Introduction
Endometriosis (EMS), a common, benign,
estrogen-dependent disease affecting 5–10% of women of reproductive
age, is characterized by the presence of ectopic endometrial glands
and stroma outside of the uterine cavity, primarily in the
peritoneum, ovaries and rectovaginal septum (1). Women suffering EMS may present with
chronic pelvic pain, dysmenorrhea, dyspareunia and/or infertility.
The incidence has been on the rise over the years (2). A number of theories, such as Sampson's
theory (3), have been proposed to
explain the etiology of EMS, but the exact mechanism of its
development remains unknown.
The novel candidate tumor suppressor the Ras
association domain family 1A (RASSF1A) gene has been recently
identified and mapped to chromosome 3p21.3 (4). It was previously demonstrated that
RASSF1A gene inactivation by promoter hypermethylation is a common
event in the development of a variety of types of human cancer, for
example in the lung, breast, ovary, endometrial and cervical
carcinomas (5–7). The role of RASSF1A as a tumor suppressor
gene has been suggested since exogenous expression of RASSF1A
decreases in vitro-colony formation, suppresses
anchorage-independent growth, and reduces the tumorigenicity of
cancer cells in vivo (4,8).
EMS is a benign gynecological disease which displays
malignant behaviors, such as enhanced proliferation and cell
invasion, ectopic implantation of distant organs similar to tumor
metastasis (9). Accumulating evidence
has indicated that DNA methylation serves definite roles in the
pathogenesis of EMS (10,11). It is therefore reasonable to
hypothesize that epigenetic inactivation of RASSF1A may have a role
in EMS. The aim of the present study was to investigate RASSF1A
protein expression and the methylation status in the ectopic and
eutopic endometrium of fertile women with EMS and normal
endometrium of women without EMS by immunohistochemistry and
methylation specific polymerase chain reaction (MSP) to better
define the possible involvement of RASSF1A in the pathogenesis of
EMS.
Materials and methods
Patients and samples
After informed consent was obtained, ectopic
endometrium from the cyst walls of ovarian EMS and the matched
eutopic endometrium from the uterus were taken from 45 patients,
aged 24–50 years. EMS stages were confirmed according to the
Revised American Fertility Society Classification (12). For controls, normal endometrium
samples were obtained from 20 healthy women, aged 34–48 years, who
underwent tubal sterilization that was confirmed laparoscopically
to be free of EMS. All of the patients underwent surgical treatment
in the Department Gynecology and Obstetrics at Liaocheng People's
hospital (Shangdong, China) between February 2012 and December
2013. None of these patients had received any
gonadotropin-releasing hormone analogue, antibiotics, radio-,
chemo-, or hormone therapy in the last 6 months prior to the
surgery. All samples were histologically confirmed, and the phase
of the menstrual cycle was determined by preoperative history and
histologic examination. The mean ages of subjects were 41±2 years
in the experimental group, and 38±2 years in the control group, and
there were no significant differences between the two groups with
respect to age. Each biopsy was divided in two fragments, which
were either frozen in liquid nitrogen (and stored in a −80°C for
DNA extraction) or fixed in 10% buffered formalin for histological
confirmation of endometriosis and immunohistochemistry. The study
was approved by the Ethics Committee of Liaocheng Hospital.
Immunohistochemistry
The expression of RASSF1A protein was evaluated by
immunohistochemistry which was performed with a standard
streptavidin-peroxidase (S-P) technique, using diamino-benzidine
(DAB) as a chromogen. The procedures were as follows: tissue
sections were dewaxed in xylene and gradient alcohol with
decreasing concentrations. Antigen retrieval was performed using
microwave irradiation at 750 W for 15 min in 10 mM citrate buffer
(pH 6.0). After treatment with 3% H2O2 for 15
min, and 5% normal milk (Solarbio, China) for 30 min to block
nonspecific protein binding. The slide were then incubated over
night with anti-RASSF1A (mouse monoclonal IgG, clone 3F3, code
number AB 23950, Santa Cruz Biotechnology, Dallas, TX, USA; 1:500
dilution). After 3 washes in PBS, sections were incubated with goat
anti-mouse IgG-HRP (sc-2031, Santa Curz Biotechnology, Dallas, TX,
USA; 1:1,000 dilution) in a streptavidin-peroxidase reagent
(ThermoFisher Scientific, Inc., Waltham, MA, USA) for 15 min, then
the peroxidase reaction was initiated by incubating tissue sections
in DAB reagent (Beyotime Insititute of Biotechnology, Inc.,
Shanghai, China) for 10–20 min at room temperature. Sections were
washed 3 times in distilled water. Slides were finally
counterstained with Mayer hematoxylin (Beyotime Insititute of
Technology, Inc.).
