Introduction
Peripheral T-cell lymphoma (PTCL) accounts for 12%
of all non-Hodgkin lymphoma (NHL) cases (1), and includes peripheral T-cell lymphoma
unspecified (PTCL-u), anaplastic large cell lymphoma (ALCL) and
angioimmunoblastic T-cell lymphoma (AITL) (1). The most common subtype is PTCL-u
(1).
As PTCL is extremely invasive, the majority of cases
are not associated with a positive prognosis (2). To date, no reliable therapeutic schedule
has been established worldwide (3).
Currently, clinicians typically refer patients with B-cell lymphoma
for CHOP or CHOP-like treatment regimens; however, the cure rate is
not particularly high (4). More
rigorous chemotherapy schemes, including HyperCVAD and stem cell
transplantation, have been tested by several clinics, however, no
markedly positive effects have been reported (3), and the side effects from such treatments
are detrimental (5,6), thus inducing patient suffering and
commonly disease relapse (7). The
overall 5-year survival rate for patients with PTCL is 20–30%
(8,9).
PTCL has no specific clinical features. The median age of onset is
60 years, and the disease is more prevalent in males, who often
present with lymphadenopathy and/or extranodal disease (10). Onset of disease is common within the
lymph nodes and also in extranodal regions, which includes the
gastrointestinal tract, nasal cavity, sinuses, nasopharynx,
oropharynx, skin, tonsils, spleen, liver and bone marrow. The
majority of cases are diagnosed during stages III–IV (10). Approximately half of patients present
with systemic symptoms, including night sweat, fever, itchy skin
and weight loss (11,12).
When air or other gases enter the pleural cavity,
this results in a pneumothorax (13).
This situation may occur spontaneously, however, the majority of
cases are induced by trauma or surgery to the lung or chest wall
(14,15). They may also be induced as a result of
other diseases, improper diagnosis or improper handling of
treatment (9). The incidence rate of
primary spontaneous pneumothorax (PSP) for males is 7.4–18 per
100,000 individuals per year, while for females is the incidence is
1.2–6 per 100,000 individuals (15,16). The
clinical behavior of PSP presents as an acute onset of local
pleuritic pain associated with short breath at rest (17). This pain may be mild, severe, sharp or
a steady ache, but can usually be resolved within 24 h even if a
pneumothorax still exists (15). When
a large pneumothorax occurs, defined as when free air occupies more
than 15–20% of the hemithorax, decreased breath sounds on
auscultation, reduced chest wall movement, hyper-resonance
(tympanic) on percussion and reduced tactile fremitus on palpation
of the chest may often be detected during clinical diagnosis
(14). If patients experience
discomfort in the form of circulatory or respiratory compromise,
this is often a result of reflex tachycardia (13). This will induce rapid pulmonary
re-expansion following decompression, which is life-threatening
(18). Pneumothorax is frequently
induced by pulmonary inflammation, tuberculosis and tumors
(15). Clinically, pulmonary
lymphomas rarely induce a pneumothorax, and a very limited number
of relevant reports are available in the literature. Primary and
secondary pulmonary lymphomas are predominantly B-cell lymphomas,
with only one case of primary pneumothorax as a result of B-cell
lymphoma identified in the current literature (19). Pulmonary T-cell lymphoma is
particularly rare, and no PTCL-u cases presenting with a
pneumothorax as the primary manifestation have been reported. The
current study describes a case of PTCL-u with pulmonary
infiltration that resulted in a pneumothorax. In addition, a
systematic review of the relevant literature is presented with the
aim of aiding clinicians in the diagnosis and treatment of PTCL-u
pulmonary infiltration.
Case report
A 51-year-old male was admitted to the Department of
Pneumology at the Hangzhou Red Cross Hospital (Hangzhou, China) on
June 27, 2013, presenting with a 2-month history of shortness of
breath after exercise. At 2 months prior to referral, the patient
had visited a local hospital for consultation. A computed
tomography (CT; Philips Brilliance 16 Slice; Philips Medical
Systems, Inc., Bothell, WA, USA) scan of the chest was performed
and the results indicated the possibility of interstitial
pneumonia. The patient was subsequently treated with oral
clarithromycin; however, the symptoms did not improve and were
instead exacerbated. The patient was therefore hospitalized at the
Hangzhou Red Cross Hospital.
Physical examination indicated the following: No
evidence of mucosal xanthochromia or superficial enlargement of
lymph nodes palpable in the systemic skins, slight cyanosis of the
lips, a symmetric thoracic cage, and Velcro rales audible under the
arm and subscapularis bilaterally. The patient had a heart rate of
72 bpm, with a regular heart rhythm, low heart sounds and no
pathological murmur. Upon palpation, the patient had a soft
abdomen, with no pressing pain or rebound tenderness, no coastal
liver or spleen, and no edema in the bilateral lower limbs. The
patient was bilateral Babinski sign-negative and all 10 fingers
were slightly clubbed. Auxiliary examination revealed the
following: White blood cell count, 5.7×109/l (normal
range, 4–10×109/l); hemoglobin, 119 g/l (normal range,
10–16 g/l); platelet count, 203×109/l (normal range,
100–300×109/l); prothrombin time, 10.8 sec (normal
range, 10.5–14.3 sec); activated partial thromboplastin time, 31.4
sec (normal range, 18–34 sec); fibrinogen, 207 mg/dl (normal range,
170–500 mg/dl); D-dimer, 621.0 µg/l (normal range, 0–750 µg/dl);
alanine aminotransferase and creatinine, normal; lactate
dehydrogenase, 217 U/l (normal range, 60–245 U/l); hepatitis B
surface antibody, positive; and normal carcinoembryonic antigen,
α-fetoprotein and cancer antigen 125 levels. On July 2, 2013, the
patient underwent a fiber bronchoscopy; as no abnormalities were
initially observed, a biopsy was subsequently performed.
