraoperatively, prior to dural incision.
As previously described (13,14,18–20,23,30),
all patients were operated on in the prone position with the head
flexed at the craniocervical junction. A vertical midline incision
was performed, followed by a suboccipital craniotomy/craniectomy,
with a bone window of ~5.0×4.0 cm. If the lesion was unilateral,
the craniotomy/craniectomy had to be extended to the contralateral
side just across the midline. A ~1.5–2.0-cm wide section of the rim
of the foremen magnum was removed, and then the posterior arch of
the atlas was removed (~1.5 cm in width) if the tonsils or lesion
extended distally to the spinal canal. The dura was opened with a
Y-shaped incision. Following the identification of the foramen of
Magendie and the posterior inferior cerebellar artery (PICA), the
tonsils were dissected from the vermis and the CMF was opened. The
CMF is composed of two slit spaces, the uvulotonsillar and
medullotonsillar spaces (14,19,23,26,27,32).
These two spaces were sharply dissected to release the tonsils and
the uvula, which enabled the easy retraction of the uvula
superiorly and of the tonsils laterally, thereby exposing the
inferior roof of the fourth ventricle, which is composed of the
tela choroidea, inferior medullary velum and lateral recess
(14,18,24).
Caution is required when dividing the attachments between the
tonsil, fourth ventricular floor and inferior medullary velum. When
the CMF was further opened, the exposure extended from the foramen
of Magendie to the foramen of Luschka and four lower cranial nerves
in the cerebellomedullary angle, depending on the tumor location
and extension. In fact, less dissection of these structures was
required in cases of large tumors, due to the distension at the
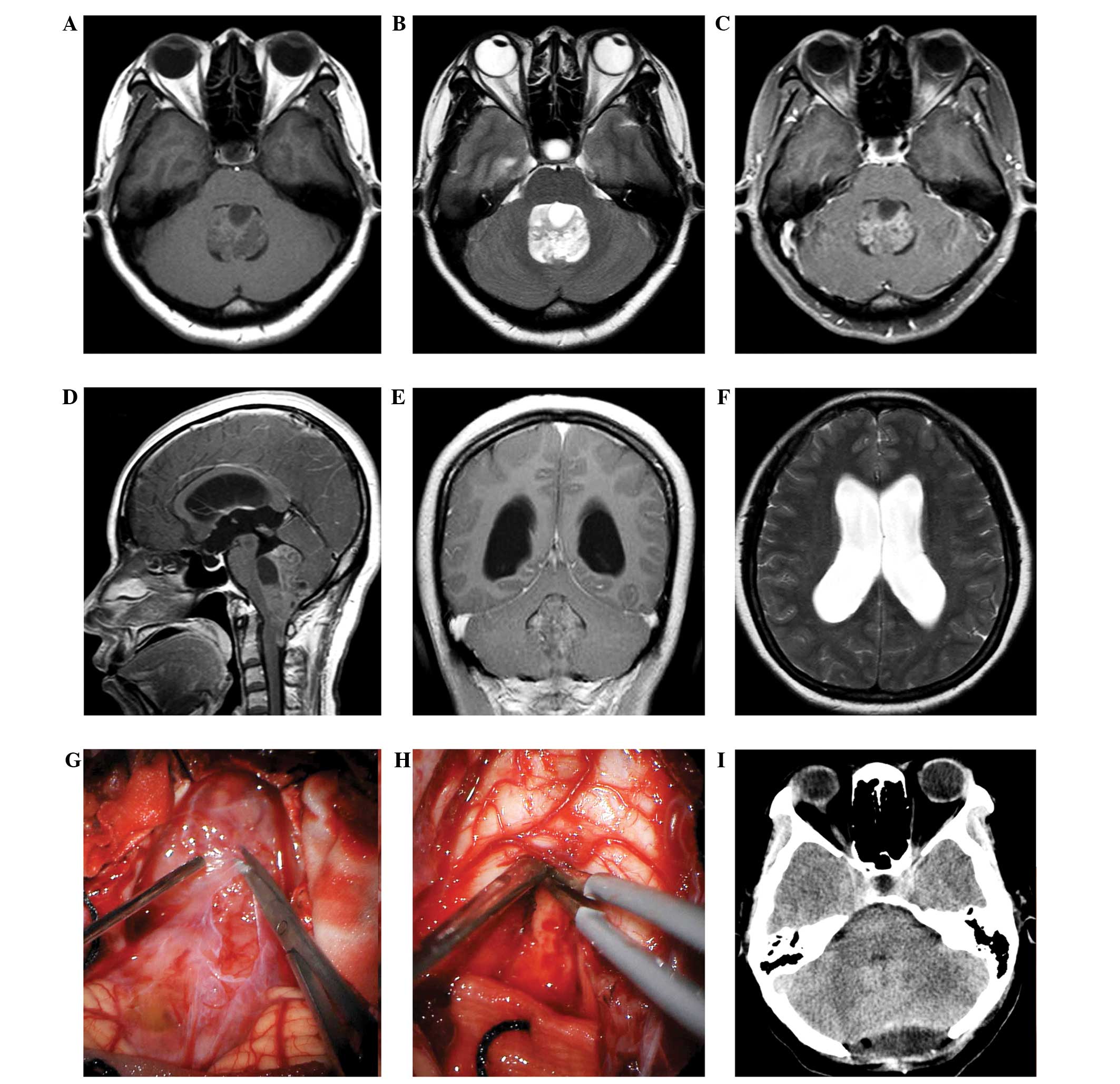
foramen of Magendie produced by the tumor (Fig. 1). If the lesions were primarily
located in the lower 3/4 region of the ventricle, only the tela
choroidea was opened; however, for tumors invading the upper 1/4
area of the ventricle, both the tela choroidea and inferior
medullary velum were opened to gain satisfactory exposure. When the
tonsil was gently elevated, the tumor bulk was exposed and then
dissected from the surrounding cerebellar and brainstem structures
using microsurgical techniques. The suboccipital bone flaps were
replaced in patients who did not present with brain swelling during
surgery. All ependymomas were pathologically confirmed using
hematoxylin and eosin staining and immunohistochemistry. Briefly,
all excised tumors were formalin (Sigma-Aldrich, St. Louis, MO,
USA)-fixed, paraffin (Sigma-Aldrich)-embedded and cut into 4-µm
sections followed by deparaffinization. The sections were stained
with hematoxylin and eosin (Beyotime Institute of Biotechnology,
Haimen, China) and reviewed by two experienced neuropathologists.