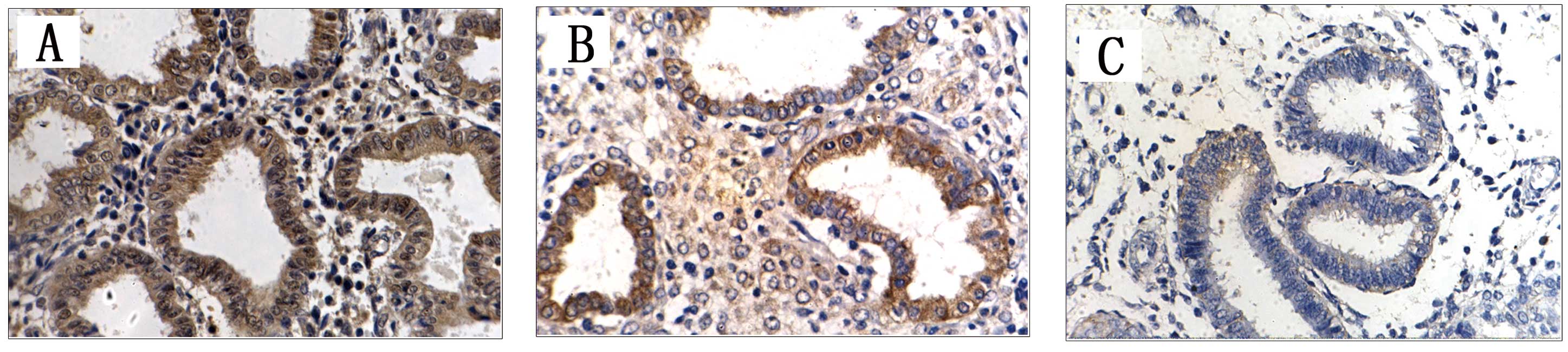
Brown staining was considered positive. Sections
known to express RASSF1A were used as the positive control, and PBS
was used as the negative control. RASSF1A protein expression
appeared in the cytoplasm of the cells. Sections were observed
under an optical microscope (Nikon, Tokyo, Japan) in ×40
magnification. A total of 5 visual fields were randomly selected in
each section, and they were graded at 4 levels according to the
percentage of positively stained cells: negative (−), <5%;
positive (+), 5–25%; positive (++), 25–50%; and positive (+++),
>50%.
DNA extraction and bisulfite
modification
DNA was extracted from frozen preserved tissues by
proteinase K digestion (Beyotime Insititute of Biotechnology, Inc.)
and a phenol/chloroform extraction method (Beyotime Insititute of
Biotechnology, Inc.) (13), denatured
with NaOH and treated with sodium bisulphate as previously
described (14).