On July 5, 2013, the patient experienced sudden
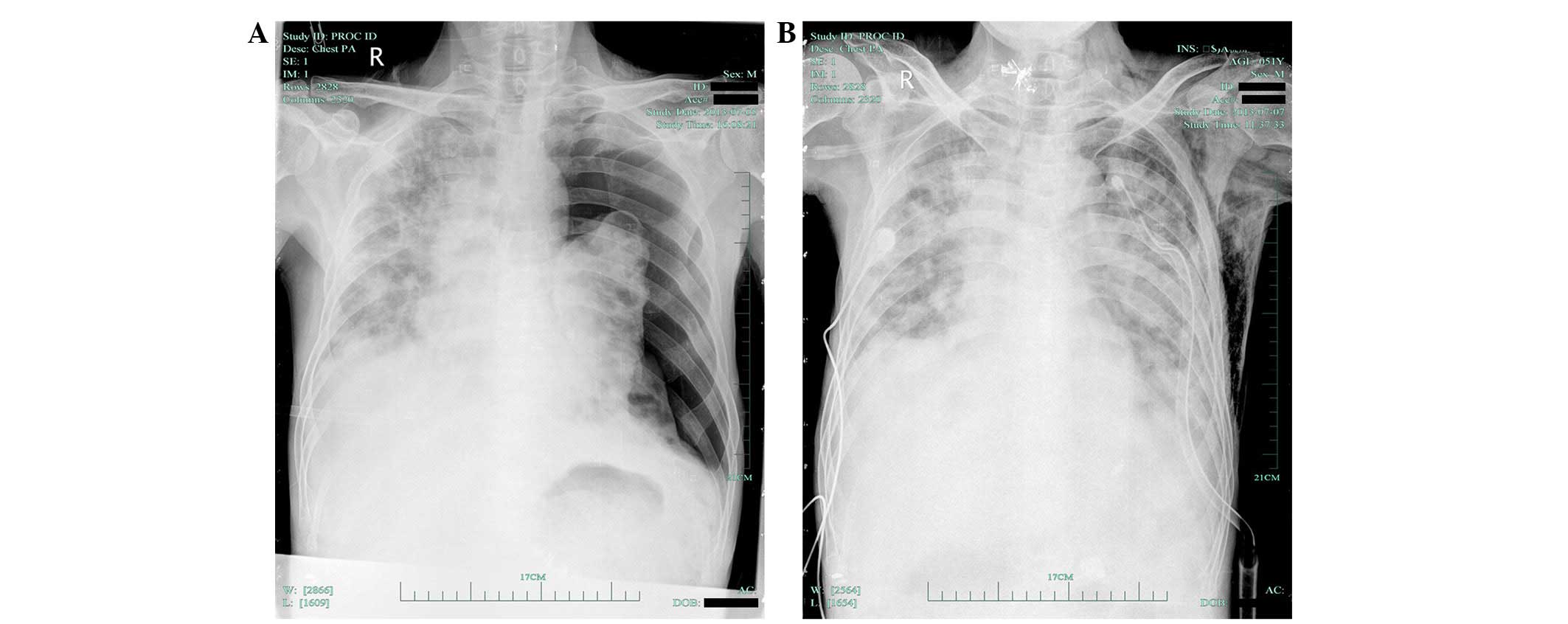
chest pain and shortness of breath, and chest radiography indicated
the presence of a left pneumothorax (Fig.
1A). The patient underwent closed thoracic drainage and the
symptoms gradually improved. On July 7, 2013, an X-ray (OpiTop
150/40/80HC-100 3PH; Siemens AG, Munich, Germany) of the chest
demonstrated that the left pneumothorax had nearly recovered
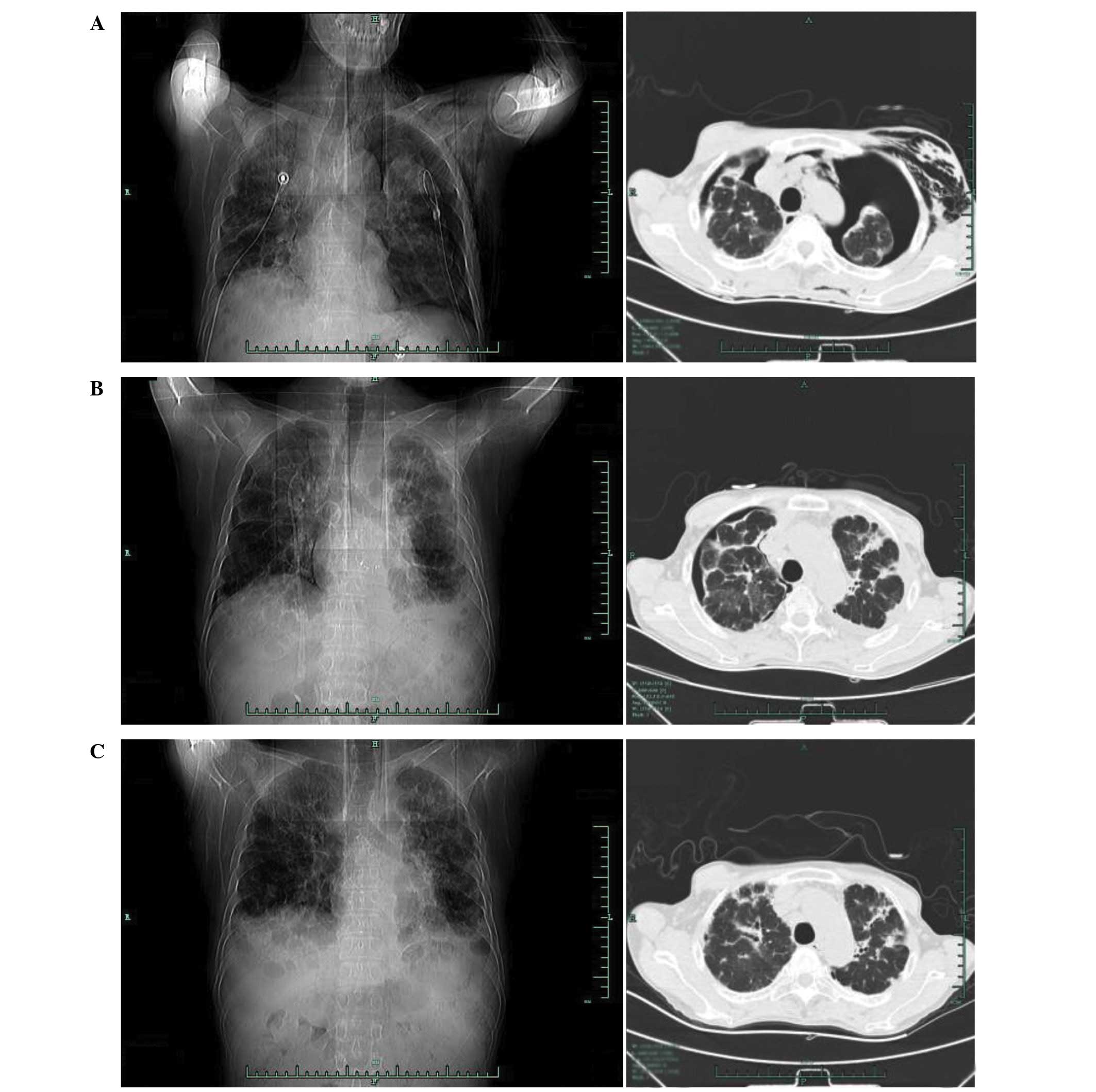
(Fig. 1B). However, a chest CT scan
(Fig. 2) performed on July 8, 2013,
indicated that the right lung and left lung had compressed by 5%
and 60%, respectively, in addition to interstitial inflammation of
each lung and pleural pathological changes, including pleural
thickening and a small amount of pleural effusion (Fig. 2A). Following several air extractions,
the condition of the patient stabilized. Chest CT was repeated on
August 5, 2013, and revealed interstitial inflammation of each lung
with left pleural effusion; the right lung had now compressed by
~20% (Fig. 2B). Furthermore, the body
temperature of the patient continued to increase during
hospitalization. Following anti-infective treatment with cefepime
(1.0 g, ivgtt q12 h, 7 days) and teicoplanin (400 mg, ivgtt qd, 7
days), the symptoms of fever had not alleviated; however, the blood
culture, C-reactive protein, endotoxin and procalcitonin levels
were normal. To test for tuberculosis, a T-SPOT®.TB test
(Shanghai Fosun Long March Medical Science Co.,Ltd., Shanghai,
China) and purified protein derivative (PPD) test (Chengdu
Institute of Biological Products Co., Ltd., Chengdu, China) were
performed; the T-SPOT®.TB test yielded positive results,
whilst the PPD test results were negative.
