Introduction
Thymoma is a tumor originating from the epithelial
cells of the thymus and is a relatively rare neoplasm with an
incidence of 0.13 cases per 100,000 individuals per year (1,2). The
prognosis of patients with thymoma largely depends on the Masaoka
stage of disease and prognosis is poorer for patients with stage
III or IV compared with patients with stage I and II tumors
(3). The 10-year survival rates are
90, 70, 55 and 35% for stages I, II, III, and IV thymoma,
respectively (4). Surgical resection
is the primary treatment method for thymoma and a sternotomy is the
optimal surgical procedure for thymoma, since it may not be
possible to perform a complete thymectomy via thoracotomy on
locally advanced lesions, particularly those that are stage III–IV
(5,6).
Normally, thymoma is a slow-growing tumor, but 40% of thymomas
exhibit a locally invasive growth pattern. In addition, thymomas
often result in the development of pleural dissemination and
distant metastasis (7,8). Therefore, complete resection should be
the primary goal during surgical treatment. The International
Thymic Malignancies Interest Group recommends en bloc
resection, also known as extended thymectomy (ET), including
complete thymectomy and resection of the surrounding mediastinal
fat, due to the possibility of macroscopically invisible invasion
of the tumor (5). Due to the limited
case number of thymomas, survival analysis following complete
thymectomy based on large cohorts is limited. Therefore, gaining an
improved understanding of survival prognosis may be beneficial for
the management of the patients with thymoma.
Aside from disease stage, other factors that affect
the outcome of patients following complete thymectomy remain
largely unknown. Thymoma is frequently associated with parathymic
diseases, including myasthenia gravis (MG) (9). MG is the most commonly associated
paraneoplastic disease in thymoma patients and 30–50% of thymoma
patients have MG (10). However,
whether MG is a determining factor for the outcome of patients with
thymoma following complete thymectomy remains unknown. The present
study demonstrated that the prognosis of patients with thymomas and
MG is poorer compared with patients without MG. Therefore, MG
status should be considered in the management of patients with
thymomas.
Materials and methods
Patients
In total, 104 patients with thymoma were
consecutively recruited to the present study between January 2005
and January 2010. All the patients were recruited at two centers
(The First Hospital of Qinhuangdao, Qinhuangdao; the First Hospital
of Jilin University, Changchun, China). The clinical
characteristics of the patients were recorded, including gender,
age, presence of MG, computed tomography (CT) of the chest, World
Health Organization (WHO) type, Masaoka stage, myasthenic crisis
presence and surgical treatment procedure. Follow-up was performed
for all patients every 3 months through office visits or telephone
interviews and the follow-up information consisted of postoperative
MG status and physical examination. In addition, chest
roentgenography and chest CT scans were performed every 6 months
for the first 2 years following surgery and annually thereafter.
The present study was approved by The First Hospital of Qinhuangdao
Ethics Committee and all patients provided signed informed consent
prior to participation.
Clinical and histological staging
The clinical stage of the tumors was evaluated
according to the Masaoka staging system classification (11): Stage I, completely encapsulated and
lacking microscopic capsular invasion; Stage II, microscopic
capsular invasion or macroscopic invasion into mediastinal pleura
or surrounding fatty tissue; stage III, macroscopic invasion into
adjacent organs; stage IV, tumor with pleural or pericardial
dissemination (IVa) or hematogenous or lymphogenous distant
metastasis (IVb). The histological subtype of thymoma was defined
according to the 2004 WHO histological classification (12). The WHO classification system
categorizes tumors as follows: Type A, comprised of a homogenous
population of neoplastic epithelial cells with spindle/oval shape,
lacking nuclear atypia, and accompanied by few or no non-neoplastic
lymphocytes; type AB, foci possessing features of type A thymoma
are admixed with foci rich in lymphocytes, the segregation of two
patterns can be sharp or indistinct; type B1, resembles the normal
functional thymus in that it combines large expanses with an
appearance practically indistinguishable from that of normal thymic
cortex, with areas resembling the thymic medulla; type B2, the
neoplastic epithelial component appears as scattered, plump cells
with vesicular nuclei and distinct nucleoli among a heavy
population of lymphocytes, and perivascular spaces are common; type
B3, comprised predominantly of epithelial cells with a round or
polygonal shape that exhibit mild atypia admixed with a minor
component of lymphocytes, and foci of squamous metaplasia and
perivascular spaces are common. Masaoka stage and WHO
classification were confirmed following histological examination of
hematoxylin and eosin-stained sections (5 µm) derived from paraffin
embedded blocks.
Surgical procedures
Two surgical procedures were used: Median sternotomy
and video-assisted thoracoscopic surgery (VATS). ET was defined as
the resection of the entire thymus and mediastinal fat tissue
between the two phrenic nerves. For Masaoka stages III and IV,
combined organ and tissue resection was required and performed.