Methylation specific polymerase chain
reaction (MSP) for RASSF1A
Promoter hypermethylation of the RASSF1A gene was
determined by the MSP as previously described (13). PCR was performed using the primer
sequences of RASSF1A listed below: The methylated reaction primer
sequences were 5′-GTGTTAACGCGTTGCGTATC-3′ (sense) and
5′-AACCCCGCGAACTAAAAACGA-3′ (antisense), and the produce size was
93 bp. For the unmethylated reaction, the primer sequences were
5′-TTTGGTTGGAGTGTGTTAATGTG-3′ (sense) and
5′-CAAACCCCACAAACTAAAAACAA-3′ (antisense), and the produce size was
103 bp. Primers and PCR reagents were purchased from Shanghai
Sangon Biological Engineering Technology (Shanghai, China). The PCR
parameters was as follows: 94°C for 10 min; 40 amplification cycles
of 30 sec at 94°C, 30 sec at the annealing temperature (59°C), 30
sec at 72°C, and finally 72°C for 10 min. Products were loaded onto
2% agarose gels (Santa Cruz Biotechnology, Inc.), stained with
ethidium bromide (Beyotime Insititute of Biotechnology, Inc.) and
visualized under ultraviolet illumination.
Statistical analysis
All statistical analysis were performed using the
SPSS software, Version 13.0 (SPSS, Inc., Chicago, IL, USA).
χ2-test and Fisher's exact were used to compare RASSF1A
protein expression and methylation status between the cases, and
with menstrual cycle phase and clinical stage. Pearson's
correlation analysis was used to evaluate the relationship between
RASSF1A protein expression and methylaion status in EMS. P<0.05
was considered to indicate a statistically significant
difference.
Results
Cycle phase
All surgeries were performed during the
proliferative and secretory phase in 2 groups. In EMS group,
surgery was performed during the proliferative phase in 21 patients
and during the secretory phase in 24 patients. In the control
group, surgery was performed during the proliferative phase in 5
patients and during the secretory phase in 15 patients. There were
no significant differences between the 2 groups with cycle phase
(χ2=2.708, P=0.100).
The RASSF1A protein expression in different
endometrium tissues. To determine RASSF1A protein expression in
EMS, ectopic and eutopic endometrium samples from 45 patients with
EMS and 20 controls without EMS were examined by
immunohistochemistry. Immunohistochemical results showed that
RASSF1A was mainly expressed in the cytoplasm of glandular
epithelial cells in the endometrial tissues.
In normal endometrium samples, immunohistochemistry
showed that RASSF1A protein expression was significantly higher at
the secretory phase than at the proliferative phase (P<0.05).
Either in ectopic or eutopic endometrium samples, the protein level
of RASSF1A at the secretory phase was higher than at the
proliferative phase, although this was not significant (P>0.05).
The results are presented in Table
I.
 | Table I.The protein expression of the RASSF1A
in both ectopic and eutopic endometrium of EMS and normal
endometrium of non-EMS and the relationship with the menstrual
cycle phase. |
Table I.
The protein expression of the RASSF1A
in both ectopic and eutopic endometrium of EMS and normal
endometrium of non-EMS and the relationship with the menstrual
cycle phase.
|
|
| RASSF1A protein
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | − | + | ++ | +++ |
χ2-value | P-value |
|---|
| Ectopic endometrium
of EMS | 45 | 28 | 11 | 6 | 0 |
|
|
|
Proliferative phase | 21 | 15 | 4 | 2 | 0 | 1.420 | 0.233 |
| Secretory
phase | 24 | 13 | 7 | 4 | 0 |
|
|
| Eutopic endometrium
of EMS | 45 | 18 | 17 | 9 | 1 |
|
|
|
Proliferative phase | 21 | 11 | 7 | 3 | 0 | 2.515 | 0.113 |
| Secretory
phase | 24 | 7 | 10 | 6 | 1 |
|
|
| Normal Endometrium of
non-EMS | 20 | 3 | 4 | 7 | 6 |
|
|
|
Proliferative phase | 5 | 3 | 1 | 1 | 0 |
| 0.009 |
| Secretory
phase | 15 | 0 | 3 | 6 | 6 |
|
|
Fig. 1 demonstrated
different degrees of RASSF1A protein expression in the 3 groups.
The positive expression rates of RASSF1A in the endometrial tissues
of each group were calculated and compared (Table I). The rates were 37.78% (17/45) and
60% (27/45) in the EMS ectopic and eutopic endometrium samples,
respectively. In the control group, the positive expression rate
was 85% (17/20). The expression level of RASSF1A was significantly
lower in ectopic endometrium (χ2=12.377, P<0.0001)
and eutopic endometrium (χ2=3.957, P=0.047) as compared
with control subjects. The difference between ectopic and eutopic
endometrium also was statistically significant
(χ2=4.447, P=0.035).