The EnVision two-step staining method with
3,3′-diaminobenzidine (DAB) was used for immunohistochemical
analysis throughout the case. The primary antibodies used were
monoclonal mouse antibodies (anti-CD2, clone AB75, 1:300 dilution,
catalog no. M7309; anti-CD3, clone F7.2.38, 1:600 dilution, catalog
no. A0452; anti-CD4, clone 4B12, 1:400 dilution, catalog no. M7310;
anti-CD5, clone 4C7, 1:100 dilution, catalog no. M3641; and
anti-CD8, clone C8/144B, 1:50 dilution, catalog no. M7103;
anti-CD43, clone DF-T1, 1:2,500 dilution, catalog no. M0786;
anti-CD20, clone L26, 1:200 dilution, catalog no. M0755; anti-CD10,
clone 56C6, 1:50 dilution, catalog no. M7308; anti-CD30, clone
Ber-H2, 1:1200 dilution, catalog no. M07G1; anti-CD56, clone 123C3,
1:400 dilution, catalog no. M7304; anti-Ki-67, clone MIB-1, 1:2,000
dilution, catalog no. M7240; and anti-CD45, clone 2B11+PD7/26;
1:4,000 dilution, catalog no. M0701) purchased from Dako
(Carpinteria, CA, USA), and a rabbit monoclonal antibody
(anti-PAX5, clone SP34, 1:200 dilution, catalog no. GT209629) and a
mouse monoclonal antibody (anti-TDT, clone SEN28, 1:100 dilution,
catalog no. GT202G29) purchased from Gene Tech Shanghai Co., Ltd.
(Shanghai, China).
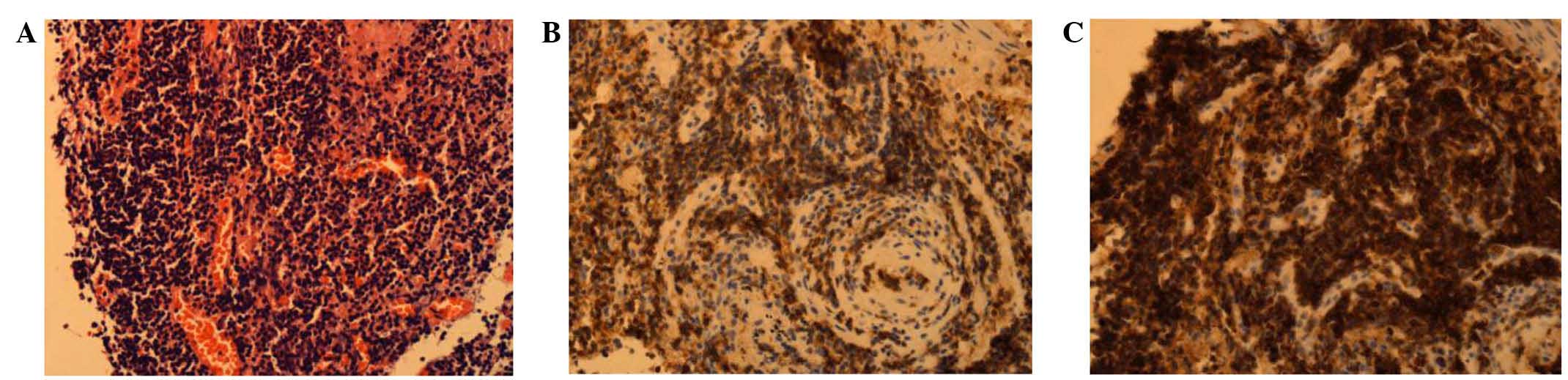
A fiber bronchoscopy biopsy sample was collected on
July 2, 2013. The sample was fixed in formalin, dehydrated, cut
into 3-µm thick sections and then embedded in paraffin prior to
hematoxylin and eosin staining. Histopathological analysis
indicated the presence of chronic mucosal inflammation accompanied
by mild to moderate small lymphoid tissue hyperplasia. Acid-fast
and periodic acid-Schiff staining was negative. Immunoenzymatic
labeling of the tissue demonstrated that it was positive for
cluster of differentiation (CD)3 and CD43, and negative for CD20
and PAX-5, with a Ki-67 index of 5%. As a result, the pathologist
suspected a diagnosis of non-Hodgkin lymphoma (NHL) (Fig. 3). The patient subsequently underwent a
number of examinations to validate this possibility. An abdominal
B-mode ultrasound indicated that the liver was of a normal size and
that the spleen had a thickness of ~4.6 cm. Several lymph gland
resounds were observed adjacent to the hepatic hilar region and
pancreas and surrounding the abdominal aorta (the largest being
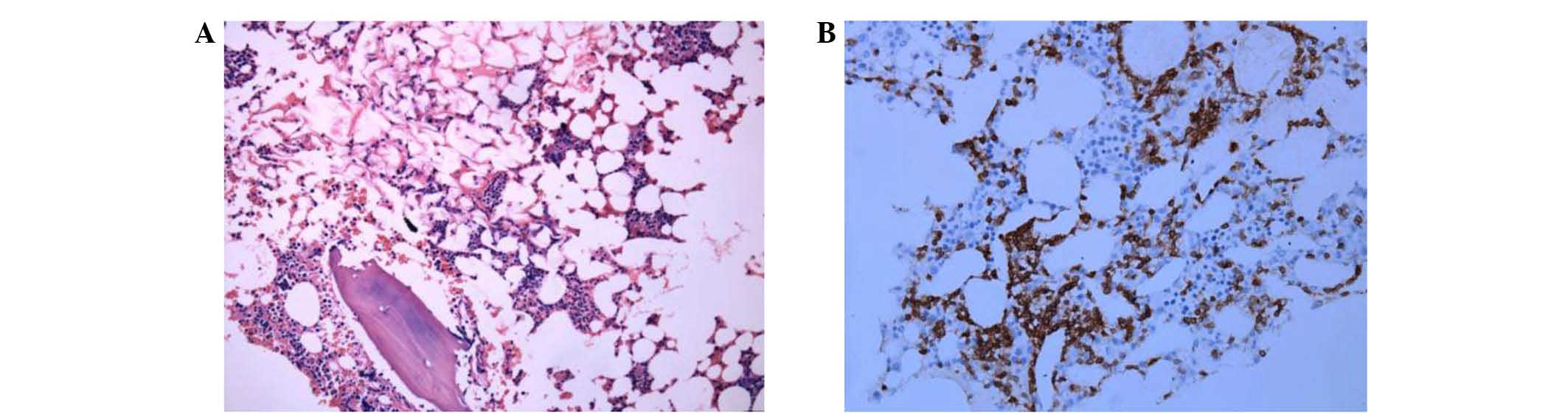
2.7×1.3 cm), in addition to an unclear hilar lymph node. A bone
marrow sample was obtained, and then fixed in Bouin solution for 24
h and decalcified using nitrate acid for 12 h. The sample was then
washed with water for 30 min to remove all the excess chemicals.