Immunohistochemical staining was performed using a Universal Dako
LSAB2/HRP Kit (Dako, Glostrup, Denmark) according to the
manufacturer's instructions, with 3,3′-diaminobenzidine (DAB; Dako)
as a chromogen. Selected antibodies, including glial fibrillary
acidic protein (GFAP; monoclonal mouse anti-human; 1:100; cat. no.
M0761; Dako), epithelial membrane antigen (EMA; monoclonal mouse
anti-human; 1:100; cat. no. M0613; Dako), S-100 (polyclonal rabbit
anti-human; 1:1,000; cat. no. Z0311; Dako), vimentin (monoclonal
mouse anti-human; 1:100; cat. no. M0725; Dako), Ki-67 (monoclonal
mouse anti-Human; 1:50; cat. no. M7240; Dako), cytokeratin
(monoclonal mouse anti-human; 1:100; cat. no. M0821; Dako),
synaptophysin (monoclonal mouse anti-human; 1:50; cat. no. M7315;
Dako), D2-40 (Podoplanin; monoclonal mouse anti-human; 1:100; cat.
no. M3619; Dako) were used for antigen detection. All staining was
visualized using an optical microscope (BX40; Olympus Corporation,
Tokyo, Japan).
Extent of resection (EOR)
The extent of resection was divided into three
categories: Gross-total resection (GTR), near-total resection (NTR)
and subtotal resection (STR). NTR was defined as a resection
following which only residual tumor <5-mm thick was visible on
postoperative neuroimaging. STR was defined as resection that left
behind residual tumor >5-mm thick on postoperative neuroimaging.
GTR was defined as a resection following which the tumor cells that
remained were not visible under the operating microscope (Leica OH3
FL800; Leica Microsystems GmbH, Wetzlar, Germany); patients who
achieved GTR did not display any evidence of disease on
postoperative contrast-enhanced MRI. NTR was defined as a resection
following which only 5-mm thick residual tumor was visible on
postoperative neuroimaging. STR was defined as a resection
following which 5-mm thick residual tumor was visible on
postoperative neuroimaging (8).
Postoperative care and follow-ups
Postoperative external ventricular drainage was not
routinely used, since the normal cerebrospinal fluid (CSF)
circulation was mandatorily restored in all patients during
surgery. The patients received continuous intensive monitoring for
respiratory and cardiovascular complications, and were
symptomatically treated following surgery. In cases exhibiting
shortness of breath and expectoration, tracheostomy was often
required. For patients with residual tumors, postoperative
radiotherapy was recommended, which was usually at a dose of 45–54
Gy administered in 30 fractions, with 5 fractions per week over 6
weeks. Patients were followed-up for 3, 6 and 12 months
postoperatively, and annually thereafter. Follow-up CT and/or MRI
scans were performed for the evaluation of the EOR and surgical
strategy. Telephone interviews with patients and their families
were also conducted.