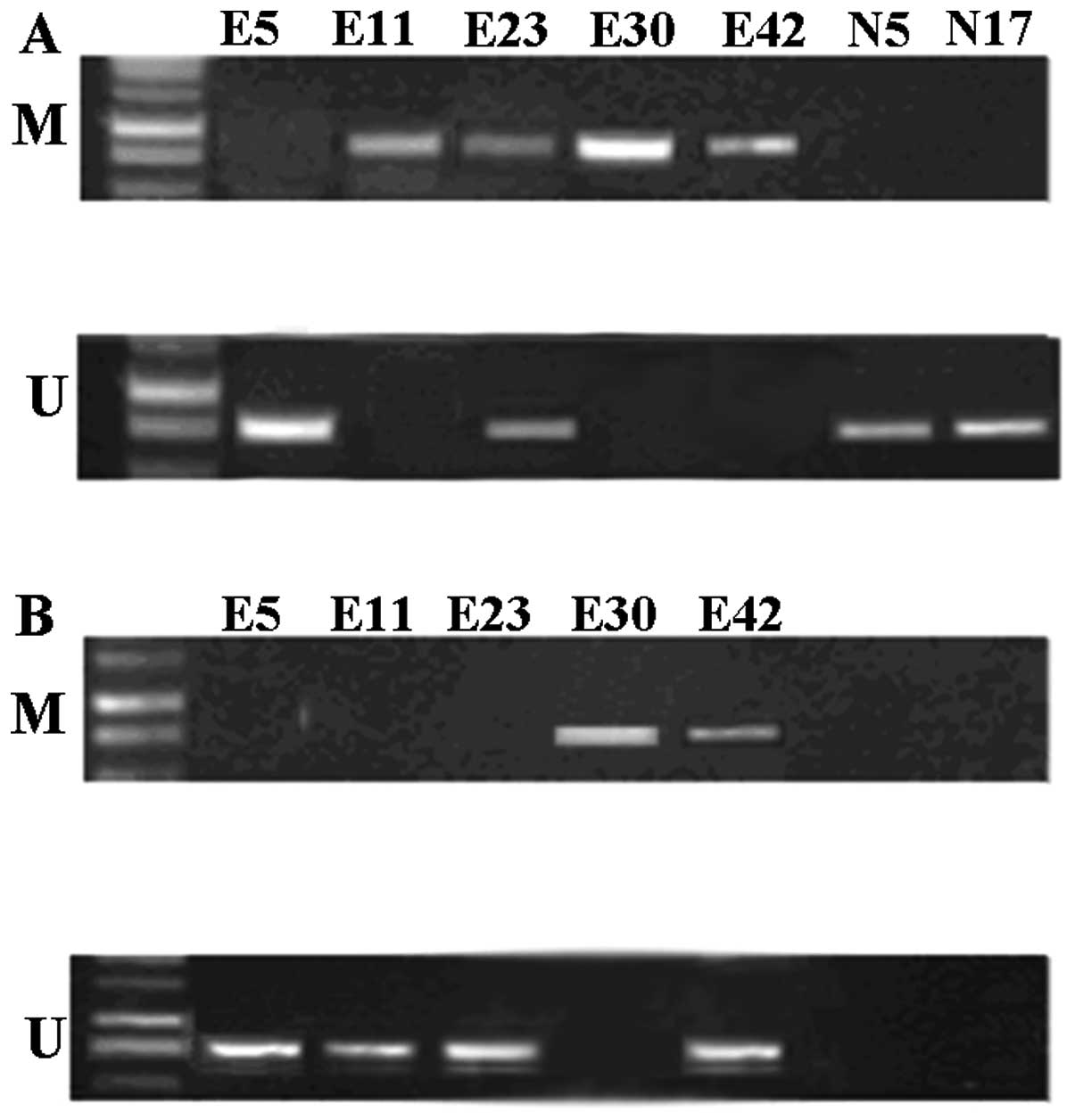
Methylation status of RASSF1A in
different endometrial samples
To determine the correlation between DNA methylation
and RASSF1A protein expression in endometrial tissues, the
methylation status of RASSF1A was evaluated in the above three
endometrial tissues by using MSP. The results demonstrated that the
promoter was hypermethylated in 55.56% (25/45) of RASSF1A genes in
ectopic endometrium and 33.33% (15/45) in eutopic endometrium. In
contrast, no RASSF1A promoter hypermethylation was observed in the
control subjects. The difference was statistically significant
between the above three groups (P<0.05). Thus, the RASSF1A
promoter hypermethylation occurred exclusively in EMS.