The sample was fixed in formalin, dehydrated, cut into 3-µm thick
sections and then embedded in paraffin prior to hematoxylin and
eosin or DAB staining. Bone marrow analysis detected an increased
proportion of mature lymphocytes, and bone marrow cell
immunophenotyping (with threshold analysis set on a CD45/side
scatter point diagram) demonstrated that lymphocytes accounted for
~31% of karyocytes (CD3+CD4−CD8−
cells accounted for ~55.16% of lymphocytes). Immunophenotyping also
indicated that human leukocyte antigen-DR, CD2, CD20 and T-cell
receptor (TCR α/β were positively expressed, whilst CD5 and CD7
were partially expressed; therefore, the most likely diagnosis was
abnormal T cell lymphocyte hyperplasia. The bone marrow biopsy
demonstrated low proliferation of the hematopoietic tissues
(accounting for ~40% of the bone bone marrow), a decreased
myeloid:erythroid ratio, scattered immature granulocytes,
proerythrocyte clusters, scattered megakaryocytes and interstitial
scattered CD3+ lymphocytes. No abnormalities were noted
during histopathological analysis of the bone marrow tissue
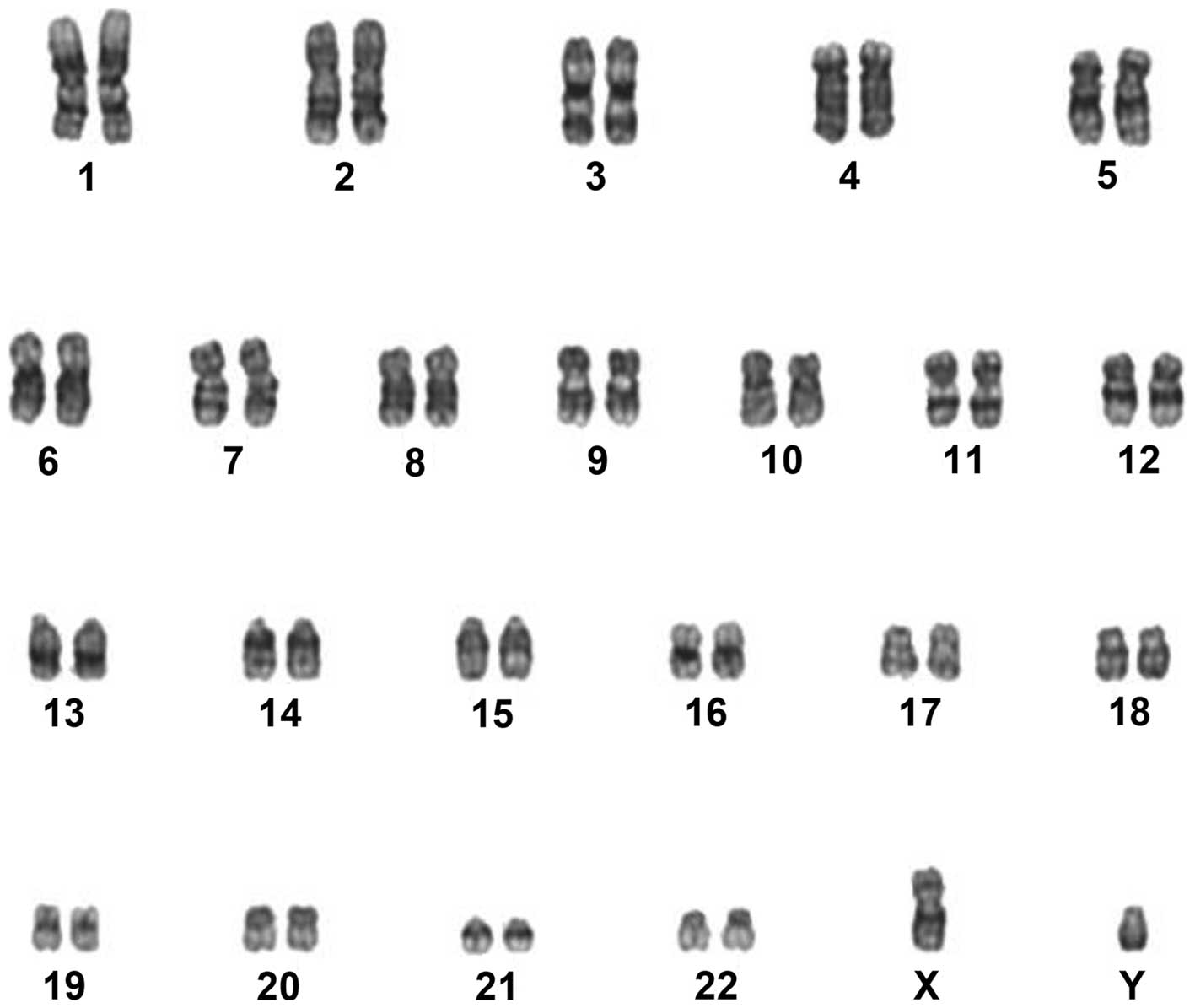
(Fig. 4), and the chromosomes
exhibited normal karyotypes (Fig. 5).
Therefore, the patient was transferred to the Department of
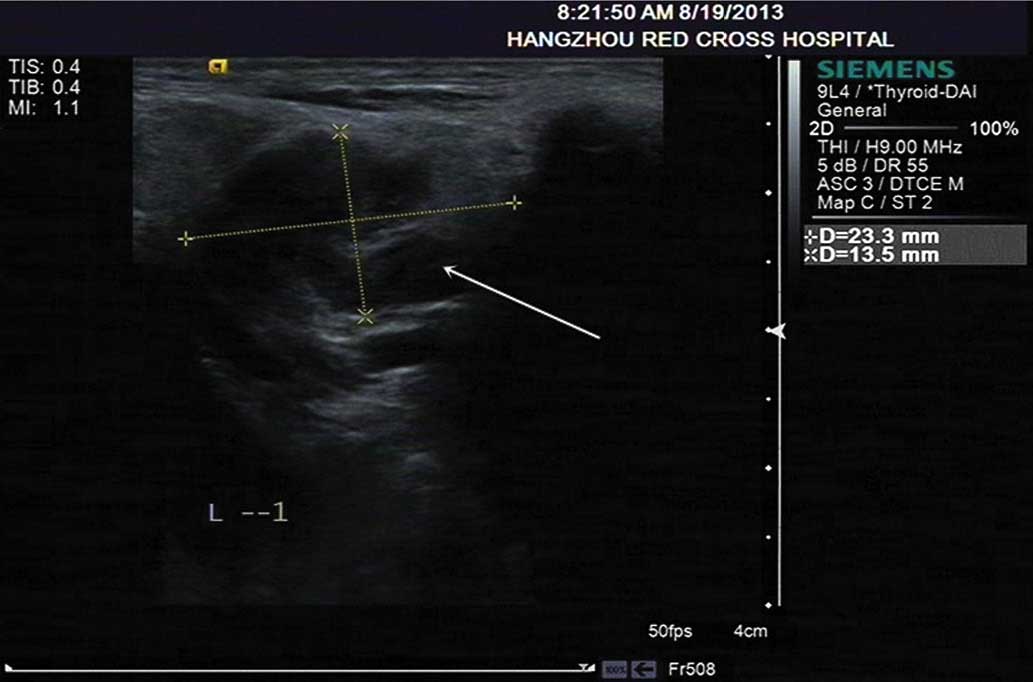
Hematology. Physical examination indicated enlargement of the lymph
nodes in the neck; a B-mode ultrasound (ACUSON S2000™; Siemens AG)
demonstrated that the lymph node located in the left side of the
neck was 2.3×1.3 cm in size, and presented with an unclear hilar
lymph node and non-rich color signals (Fig. 6).