Results
Among the 26 patients, simple tela choroidea opening
was performed in 21 patients, while the other 5 patients received
both tela choroidea and inferior medullary velum opening due to the
excessive tumor invasion into the upper ventricle. Unilateral and
bilateral approaches were performed in 17 and 9 cases,
respectively. All ependymomas were pathologically confirmed. Of the
26 ependymomas, 19 were low-grade and 7 high-grade. No
intraoperative mortality was recorded. One patient underwent
permanent ventriculoperitoneal shunting for persistent
hydrocephalus. Each ependymoma was well exposed during surgery, and
GTR was achieved in 22 cases, NTR in 3 and STR in 1. Two patients
who underwent the bilateral trans-CMF approach developed temporary
mutism, with the symptom disappearing within 4 weeks. Other
complications included ataxia, weakness in the extremities, mild
facial or abducent palsy and dysphagia, which steadily improved and
eventually recovered within 1 month following symptomatic
treatment. No surgery-induced permanent neurological deficit was
recorded. One patient succumbed to pulmonary infection 3 months
subsequent to surgery. Over the 3.0–7.5-year follow-up period
(average, 4.8 years), the preoperative symptoms disappeared in 20
cases while persisted in 1. Tumor relapse occurred in 1 case with
GTR, 2 cases with NTR and 1 case with STR. In total, 3 patients
succumbed to tumor relapse and 4 were lost to follow-up.
Discussion
The fourth ventricle is situated in front of the
cerebellum and behind the pons and upper half of the medulla
oblongata, and it consists of several vital structures in the
ventricle wall and floor, thus being a surgically challenging area
due to the severe deficits that may occur if these delicate
structures are injured (9,14,19,32,34).
Ependymoma is one of the most common tumors of the fourth
ventricle, particularly in pediatric patients (1,2,9). Due to its deep location and close
association with the surrounding brain stem, important peripheral
nerves and blood vessels, the radical surgical removal of
ependymomas of the fourth ventricle is considerably risky (9,14,19,30,34,35).
Since pediatric patients are constantly growing and developing,
their clinical manifestations lack specificity and accuracy in
description (35). This feature was
also observed in the present cases. Early manifestations of nervous
system injuries are usually ignored, as noted in the current study,
which is why tumors are generally large at diagnosis (35). In the present study, increased
intracranial pressure and obstructive hydrocephalus were the most
common early symptoms of ependymomas of the fourth ventricle. Due
to the advances in MRI technology, it is possible to detect the
precise location and extension of an ependymoma of the fourth
ventricle (2,36,37). Once
a diagnosis of ependymoma of the fourth ventricle is confirmed,
surgical resection of the tumor is usually obligatory due to
oncological reasons, and must be as extensive as possible, in order
to reduce local recurrence and seeding rates of metastatic cells
(5,6,17,38–40).
The traditional approach for removing ependymomas of
the fourth ventricle is incision of the cerebellar vermis, which,
however, is often associated with a higher incidence of cerebellar
mutism or posterior fossa syndrome, along with chronic
neurocognitive sequelae, compared with the trans-CMF approach
(13,18–21,24,25,41–43).
The trans-CMF approach to the fourth ventricle, which does not
cause significant injury to the neural tissue, was first proposed
by Matsushima et al (36), and
is currently widely accepted (8,13,14,18–20,22–26,29–32,36,43).
As a natural cleft between the cerebellum and medulla oblongata,
the CMF provides sufficient exposure of the fourth ventricle
without causing neurological deficits, following incisions in the
tela choroidea and inferior medullary velum (14,23,27).