Representative examples are presented in Fig. 2.
As shown in Table II
that frequency of promoter hypermethylation of RASSF1A did not vary
significantly throughout the menstrual cycle either in ectopic
endometrium or in eutopic endometrium.
 | Table II.The promoter hypermethylated status of
the RASSF1A gene in ectopic and eutopic endometrium of EMS and the
relationship with the menstrual cycle phase and clinical
stages. |
Table II.
The promoter hypermethylated status of
the RASSF1A gene in ectopic and eutopic endometrium of EMS and the
relationship with the menstrual cycle phase and clinical
stages.
|
|
| RASSF1A promoter
methylation |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | M | U |
χ2-value | P-value |
|---|
| Ectopic endometrium
of EMS | 45 | 25 | 20 |
|
|
|
Proliferative phase | 21 | 14 | 7 | 1.969 | 0.161 |
| Secretory
phase | 24 | 11 | 13 |
|
|
| Eutopic endometrium
of EMS | 45 | 15 | 30 |
|
|
|
Proliferative phase | 21 | 8 | 13 | 0.402 | 0.526 |
|
Secretory phase | 24 | 7 | 17 |
|
|
| Clinical stages of
EMSa | 45 | 25 | 20 |
|
|
| Stage
I, II | 16 | 5 | 11 | 5.940 | 0.015 |
| Stage
III, IV | 29 | 20 | 9 |
|
|
| Clinical stages of
EMSb | 45 | 15 | 30 |
|
|
| Stage
I, II | 16 | 11 | 5 | 1.751 | 0.186 |
| Stage
III, IV | 29 | 14 | 15 |
|
|
The EMS stages were between stage I to IV. There
were 16 cases of stage I and II, 29 cases of stage III and IV. As
Table III demonstrates, in the
ectopic endometrium, the frequency of promoter hypermethylation of
RASSF1A at advanced stage (stage III, IV) was significantly higher
than at early stage (stage I, II) (P<0.05). However, in the
eutopic endometrium, frequency of promoter hypermethylation of
RASSF1A at different clinical stages was not significantly
different.
 | Table III.Correlation between the protein
expression and methylation of RASSF1A in ectopic and eutopic
endometrium of EMS. |
Table III.
Correlation between the protein
expression and methylation of RASSF1A in ectopic and eutopic
endometrium of EMS.
|
|
| RASSF1A promoter
methylation |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
|
| RASSF1A | M | U |
χ2-value | r | P-value |
|---|
| Ectopic endometrium
of EMS |
|
|
|
|
|
|
|
| + | 3 | 14 | 15.901 | −0.594 | 0.000 |
|
| − | 22 | 6 |
|
|
|
| Eutopic endometrium
of EMS |
|
|
|
|
|
|
|
| + | 3 | 24 | 15 | −0.577 | 0.000 |
|
| − | 12 | 6 |
|
|
|
Correlation between RASSF1A protein
expression and promoter hypermethylation
Finally, the correlation between RASSF1A
immunoexpression and the methylation status in EMS was determined.