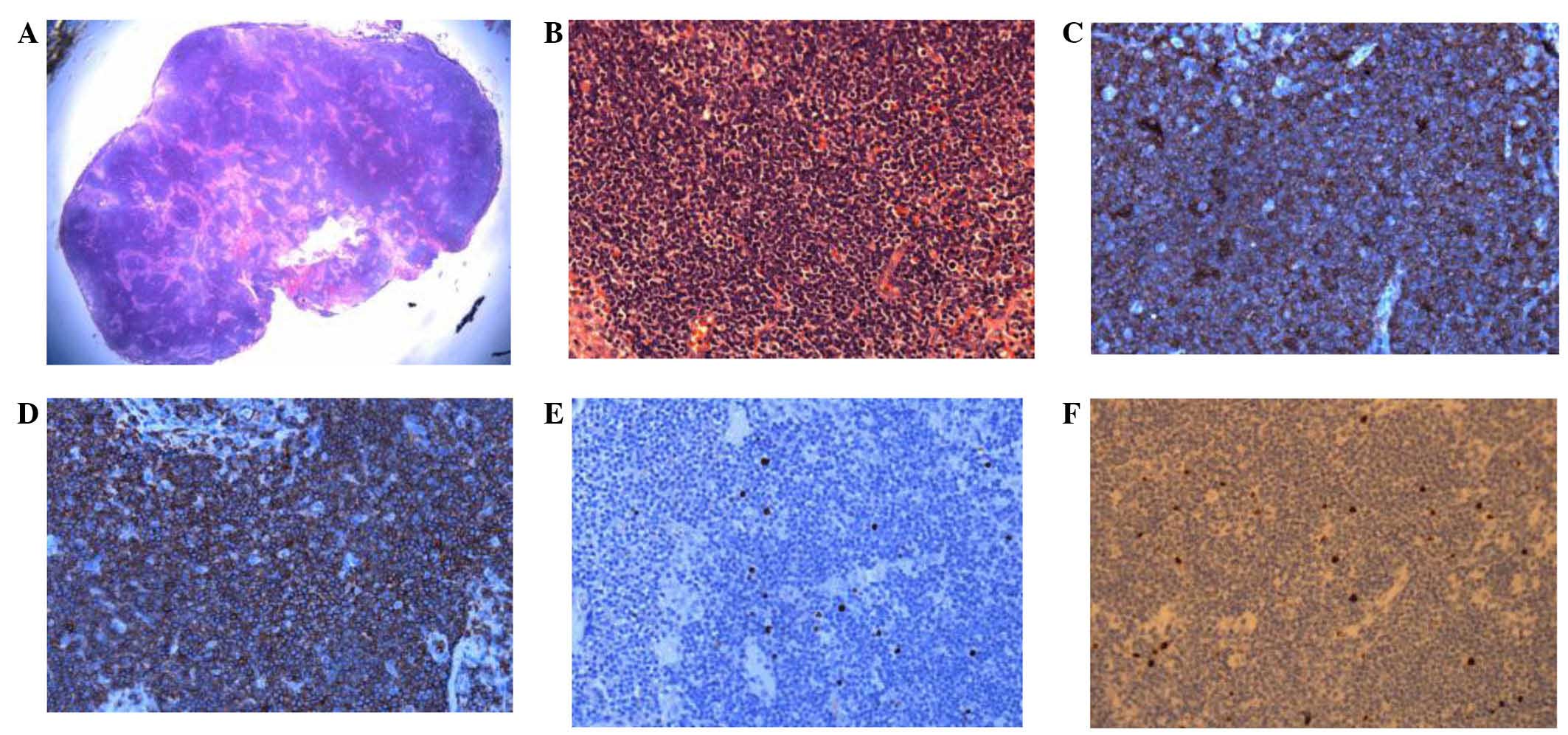
The patient underwent a cervical lymph node biopsy
in the left of the neck under local anesthesia on August 30, 2013.
The sample was prepared as aforementioned for the bronchoscopy
sample. Post-operative pathological examination indicated that the
normal structure of the lymph node had disappeared. Additionally,
mild to moderate-sized diffusive, irregular leukomonocytes were
observed; the envelope and extranodal soft tissue had been
infiltrated, and hyperplasia of the endothelial vein had been
noted, alongside a small number of plasmocytes. Immunoenzymatic
labeling indicated that CD2, CD3, CD5, CD8 and CD43 were positive,
whilst CD20, CD10, PAX5, CD30, TDT and CD56 were negative, with a
Ki-67 index of 5%. Due to the combination of these
immunohistochemical results, a diagnosis of PTCL-u was suspected
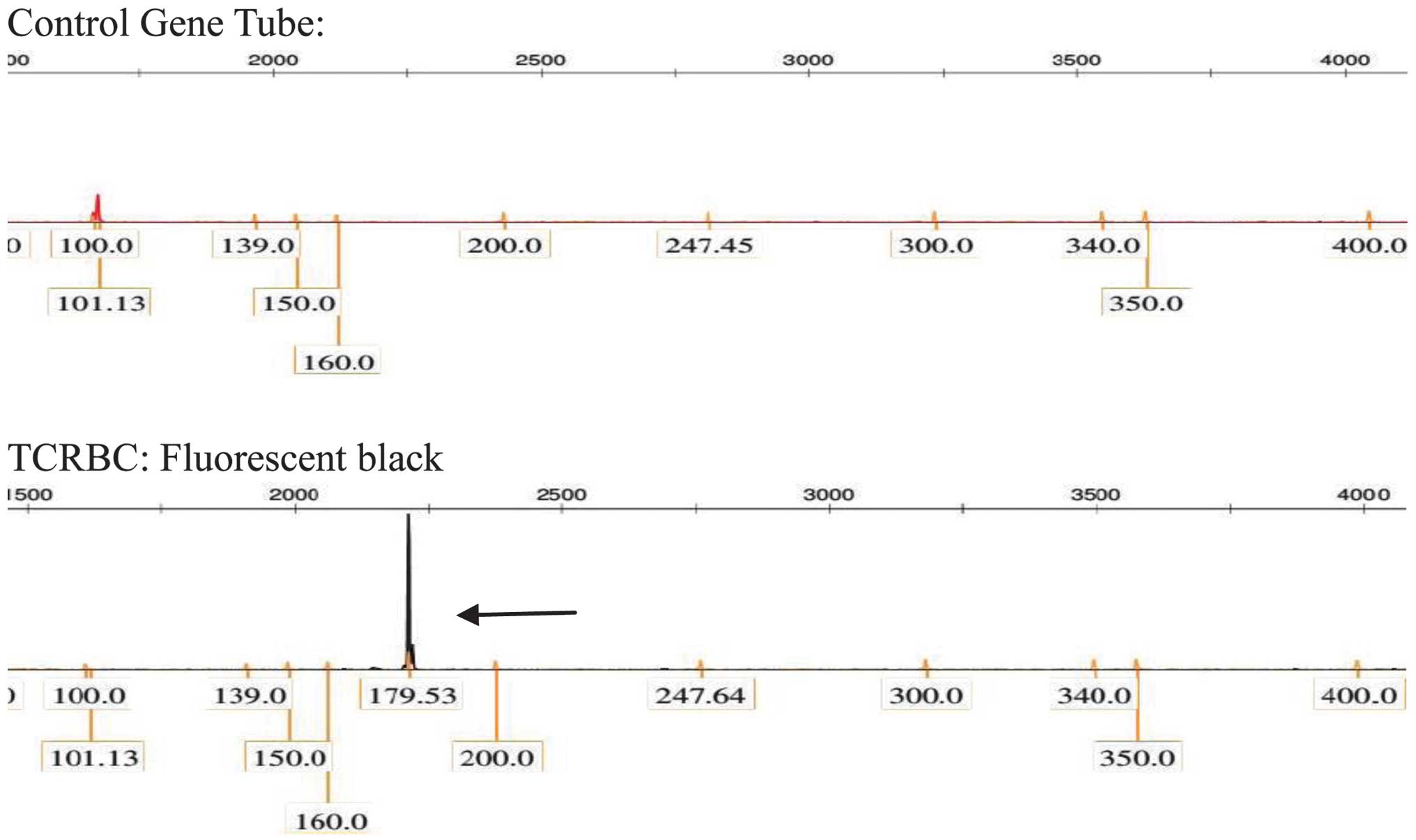
(Fig. 7). TCR gene rearrangement in
the lymph node tissues was positive (Fig.
8), and immunoglobulin gene rearrangement was negative.
Re-examination of bone marrow cell immunophenotyping was also
consistent with a possible diagnosis of T-cell lymphoma
(CD3−CD4+CD8+). Epstein-Barr viral
antibody was negative. As the patient presented with symptoms that
included night sweats, a fever and a reduction in body weight
during hospitalization, the final diagnosis was confirmed as PTCL-u
stage IVB (20), and the pneumothorax
was considered to have occurred as a result of PTCL-u pulmonary
infiltration.
On September 9, 2013, the patient received the first
cycle of CHOP chemotherapy [cyclophosphamide (CTX), 750
mg/m2, day 1; vindesine (VDS), 4 mg, day 1;
epi-adriamycin (ADM), 50 mg, day 1; dexamethasone (DXM), 15 mg,
days 1–5]. The second T-SPOT.TB test was positive and, following a
group consultation by the Tuberculosis Department, it was advised
that the patient should begin preventative antituberculosis therapy
(rifampin, 0.45 g/day/adult qd; isoniazide, 0.3 g/day/adult qd). On
October 16, 2013, the patient received a second cycle of CHOP
chemotherapy (CTX, 1.0 g, day 1; VDS, 4 mg, day 1; epi-ADM, 60 mg,
day 1; DXM, 15 mg, days 1–5). Following treatment, the symptoms of
fever and night sweats were alleviated, and body weight increased.
A further CT scan of the chest on December 3, 2013, showed that the
lung tissue exhibited an appearance similar to diffused ground
glass, and high density patches were observed with shadows in the
form of accumulated strips. There was no obvious evidence of
stenosis in the bronchia or trachea, and no sign of pleural
effusion or pneumothorax (Fig. 2C).
In addition, several enlarged lymph nodes were observed in the
mediastinum. Although the disease had evidently alleviated
following chemotherapy, the patient withdrew from further treatment
due to financial difficulty, and succumbed to the disease 6 months
later.
Discussion
The development of pneumothorax is common, with the
majority of cases occurring as a result of pulmonary infection,