Furthermore, the trans-CMF approach is able to achieve a greater
angle of exposure and more capacious working space than the
trans-vermian approach (14,18,22). There
are two types of trans-CMF approach: The simple tela choroidea
opening approach and the telovelar approach, and the decision to
use intraoperatively one or the other depends on the location of
the upper pole of the tumor in the fourth ventricle (14). Of the 26 ependymomas of the fourth
ventricle reviewed in the present study, 21 (80.8%)were removed
using a simple tela choroidea opening, while 5 (19.2%) required an
incision in the inferior medullary velum in order to gain
satisfactory exposure of the upper ventricle. Matsushima et
al (18) introduced three opening
methods for the trans-CMF approach: Extensive (aqueduct type),
lateral wall and lateral recess opening types. According to our
experience in the present study, the simple tela choroidea opening
approach is sufficient in the majority of cases. When the tela
choroidea is completely opened and the tonsils and uvula are
properly retracted, the lateral recess and at least the lower 3/4
parts of the fourth ventricle are adequately visualized (14). Caution should be taken in order to
prevent excessive stretching of the tonsils, which could lead to
the compression of the associated dentate nuclei and cerebellar
peduncles (25,27,32,44).
If an ependymoma is relatively large, it frequently
protrudes through the ventricular outlets into the subarachnoid
space, expanding the operating space (20), as indicated in Fig. 1. In fact, the larger the tumor is
preoperatively, the more stretched and thinned the inferior
medullary velum and tela choroidea tend to be, which enables easier
and wider access to the tumor within the ventricle (13,20).
Furthermore, additional operating space in large ependymomas may be
gained by central debulking; however, 5 ependymomas of those
reviewed in the present study involved the upper part of the fourth
ventricle (upper 1/4 region, generally), which rendered an incision
in the inferior medullary velum necessary in order to obtain
adequate exposure (31). It has been
previously reported that tonsillar resection in the telovelar
approach could provide a better operative perspective compared with
tonsillar retraction (45); however,
according to the experience of the present study, complete
dissection of the tela choroidea and flexible retraction of the
brain tissue are more important than tonsillar retraction.
Of the 26 cases of ependymoma of the fourth
ventricle reviewed in the present study, 17 underwent the
unilateral approach and 9 the bilateral approach. In general,
following the dissection of the ipsilateral CMF and contralateral
tonsillar retraction, the majority of tumors were sufficiently
exposed, unless there was obvious invasion into the contralateral,
superolateral and/or lateral recess. In the present study,
extensive involvement of the fourth ventricle was observed in 9
cases, where the bilateral approach was performed; however,
temporary postoperative mutism occurred in 2 patients (7.7%), both
of whom underwent the bilateral trans-CMF approach. According to
the existing literature (19,29), the bilateral trans-CMF approach
appears to be associated with a high rate of postoperative mutism,
compared with the unilateral approach. Despite the controversy
surrounding the pathophysiological mechanisms, it is considered
that the dentate nuclei and their connections with the thalamus
through the brainstem participate in speech function; therefore
injury to this pathway may result in mutism (12,14,20,21).
Another explanation for this observation may be that the
preservation of the contralateral CMF reduces the likelihood of
blood vessel injury (particularly in branches of the PICA) during
the unilateral approach. Thus, the unilateral approach could be an
effective way to avoid postoperative neurological deficits;
however, the patient number in the present study was too small to
draw any conclusions. In the present study the bilateral approach
was applied to large tumors, the resection of which was essentially
more invasive than the unilateral approach, due to more anatomical
structure being resected, leading to an increased chance of tissue
injury. With regard to other complications, such as ataxia and
cranial nerve and/or brainstem dysfunction, no pattern of
unilateral or bilateral predominance was identified, which could be
due to the small number of patients evaluated in the present study.
Furthermore, the postoperative complications gradually improved and
eventually disappeared, indicating the safety and usefulness of
this minimally invasive approach.
Considering the particular nature and pathogenesis
of ependymoma, the detailed microsurgical procedures performed for
ependymomas of the fourth ventricle are relatively different from
those performed for other tumor types (23). The extent of surgical resection is a
major determinant of the outcome (1,6,8,11,13,17,38–40).
It is known that the majority of ependymomas arise from ependymal
cells present in the floor of the fourth ventricle (1,2,37). In the current study, the majority of
tumors derived from or close to the obex, or close to the midline
and located at the lower part of the ventricle (the midfloor type)
(2,13,22,37), which
facilitated their exposure easier using the unilateral approach or
limited dissection of the tela choroidea. Ependymomas are normally
slow-growing, well-circumscribed, soft and often frond-like. In
addition, they have a propensity for plastic growth and may
protrude into the cerebellopontine cistern (1,2).
Therefore, ependymomas of the fourth ventricle may be well-exposed
and easily recognized, following their separation from the
surrounding structures and management of the tumor base. The
well-delineated tumors exhibited distinct boundaries from the
surrounding tissues, and the soft and heterogeneous nature of the
parenchyma facilitated the tumor removal. Intraoperatively, the
detachment of the tumor base is of particular importance, since any
injury to the medulla oblongata could have serious consequences.