In the ectopic endometrium, RASSF1A was methylated in 22 of 28
cases and negative for RASSF1A immunostaining. Meanwhile, in
eutopic endometrium, RASSF1A protein expression was dramatically
reduced in cases with RASSF1A promoter mythylation as compared with
unmethylated cases. There was generally a negative correlation
between hypermethylated RASSF1A and protein expression in both
ectopic endometrium and eutopic endometrium. These data suggest
that hypermethylation of RASSF1A promoter may be associated with
abnormally low or absent expression in EMS. The results are shown
in Table III.
Discussion
Epigenetics refers to heritable changes in DNA and
chromatin that impact gene expression without changes in DNA
sequence (15). There are 2 basic
epigenetic regulatory mechanisms: DNA methylation and histone
modifications. DNA methylation is the best understood and most
extensively studied epigenetic mechanism and refers to the covalent
modification of post-replicative DNA, when a methyl group is added
to the cytosine ring to form methyl cytosine (16). DNA methylation serves a critical role
in the regulation of gene expression in development,
differentiation, and complex diseases, cancer being the most
prominent example (15,16). EMS shares some common features with
cancer. For instance, the loss of the tumor suppressor PTEN in
endometrial cancer was well-established in 2010 (17) and decreased PTEN expression has also
been reported in the eutopic and ectopic endometrial of patients
with EMS as well (18).
In women of reproductive age, EMS mainly involves
the ovary (2); an endometriotic cyst
of ovary is the most common type of EMS. Ovarian EMS was selected
as the focus of the present study. Meanwhile, patients who
underwent tubal sterilization that were confirmed to be
laparoscopically to be free of EMS were selected as the control
group.
The present study investigated the protein
expression of RASSF1A in EMS. RASSF1A is localized in the cytoplasm
of glandular epithelial cells in both ectopic and eutopic
endometrium of EMS patients and in normal endometrium from women
with non-EMS. It was demonstrated that RASSF1A was stronger
expressed in normal endometrium. This is similar to Pallarés et
al (19). In the present study,
RASSF1A protein expression was significantly higher at the
secretory phase than at the proliferative phase, but this is
different from Pallarés et al (19), who found RASSF1A immunoexpression was
significantly higher in the proliferative phase than in the
secretory endometrium. This difference may be due in part to
different patients and agents used.
In the present study, experimental evidence was
presented that indicated that the frequency of methylation of the
RASSF1A gene in ectopic endometrium and eutopic endometrium were
55.56 and 33.33%, respectively, whereas such methylation was not
detected in normal endometrium. Combined with the
immunohistochemical results, the positive expression rate of
RASSF1A protein in the ectopic and eutopic endometrium were lower
than in normal endometrium. In addition, a clear inverse
relationship was observed between the extent of methylation in the
RASSF1A promoter CpG island and its protein levels, either in
ectopic or in eutopic endometrium. In addition, endometrium with
RASSF1A methylation exhibited weak or lost RASSF1A protein
expression in comparison to endometrium with RASSF1A unmethylation.
Taken together, these results provide strong evidence that RASSF1A
hypermethylation may constitute the mechanism through which RASSF1A
expression is downregulated in endometriosis. Epigenetic
inactivation of RASSF1A through aberrant promoter methylation may
serve an important role in the pathogenesis of EMS.
The present data also demonstrated that there were
statistical differences between eutopic and normal endometrium with
regards to the methylation status and protein levels of RASSF1A. In
addition, DNA methylation was observed in eutopic and ectopic
endometrium of EMS. Eutopic endometrium is readily available and
gene alteration in the eutopic endometrium can be easily detected.
Identification EMS-related genes in eutopic endometrium will
further reveal the pathogenesis of EMS and offer the basis for
targeted gene diagnosis and therapy of EMS (20). Assessment of RASSF1A methylation
status in eutopic endometrium of EMS may be a potentially useful
biomarker to enhance the early detection of EMS.