tuberculosis or bronchogenic carcinoma (21). On rare occasions, pneumothorax may
also be induced by pulmonary lymphoma, the majority of these being
B-cell lymphoma (21).
When diagnosing a pulmonary lymphoma, it is
important to first determine whether it is primary or secondary
(22). Primary pulmonary lymphomas
originate from bronchial submucosal and arteriovenous lymphatic
tissues. A definitive diagnosis should include the following: i)
Clear histopathological diagnosis; ii) pathological changes limited
to the pulmonary area, with or without mediastinal and hilar lymph
node implications; and iii) 3 months after diagnosis, absence of
lymphoma in the tissues or organs outside the lungs and bronchi.
Primary pulmonary lymphoma is particularly rare, accounting for
<1% of all pulmonary tumors and 3–4% of extranodal lymphoma
cases (15). The average age of
primary pulmonary lymphoma development is 60–70 years, and this
type of disease comprises mucosa-associated B-cell lymphoma,
diffusive B-cell lymphoma, mantle cell lymphoma and follicular
lymphoma (21). There are two
pathological types of pulmonary lymphoma, Hodgkin's lymphoma and
NHL, with NHL occurring more prevalently (23). Clinical symptoms may include coughing,
expectoration, blood-stained sputum, fever, chest pain and chest
oppression. The disease may also manifest differently by affecting
various areas of the body, including the lungs, the alveolar septum
and the pleural area, identified by imaging analysis (24). The pathological changes commonly
observed inside the lungs predominantly affect the pulmonary
interstitium and bronchial submucosal tissues, in addition to the
bronchial wall (primarily the extra-wall pulmonary interstitium,
such that the bronchial lumen remains unobstructed or experiences
only slight stenosis) (25).
Bronchial submucosal lymphoma involvement may form inner lumen
nodular protrusions, or its growth around the bronchial wall may
result in limited or extensive bronchial lumen narrowing, or even
complete luminal obstruction complicated by pulmonary consolidation
and atelectasis (25). When the
alveolar septum is affected, the pulmonary septum initially
thickens and, as the disease progresses, the alveolar space
gradually becomes smaller or completely obstructed (25). The invasions of the pleura is
manifested as pleural thickening, patchy infiltrates or nodules,
which tend to be distributed with a lack of aggregation (25).
Secondary pulmonary lymphoma is a form of systemic
lymphoma (22). In addition to the
common symptoms, including coughing, expectoration, hemoptysis,
chest pain and pleural effusion, which are clinically manifested by
corresponding respiratory, secondary pulmonary lymphoma also
presents with systemic superficial lymph node enlargement and/or B
symptoms (22). The imaging
characteristics associated with these symptoms are similar to those
of pneumonia and tuberculosis, and may therefore lead to
misdiagnosis (22).