Conversely to astrocytomas, whose infiltrating nature hinders a
precise estimation of tumor removal both intraoperatively and on
postoperative imaging, the ependymomas reviewed in the present
study (particularly the low-grade ependymomas), usually displayed a
cleavage plane between tumor tissue and brainstem, which
facilitated the tumor removal. Since the tumor was minimally
adherent and could be easily dissected from the base, the EOR of
the ependymomas improved, compared with other types of tumors of
the fourth ventricle, including high-grade ependymomas. However,
great care should be taken when the tumor base is near the obex,
which is pivotal to respiratory function (23). In one of the reviewed cases in the
present study, the tumor had extensively invaded the obex, and was
therefore managed aggressively. The patient suffered from
respiratory and expectoration dysfunction, and eventually succumbed
to pulmonary infection. Therefore, if the floor of the fourth
ventricle, including the obex, is obviously infiltrated, then GTR
should not be a mandatory goal, in order to avoid vital damage
(Fig. 1). In addition, as represented
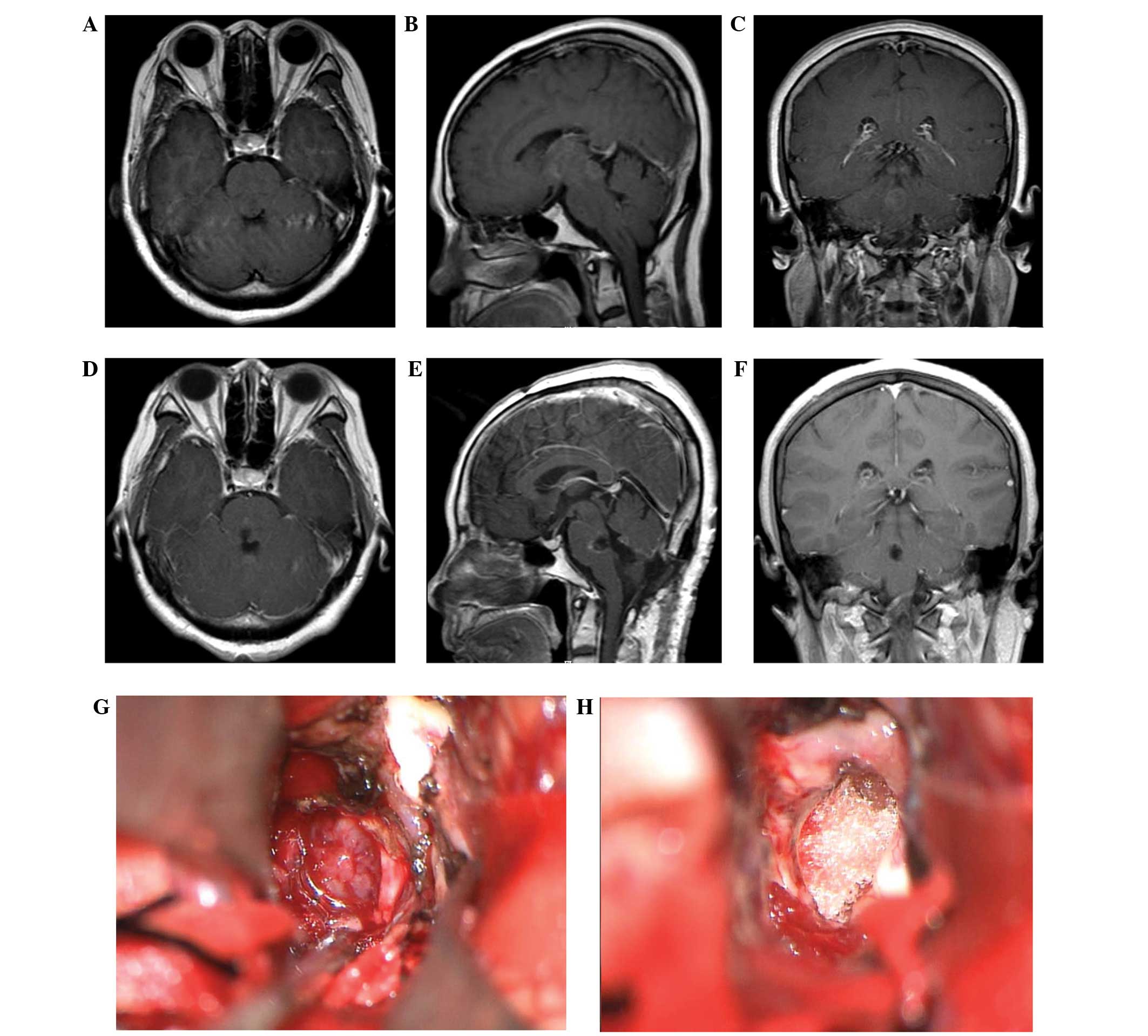
in Fig. 2, one of the ependymomas
reviewed in the present study was preoperatively diagnosed as an
intrinsic brainstem tumor (low-grade astrocytoma), since it did not
display typical imaging features of ependymoma and was located
within the pons. Intraoperatively, the tumor was well demarcated,
and was easily separated from the surrounding tissue following a
longitudinal incision of the pons. The final pathological diagnosis
confirmed a low-grade ependymoma.