It has previously been reported that
hypermethylation of RASSF1A is associated with advanced tumor stage
in bladder, esophageal, stomach and endometrial cancer (6,21–23). The severity of endometriosis has been
clinically classified by numerous systems. The revised
classification system of the American Society for Reproductive
Medicine, currently widely used, consists of 4 stages (I–IV), with
stage III and IV representing more serious cases (12). In the present study, comparing
patients with early clinical stage to patients with advanced
clinical stage there were statistical differences with regards to
RASSF1A methylation in ectopic endometrium. However no
statistically significant differences were observed in eutopic
endometrium, which suggests that the methylation status of RASSF1A
in the EMS eutopic endometrium tissues would not alter with
clinical stage; promoter methylation of RASSF1A in the ectopic
endometrium may therefore serve a more prominent role in the
development and progression of EMS. This is a noteworthy topic that
may merit further study.
Transient treatment of cells with demethylating
agents can lead to a long-lasting demethylation effect (24). The epigenetic mechanisms represent a
heritable dynamic and reversible means of modulating gene
expression. The reversible nature of epigenetic aberrations is an
important characteristic, since it allows us to search for
appropriate pharmacological treatments, or ‘epigenetic therapies’
(25). In a number of studies it was
demonstrated that RASSF1A expression was significantly increased or
markedly upregulated in a number of types of cancer, including
ovarian cancer and endometrial carcinoma, after treatment with
5-aza-DC (26–28). Therefore, DNA demethylation agents may
be used in the future to restore methylation aberrations of RASSF1A
in EMS.
However, how methylation of the RASSF1A promoter
results in a reduction of protein expression in EMS needs further
study. Further studies are necessary to confirm the findings in
larger samples and to investigate the promoter in vivo prior
to and following 5-aza-DC treatment.
In summary, the present study demonstrated, to the
best of our knowledge, for the first time that the high frequency
of promoter hypermethylation of the RASSF1A gene contributes to the
loss or absent of protein expression in EMS. The results indicated
that hypermethylation of the RASSF1A CpG island was involved in the
pathogenesis of EMS. This also provides another piece of evidence
that EMS may be ultimately an epigenetic disease. This may open up
a novel avenue for the development of therapeutics for EMS and
provide a potentially promising way for epigenetic reprogramming
that restores dysregulated gene expression.
Acknowledgements
The Central Laboratory of Liaocheng People's
Hospital is thanked for providing technical support for the
experimental process. The authors would also like to thank all the
women who participated in the study.
References
|
1.
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Farquhar C: Endometriosis. BMJ.
334:249–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sampson JA: Ovarian hematomas of
endometrial type (perforating hemorrhagic cysts of the ovary) and
implantation adenomas of endometrial type. Boston Med Surg J.
186:445–456. 1922. View Article : Google Scholar
|
|
4.
|
Dammann R, Yang G and Pfeifer GP:
Hypermethylation of the cpG island of Ras association domain family
1A(RASSF1A), a putative tumor suppressor gene from the 3p21.3
locus, occurs in a large percentage of human breast cancers. Cancer
Res. 61:3105–3109. 2001.PubMed/NCBI
|
|
5.
|
Agathanggelou A, Honorio S, Macartney DP,
et al: Methylation associated inactivation of RASSF1A from region
3p21.3 in lung, breast and ovarian tumours. Oncogene. 20:1509–1518.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jo H, Kim JW, Kang GH, et al: Association
of promoter hypermethylation of the RASSF1A gene with prognostic
parameters in endometrial cancer. Oncol Res. 16:205–209.
2006.PubMed/NCBI
|
|
7.
|
Kuzmin I, Liu L, Dammann R, et al:
Inactivation of RAS association domain family 1A gene in cervical
carcinomas and the role of human papillomavirus infection. Cancer
Res. 63:1888–1893. 2003.PubMed/NCBI
|
|
8.
|
Burbee DG, Forgacs E, Zöchbauer-Müller S,
et al: Epigenetic inactivation of RASSF1A in lung and breast
cancers and malignant phenotype suppression. J Natl Cancer Inst.