Primary and secondary pulmonary lymphomas present
with multiple imaging manifestations; therefore a comprehensive
analysis of the clinical pathology, vital signs and symptoms is
required to distinguish between the two (22). In the present case, analysis of the
lymph node biopsy resulted in the diagnosis of PTCL-u. The patient
was experiencing a fever, night sweats, decreased body weight and a
pneumothorax and, as the disease was not limited to the lungs, this
lead to the induction of hepatosplenomegaly. As a result, the
patient was diagnosed with pneumothorax induced by PTCL-u pulmonary
infiltration.
Regarding the present case, there are a number of
mechanisms that may explain how pulmonary lymphoma induced a
pneumothorax. Firstly, it may occur as a result of lymphoma cells
invading the visceral pleura, or by necrosis exposing the bronchium
leading to air leakage (26).
Secondly, metastasis may obstruct the surrounding airway, resulting
in obstructive emphysema and unstable pulmonary bullae, inducing
pneumothorax as a secondary pulmonary disease (26). The present case was considered to
occur due to a combination of comprehensive factors. Therefore, to
further confirm the underlying mechanisms, pathological methods
should be utilized to examine a direct association between the
pleura rupture site and the pulmonary lymphoma.
PTCL accounts for 5–15% of all NHL cases and
exhibits marked heterogeneity (12).
PTCL-u is the most prevalent form of PTCL and occurs most commonly
in the elderly. The disease exhibits high degrees of invasion and
malignancy, and often affects the systemic lymph nodes and
extranodal sites (typically invading the spleen, skin, digestive
tract and bone marrow) (11). As the
lungs contain rich lymphatic tissues, PTCL-u should theoretically
infiltrate these; however, infiltration of PTCL-u to the lungs is
extremely rare (27). Wang et
al (28) reported a rare case of
cough-variant asthma secondary to angioimmunoblastic T-cell
lymphoma; however, to date, there have been no reports of PTCL-u
occurring with pneumothorax as the primary manifestation.
The definitive diagnosis of PTCL-u depends upon the
pathological analysis of biopsy tissue, with typical manifestations
including the dispersive distribution of oncocytes, structural
damage of lymph nodes, inflammatory polymorphic background of
lymphocytes, eosinophil granulocytes, plasmocytes and a large
number of epithelioid tissue cells (27). Oncocytes exist in a number of
different forms and may be small, medium or large cells. However,
in the majority of cases, the cells are medium to large in size,
and have a polymorphic and irregular nucleus with vesicular
chromatin and an obvious nucleolus, and frequently form mitotic
figures (27). Transparent cells or
Reed-Sternberg-like cells are always present, but not true
Reed-Sternberg cells. A limited number of cases report of tissue
biopsies predominantly containing small lymphocytes with irregular
nuclei. There are also increased small vessels, endothelial cell
hypertrophy and branching vessels. PTCL-u expresses T
cell-associated antigens, with CD3+ serving as a
reliable marker for this mechanism, whilst CD45RO and CD43, which
are not specific T cell-associated antigens, are also expressed. In
the majority of cases, lymph nodes have been identified as
CD4+CD8– (29).
Therefore, the diagnosis of lymphoma primarily depends on biopsy of
the entire lymph nodes, and clear structure of the lymphocytes and
associated immunophenotyping is required (27). In the present case, the lymph node
sample from the left of the neck was of good quality and conformed
to the diagnostic requirements; however, the bronchial mucosa
sample was poor and did not reach the diagnostic requirements for
lymphoma. Therefore, the pathological analysis of the lymph node
was used to confirm the diagnosis of PTCL-u, and flow cytometry
indicated T lymphocyte proliferation. Taking into consideration the
clinical manifestations of the present case, a diagnosis of PTCL-u
was apparent, and the CD20+ lymphocytes present in the
bronchial mucosa were considered to demonstrate increased
reactivity.
At present, there are no standard guidelines in
place for the treatment of PTCL-u, with therapy primarily based on
the CHOP chemotherapy regimen. The disease-free and overall
survival times of patients with PTCL-u following conventional CHOP
treatment are markedly lower than those of patients with B-cell
lymphoma (30,31). Novel therapeutic approaches aiming to
improve upon the short-term and long-term efficacy of the currently
available PTCL-u treatments are being investigated at present. For
example, applications of hematopoietic stem cell transplantation,
lenalidomide and cytarabine derivatives (32–34) all
demonstrate efficacy; however, further clinical studies are
required to determine the safest and most reliable treatment. In
the present case, following two cycles of CHOP regimen, the body
temperature and weight of the patient returned close to normal,
night sweats ceased and CT imaging of the chest revealed no
evidence of pneumothorax manifestations. A further chemotherapy
regimen was considered (L-asparaginase, etoposide and
bendamustine); however, the patient withdrew from treatment due to
financial difficulty. A relapse was predicted, and the patient
succumbed to the disease 6 months later.
In conclusion, as pneumothorax is a particularly
common clinical disease, when forming a diagnosis, physicians
should pay attention to the medical history of the patient, the
clinical symptoms and the results of physical examination. If the
pneumothorax-associated symptoms cannot be explained by a common
disease, the possibility of pulmonary lymphoma should be taken into
consideration, and the conduction of a biopsy followed by
pathological analysis may prevent misdiagnosis. The treatment of
PTCL-u remains a challenge in the clinical setting, as the disease
is commonly resistant to chemotherapy and is prone to relapse. Stem
cell transplantation may be considered as a preferred therapeutic
approach for young patients; however, the prognosis is commonly
poor. There are currently no existing unified treatment guidelines
for PTCL-u. As the amount of research in this area increases, it is
anticipated that an effective treatment may be developed in the
future.
References
|
1.
|
Vose J and Armitage J: and Weisenburger.
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: Pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Federico M, Rudiger T, Bellei M, Nathwani
BN, Luminari S, Coiffier B, Harris NL, Jaffe ES, Pileri SA, Savage
KJ, et al: Clinicopathologic characteristics of angioimmunoblastic
T-cell lymphoma: Analysis of the international peripheral T-cell
lymphoma project. J Clin Oncol. 31:240–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lunning MA, Moskowitz AJ and Horwitz S:
Strategies for relapsed peripheral T-cell lymphoma: The tail that
wags the curve. J Clin Oncol. 31:1922–1927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schmitz N, Trümper L, Ziepert M, Nickelsen
M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A and
Pfreundschuh M: Treatment and prognosis of mature T-cell and
NK-cell lymphoma: An analysis of patients with T-cell lymphoma
treated in studies of the German High-Grade Non-Hodgkin Lymphoma
Study Group. Blood. 116:3418–3425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rodríguez J, Conde E, Gutiérrez A, Arranz
R, León A, Marín J, Bendandi M, Albo C and Caballero MD: The
results of consolidation with autologous stem-cell transplantation
in patients with peripheral T-cell lymphoma (PTCL) in first
complete remission: The Spanish Lymphoma and Autologous
Transplantation Group experience. Ann Oncol. 18:652–657. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Feyler S, Prince HM, Pearce R, Towlson K,
Nivison-Smith I, Schey S, Gibson J, Patton N, Bradstock K, Marks DI
and Cook G: The role of high-dose therapy and stem cell rescue in
the management of T-cell malignant lymphomas: A BSBMT and ABMTRR
study. Bone Marrow Transplant. 40:443–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moskowitz AJ, Lunning MA and Horwitz SM:
How I treat the peripheral T-cell lymphomas. Blood. 123:2636–2644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Savage KJ, Chhanabhai M, Gascoyne RD and
Connors JM: Characterization of peripheral T-cell lymphomas in a
single North American institution by the WHO classification. Ann
Oncol. 15:1467–1475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Escalón MP, Liu NS, Yang Y, Hess M, Walker
PL, Smith TL and Dang NH: Prognostic factors and treatment of
patients with T-cell non-Hodgkin lymphoma: The M. D. Anderson
Cancer Center experience. Cancer. 103:2091–2098. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Barbarotta L and Hurley K: Romidepsin for
the treatment of peripheral T-cell lymphoma. J Adv Pract Oncol.