In addition, several aspects should be taken into
consideration in order to achieve a safe GTR: The branches of the
PICA will be encountered during the dissection of the CMF, and
these arteries should be carefully protected (with the exception of
those well-defined tumor feeding arteries). If any damage occurs to
the PICA branches distal to the medullary branches at the level of
the roof of the fourth ventricle, inability to stand or walk and
nystagmus without appendicular dysmetria may occur (19,31).
Furthermore, any reflux vein should be managed with great caution,
particularly those near the obex. Intraoperatively, it is also
important to ensure the patency of the fourth ventricle and
aqueduct, thereby relieving the obstructive hydrocephalus. In all
the cases included in the present study, smooth CSF circulation was
visualized; however, a patient presented with postoperative
hydrocephalus and underwent ventriculoperitoneal shunting.
Postoperative hydrocephalus may be caused by brain tissue swelling,
residual tumor or arachnoid adhesion due to incomplete bipolar
coagulation hemostasis. Neurosurgeons should also note that
ependymomas have a tendency to disseminate through the CSF, with
CSF seeding present in 3–15% of the cases (6,11,12,28).
Therefore, during surgery, the cisterna magna and aqueduct should
be protected using brain cotton, in order to prevent tumor cells
from spreading through the ventricular system.
In conclusion, the trans-CMF approach constitutes a
favorable surgical approach for the treatment of ependymomas of the
fourth ventricle, since it does not require the splitting of the
vermis. The opening portion of the CMF and roof of the ventricle
should be determined based on the location, extension and size of
the lesion. For the majority of cases, the unilateral trans-CMF
approach with a simple opening of the tela choroidea is sufficient
and should be preferred, since it is minimally invasive and could
potentially prevent the occurrence of postoperative mutism. The
total removal of tumors focally attached to critical areas of the
fourth ventricle should not be attempted at the expense of the
patient's morbidity and mortality. Tumor removal, restoration of
the CSF circulation and preservation of brainstem function should
all be taken into consideration during surgery. If appropriate
microneurosurgical techniques are used via the trans-CMF approach,
the majority of ependymomas of the fourth ventricle may be accessed
and resected safely.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Liaoning Province (Shenyang, China; grant no.
2013021075 awarded to Professor Bo Qiu), the Fund for Scientific
Research of The First Affiliated Hospital of China Medical
University (Shenyang, China; grant no. fsfh 1304 awarded to
Professor Bo Qiu), the Program for Liaoning Excellent Talents in
University (Shenyang, China; grant no. LJQ2013085 awarded to
Professor Pengfei Wu), the Liaoning Provincial Project on Social
Development (Shenyang, China; grant no. 2013225079 awarded to
Professor Shaowu Ou) and the Science and Technology Program of
Shenyang City (Shenyang, China; grant no. F12-277-1-04 awarded to
Professor Shaowu Ou).
References
|
1.
|
Spagnoli D, Tomei G, Ceccarelli G,
Grimoldi N, Lanterna A, Bello L, Sinisi MM, De Santis A and Villani
RM: Combined treatment of fourth ventricle ependymomas: Report of
26 cases. Surg Neurol. 54:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tortori-Donati P, Fondelli MP, Cama A,
Garrè ML, Rossi A and Andreussi L: Ependymomas of the posterior
cranial fossa: CT and MRI findings. Neuroradiology. 37:238–243.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kun LE, Kovnar EH and Sanford RA:
Ependymomas in children. Pediatr Neurosci. 14:57–63. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kojima A, Yamaguchi N, Okui S, Kamiya M,
Hirato J and Nakazato Y: Parenchymal anaplastic ependymoma with
intratumoral hemorrhage: A case report. Brain Tumor Pathol.