93:691–699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Starzinski-Powitz A, Gaetje R, Zeitvogel
A, et al: Tracing cellular and molecular mechanisms involved in
endometriosis. Hum Reprod Update. 4:724–729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wu Y, Strawn E, Basir Z, Halverson G and
Guo SW: Promoter hypermethylation of progesterone receptor isoform
B (PR-B) in endometriosis. Epigenetics. 1:106–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wu Y, Strawn E, Basir Z, Halverson G and
Guo SW: Aberrant expression of deoxyribonucleic acid
methyltransferases DNMT1, DNMT3A, and DNMT3B in women with
endometriosis. Fertil Steril. 87:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Canis M, Donnez JG, Guzick DS, et al:
Revised American Society for Reproductive Medicine classification
of endometriosis:1996. Fertil Steril. 67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Darehdori AS, Dastjerdi MN, Dahim H, et
al: Lack of significance of the BRCA2 promoter methylation status
in different genotypes of the MTHFR a1298c polymorphism in ovarian
cancer cases in Iran. Asian Pac J Cancer Prev. 13:1833–1836. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wu Y, Zhang X, Lin L, et al: Aberrant
methylation of RASSF2A in tumors and plasma of patients with
epithelial ovarian cancer. Asian Pac J Cancer Prev. 15:1171–1176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Feinberg AP: Phenotypic plasticity and the
epigenetics of human disease. Nature. 447:433–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Feinberg AP: Epigenetics at the epicenter
of modern medicine. JAMA. 299:1345–1350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Monte NM, Webster KA, Neuberg D, Dressler
GR and Mutter GL: Joint loss of PAX2 and PTEN expression in
endometrial precancers and cancer. Cancer Res. 70:6225–6232. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang H, Zhao X, Liu S, Li J, Wen Z and Li
M: 17betaE2 promotes cell proliferation in endometriosis by
decreasing PTEN via NFkappаB-dependent pathway. Mol Cell
Endocrinol. 317:31–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pallarés J, Velasco A, Eritja N, et al:
Promoter hypermethylation and reduced expression of RASSF1A are
frequent molecular alterations of endometrial carcinoma. Mod
Pathol. 21:691–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang D, Chen Q, Zhang C, Ren F and Li T:
DNA hypomethylation of the COX-2 gene promoter is associated with
up-regulation of its mRNA expression in eutopic endometrium of
endometriosis. Eur J Med Res. 17:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Byun DS, Lee MG, Chae KS, Ryu BG and Chi
SG: Frequent epigenetic inactivation of RASSF1A by aberrant
promoter hypermethylation in human gastric adenocarcinoma. Cancer
Res. 61:7034–7038. 2001.PubMed/NCBI
|
|
22.
|
Maruyama R, Toyooka S, Toyooka KO, et al:
Aberrant promoter methylation profile of bladder cancer and its
relationship to clinicopathological features. Cancer Res.
61:8659–8663. 2001.PubMed/NCBI
|
|
23.
|
Kuroki T, Trapasso F, Yendamuri S, et al:
Promoter hypermethylation of RASSF1A in esophageal squamous cell
carcinoma. Clin Cancer Res. 9:1441–1445. 2003.PubMed/NCBI
|
|
24.
|
Egger G, Liang G, Aparicio A and Jones PA:
Epigenetics in human disease and prospects for epigenetic therapy.
Nature. 429:457–463. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Nasu K, Kawano Y, Tsukamoto Y, et al:
Aberrant DNA methylation status of endometriosis: Epigenetics as
the pathogenesis, biomarker and therapeutic target. J Obstet
Gynaecol Res. 37:683–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Balch C, Yan P, Craft T, et al:
Antimitogenic and chemosensitizing effects of the methylation
inhibitor zebularine in ovarian cancer. Mol Cancer Ther.
4:1505–1514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kang S, Lee JM, Jeon ES, et al: RASSF1A
hepermethylation and its inverse correlation with BRAF and/or KRAS
mutations in MSI-associated endometrial carcinoma. Int J Cancer.
119:1316–1321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Donninger H, Vos MD and Clark GJ: The
RASSF1A tumor suppressor. J Cell Sci. 120:3163–3172. 2007.
View Article : Google Scholar : PubMed/NCBI
|