6:22–36. 2015.PubMed/NCBI
|
|
11.
|
Bekkenk MW, Vermeer MH, Jansen PM, van
Marion AM, Canninga-van Dijk MR, Kluin PM, Geerts ML, Meijer CJ and
Willemze R: Peripheral T-cell lymphomas unspecified presenting in
the skin: Analysis of prognostic factors in a group of 82 patients.
Blood. 102:2213–2219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lee HJ, Im JG, Goo JM, Kim KW, Choi BI,
Chang KH, Han JK and Han MH: Peripheral T-cell lymphoma: Spectrum
of imaging findings with clinical and pathologic features.
Radiographics. 23:7–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Salazar AJ, Aguirre DA, Ocampo J, Camacho
JC and Díaz XA: Evaluation of three pneumothorax size
quantification methods on digitized chest X-ray films using
medical-grade grayscale and consumer-grade color displays. J Digit
Imaging. 27:280–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Luh SP: Review: Diagnosis and treatment of
primary spontaneous pneumothorax. J Zhejiang Univ Sci B.
11:735–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Noppen M and de Keukeleire T:
Pneumothorax. Respiration. 76:121–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sahn SA and Heffner JE: Spontaneous
pneumothorax. N Engl J Med. 342:868–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hobbs BD, Foreman MG, Bowler R, Jacobson
F, Make BJ, Castaldi PJ, San José, Estépar R, Silverman EK and
Hersh CP: COPDGene Investigators: Pneumothorax risk factors in
smokers with and without chronic obstructive pulmonary disease. Ann
Am Thorac Soc. 11:1387–1394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Schmidt-Horlohé N, Rudig L, Azvedo CT and
Habekost M: Fulminant unilateral pulmonary edema after insertion of
a chest tube: A complication after a primary spontaneous
pneumothorax. Dtsch Arztebl Int. 105:878–881. 2008.PubMed/NCBI
|
|
19.
|
Matano S, Satoh S, Sugiguchi S and
Sugimoto T: Pneumothorax associated with malignant lymphoma. Intern
Med. 49:2337–2339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Parissis H: Forty years literature review
of primary lung lymphoma. J Cardiothoracic Surg. 6:232011.
View Article : Google Scholar
|
|
21.
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. Eur Respir J. 20:750–762. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Niu X, Hu H, Gao J, Nie Y, Zhao W, Xu H,
Bai X and Chen L: A clinical analysis of 40 cases of primary and
secondary pulmonary lymphoma. Zhonghua Jie He He Hu Xi Za Zhi.
37:502–506. 2014.(In Chinese). PubMed/NCBI
|
|
23.
|
Zhu L and Zhang J: Pulmonary malignant
lymphoma: A clinicopathological analysis of 16 cases. J Diagn
Pathol. 20:740–743. 2013.
|
|
24.
|
Zhang ZY, Wu XH and Shi YX: CT study of
pulmonary lymphoma comparison with X-ray. Clin Med J China.
8:26–28. 2001.
|
|
25.
|
Hare SS, Souza CA, Bain G, Seely JM Frcpc,
Gomes MM and Quigley M: The radiological spectrum of pulmonary
lymphoproliferative disease. Br J Radiol. 85:848–864. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shan Y and Zhou Y: Recent research of
pathogenesis and causes of recurrence of primary spontaneous
pneumothorax. Surg Res New Technique. 4:52–56. 2015.
|
|
27.
|
Went P, Agostinelli C, Gallamini A,
Piccaluga PP, Ascani S, Sabattini E, Bacci F, Falini B, Motta T,
Paulli M, et al: Marker expression in peripheral T-cell lymphoma: A
proposed clinical-pathologic prognostic score. J Clin Oncol.
24:2472–2479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang XY, Xia GG, Zhang YJ, Zhang B and
Zhang PJ: Angioimmunoblastic T-cell lymphoma: A case report and
review of literature. Int J Respir 32. 6:91–94. 2012.(In
Chinese).
|
|
29.
|
Zhe W, Xie YQ and Zhu J: The advance in
diagnosis and treatment of peripheral T-cell lymphoma-unspecified.
Chin J Clin Oncol. 33:476–480. 2006.
|
|
30.
|
Morabito F, Gallamini A, Stelitano C,
Callea V, Guglielmi C, Neri S, Lazzaro A, Orsucci L, Ilariucci F,
Sacchi S, et al: Clinical relevance of immunophenotype in a
retrospective comparative study of 297 peripheral T-cell lymphomas,
unspecified, and 496 diffuse large B-cell lymphomas: Experience of
the Intergruppo Italiano Linformi. Cancer. 101:1601–1608. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Intragumtornchai T, Rotnakkarin P,
Sutcharitchan P and Wannagrairoj P: Prognostic significance of the
immunophenotype versus the International Prognostic Index in
aggressive non-Hodgk'ns lymphoma. Clin Lymphoma. 4:52–55. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gui L, Shi YK, He XH, Lei YH, Zhang HZ,
Han XH, Zhou SY, Liu P, Yang JL, Dong M, et al: High-dose therapy
and autologous stem cell transplantation in peripheral T-cell
lymphoma: Treatment outcome and prognostic factor analysis. Int J
Hematol. 99:69–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Hopfinger G, Nösslinger T, Lang A,
Linkesch W, Melchardt T, Weiss L, Egle A and Greil R: Lenalidomide
in combination with vorinostat and dexamethasone for the treatment
of relapsed/refractory peripheral T cell lymphoma (PTCL): Report of
a phase I/II trial. Ann Hematol. 93:459–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Evens AM, Rosen ST, Helenowski I, Kline J,
Larsen A, Colvin J, Winter JN, van Besien KM, Gordon LI and Smith
SM: A phase I/II trial of bortezomib combined concurrently with
gemcitabine for relapsed or refractory DLBCL and peripheral T-cell
lymphomas. Br J Haematol. 163:55–61. 2013. View Article : Google Scholar : PubMed/NCBI
|