20:85–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
El Majdoub F, Elawady M, Blau T, et al:
Intracranial ependymoma: Long-term results in a series of 21
patients treated with stereotactic (125)iodine brachytherapy. PLoS
One. 7:e472662012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Rudà R, Gilbert M and Soffietti R:
Ependymomas of the adult: Molecular biology and treatment. Curr
Opin Neurol. 21:754–761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Nazar GB, Hoffman HJ, Becker LE, Jenkin D,
Humphreys RP and Hendrick EB: Infratentorial ependymomas in
childhood: Prognostic factors and treatment. J Neurosurg.
72:408–417. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Morris EB, Li C, Khan RB, Sanford RA, Boop
F, Pinlac R, Xiong X and Merchant TE: Evolution of neurological
impairment in pediatric infratentorial ependymoma patients. J
Neurooncol. 94:391–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ferrante L, Mastronardi L, Schettini G,
Lunardi P and Fortuna A: Fourth ventricle ependymomas. A study of
20 cases with survival analysis. Acta Neurochir (Wien). 131:67–74.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kilday JP, Rahman R, Dyer S, Ridley L,
Lowe J, Coyle B and Grundy R: Pediatric ependymoma: Biological
perspectives. Mol Cancer Res. 7:765–786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kano H, Yang HC, Kondziolka D, Niranjan A,
Arai Y, Flickinger JC and Lunsford LD: Stereotactic radiosurgery
for pediatric recurrent intracranial ependymomas. J Neurosurg
Pediatr. 6:417–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bademci G, Tun K, Erden E, Evliyaoglu C
and Unlu A: Late dissemination of ependymoma: Case report.
Neurocirugia (Astur). 18:333–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shimoji K, Miyajima M, Karagiozov K,
Yatomi K, Matsushima T and Arai H: Surgical considerations in
fourth ventricular ependymoma with the transcerebellomedullary
fissure approach in focus. Childs Nerv Syst. 25:1221–1228. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Han S, Wang Z, Wang Y and Wu A:
Transcerebellomedullary fissure approach to lesions of the fourth
ventricle: Less is more? Acta Neurochir (Wien). 155:1011–1016.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Goldwein JW, Leahy JM, Packer RJ, Sutton
LN, Curran WJ, Rorke LB, Schut L, Littman PS and D'Angio GJ:
Intracranial ependymomas in children. Int J Radiat Oncol Biol Phys.
19:1497–1502. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Goldwein JW, Glauser TA, Packer RJ, Finlay
JL, Sutton LN, Curran WJ, Laehy JM, Rorke LB, Schut L and D'Angio
GJ: Recurrent intracranial ependymomas in children. Survival,
patterns of failure, and prognostic factors. Cancer. 66:557–563.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Guyotat J, Signorelli F, Desme S, Frappaz
D, Madarassy G, Montange MF, Jouvet A and Bret P: Intracranial
ependymomas in adult patients: Analyses of prognostic factors. J
Neurooncol. 60:255–268. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matsushima T, Abe H, Kawashima M and Inoue
T: Exposure of the wide interior of the fourth ventricle without
splitting the vermis: Importance of cutting procedures for the tela
choroidea. Neurosurg Rev. 35:563–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gök A, Alptekin M and Erkutlu I: Surgical
approach to the fourth ventricle cavity through the
cerebellomedullary fissure. Neurosurg Rev. 27:50–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zaheer SN and Wood M: Experiences with the
telovelar approach to fourth ventricular tumors in children.
Pediatr Neurosurg. 46:340–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Puget S, Boddaert N, Viguier D, Kieffer V,
Bulteau C, Garnett M, Callu D, Sainte-Rose C, Kalifa C, Dellatolas
G and Grill J: Injuries to inferior vermis and dentate nuclei
predict poor neurological and neuropsychological outcome in
children with malignant posterior fossa tumors. Cancer.
115:1338–1347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ziyal IM, Sekhar LN and Salas E:
Subtonsillar-transcerebellomedullary approach to lesions involving
the fourth ventricle, the cerebellomedullary fissure and the
lateral brainstem. Br J Neurosurg. 13:276–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
El-Bahy K: Telovelar approach to the
fourth ventricle: Operative findings and results in 16 cases. Acta
Neurochir (Wien). 147:137–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kawashima M, Matsushima T, Nakahara Y,
Takase Y, Masuoka J and Ohata K: Trans-cerebellomedullary fissure
approach with special reference to lateral route. Neurosurg Rev.
32:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Akakin A, Peris-Celda M, Kilic T, Seker A,
Gutierrez-Martin A and Rhoton A Jr: The dentate nucleus and its
projection system in the human cerebellum: The dentate nucleus
microsurgical anatomical study. Neurosurgery. 74:401–425. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Abla AA and Lawton MT: Cerebellomedullary
fissure dissection and tonsillar mobilization: A gateway to lesions
around the medulla. World Neurosurg. 82:e591–e592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Di Ieva A, Komatsu M, Komatsu F and
Tschabitscher M: Endoscopic telovelar approach to the fourth
ventricle: Anatomic study. Neurosurg Rev. 35:341–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kawabata Y, Takahashi JA, Arakawa Y and
Hashimoto N: Long-term outcome in patients harboring intracranial
ependymoma. J Neurosurg. 103:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Matsushima T, Inoue T, Inamura T, Natori
Y, Ikezaki K and Fukui M: Transcerebellomedullary fissure approach
with special reference to methods of dissecting the fissure. J
Neurosurg. 94:257–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Matsushima T, Kawashima M, Inoue K,
Matsushima K and Miki K: Exposure of wide cerebellomedullary
cisterns for vascular lesion surgeries in cerebellomedullary
cisterns: Opening of unilateral cerebellomedullary fissures
combined with lateral foramen magnum approach. World Neurosurg.
82:e615–e621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Mussi AC and Rhoton AL Jr: Telovelar
approach to the fourth ventricle: Microsurgical anatomy. J
Neurosurg. 92:812–823. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Rajesh BJ, Rao BR, Menon G, Abraham M,
Easwer HV and Nair S: Telovelar approach: Technical issues for
large fourth ventricle tumors. Childs Nerv Syst. 23:555–558. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Parkinson D: The posterior cranial fossa:
Microsurgical anatomy and surgical approaches. Neurosurgery.
48:11962001. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Chen D, Wei X, Yin Q, Guan J, Pan W, Wang
C and Liu Y: The microscopic surgical treatment for tumor of
posterior cranial fossa in children. Clin Oncol Cancer Res.
6:95–99. 2009. View Article : Google Scholar
|
|
36.
|
Matsushima T, Fukui M, Inoue T, Natori Y,
Baba T and Fujii K: Microsurgical and magnetic resonance imaging
anatomy of the cerebello-medullary fissure and its application
during fourth ventricle surgery. Neurosurgery. 30:325–330. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ikezaki K, Matsushima T, Inoue T, Yokoyama
N, Kaneko Y and Fukui M: Correlation of microanatomical
localization with postoperative survival in posterior fossa
ependymomas. Neurosurgery. 32:38–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Pollack IF, Gerszten PC, Martinez AJ, Lo
KH, Shultz B, Albright AL, Janosky J and Deutsch M: Intracranial
ependymomas of childhood: Long-term outcome and prognostic factors.
Neurosurgery. 37:655–667. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Robertson PL, Zeltzer PM, Boyett JM, Rorke
LB, Allen JC, Geyer JR, Stanley P, Li H, Albright AL,
McGuire-Cullen P, et al: Survival and prognostic factors following
radiation therapy and chemotherapy for ependymomas in children: A
report of the Children's Cancer Group. J Neurosurg. 88:695–703.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Sutton LN, Goldwein J, Perilongo G, Lang
B, Schut L, Rorke L and Packer R: Prognostic factors in childhood
ependymomas. Pediatr Neurosurg. 16:57–65. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Dailey AT, McKhann GM II and Berger MS:
The pathophysiology of oral pharyngeal apraxia and mutism following
posterior fossa tumor resection in children. J Neurosurg.
83:467–475. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Mastronardi L: Mutism and pseudobulbar
symptoms after resection of posterior fossa tumors in children:
Incidence and pathophysiology and transient cerebellar mutism after
posterior fossa surgery in children. Neurosurgery. 38:10661996.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Van Calenbergh F, Van de Laar A, Plets C,
Goffin J and Casaer P: Transient cerebellar mutism after posterior
fossa surgery in children. Neurosurgery. 37:894–898. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Fulton JF and Dow RS: The cerebellum: A
summary of functional localization. Yale J Biol Med. 10:89–119.
1937.PubMed/NCBI
|
|
45.
|
Jittapiromsak P, Sabuncuoglu H, Deshmukh
P, Spetzler RF and Preul MC: Accessing the recesses of the fourth
ventricle: Comparison of tonsillar retraction and resection in the
telovelar approach. Neurosurgery. 66(Suppl Operative 3): 30–40.
2010.PubMed/NCBI
|