Survival analysis
The date of surgery was considered at the time of
diagnosis. The survival durations were calculated between the date
of surgery and the date of death due to any cause or the last
follow-up day in January 2015. Overall survival (OS) time was
defined as the time between the date of diagnosis to date of death
due to any cause.
Statistical analysis
The Kaplan-Meier method was used to estimate the
probability of survival and the differences between survival in
each group was analyzed by the log-rank test. Categorical variables
were compared using the χ2-test or Fisher's exact test.
Multivariate analysis was performed using a Cox regression model.
Statistical analysis was performed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients characteristics
Out of the 104 patients, 63 were men and 41 were
women. The mean age was 54.6 years (range, 20–79 years). As shown
in Table I, all patients were divided
into two groups based on whether or not the patient presented with
MG. In addition, 30 patients presented with coronary disease,
hypertension or diabetes. In total, 8 patients (7.7%) were WHO type
A, 22 (21.2%) were AB, 17 (16.3%) were B1, 34 (32.7%) were B2 and
23 (22.1) were B3. According to the Masaoka staging system, 46
patients (44.2%) were diagnosed with stage I disease, 42 patients
(40.4%) had stage II disease, 14 patients (13.5%) had stage III
disease and 2 patients (1.9%) had stage IVa disease. In total, 38
patients (36.5%) had MG. In a comparison between the presence and
absence of MG, the prevalence of MG was significantly higher in
type B2 thymoma compared with other types of thymoma (P=0.019).
 | Table I.Characteristics of 104 thymoma
patients with and without MG. |
Table I.
Characteristics of 104 thymoma
patients with and without MG.
| Characteristic | Total | Without MG | With MG | t/χ2
value | P-value |
|---|
| Total patients,
n | 104 (100) | 66 (64) | 38 (37) |
|
|
| Age at surgery,
years | 54.6±11.0 | 56.0±10.8 | 52.2±11.1 | 1.714 | 0.090 |
| Gender, n (%) |
|
|
| 1.849 | 0.174 |
| Male | 61 (59) | 42 (64) | 19 (50) |
|
|
|
Female | 43 (41) | 24 (36) | 19 (50) |
|
|
| Tumor size, cm | 3.1±1.3 | 3.2±1.4 | 3.0±0.9 | 0.610 | 0.543 |
| Surgical procedure, n
(%) |
|
|
| 0.865 | 0.352 |
|
Sternotomy | 10 (10) | 5 (8) | 5
(13) |
|
|
| VATS | 94 (90) | 61 (92) | 33 (87) |
|
|
| Myasthenic crisis, n
(%) | 8 (8) | 0 (0) | 8
(21) | 7.538 | 0.006b |
| WHO histological
classification, n (%) |
|
|
| 11.83 | 0.019a |
| A | 8 (8) | 7
(11) | 1 (3) | 4.500 | 0.034a |
| AB | 22 (21) | 17 (26) | 5
(13) | 6.545 | 0.011a |
| B1 | 17 (16) | 12 (18) | 5
(13) | 2.882 | 0.090 |
| B2 | 34 (33) | 14 (21) | 20
(53)c | 0.471 | 0.493 |
| B3 | 23 (22) | 16 (24) | 7
(18) | 2.130 | 0.144 |
| Masaoka stage, n
(%) |
|
|
| 3.962 | 0.266 |
| I | 46 (44) | 30 (45) | 16 (42) | 6.712 | 0.010a |
| II | 42 (40) | 28 (42) | 14 (37) | 4.677 | 0.031a |
|
III | 14 (14) | 6 (9) | 8
(21) | 0.059 | 0.808 |
|
IVa | 2 (2) | 2 (3) | 0 (0) |
| Pathology of
paraneoplastic thymus, n (%) |
|
|
| 13.975 |
<0.001b |
|
Involuted | 87 (84) | 62 (94) | 25 (66) |
|
|
|
Hyperplastic | 17 (16) | 4 (6) | 13 (34) |
|
<0.001b |
| Radiotherapy, n
(%) | 49 (47) | 25 (38) | 24 (63) | 6.185 | 0.013a |
| Recurrence, n
(%) | 3 (3) | 1 (2) | 2 (5) | 92.346 |
<0.001b |
| Mortality, n
(%) | 11 (11) | 3 (5) | 8
(21) | 9.013 | 0.003b |
Masaoka staging and WHO
classification
The association between Masaoka stage and WHO
classification was analyzed and is presented in Table II. The percentage of patients with
Masaoka stages I, II, III and IVa was 42.3, 39.4, 16.3 and 1.9%,
respectively. The percentage of patients with WHO type A, AB, B1,
B2 and B3 was 7.7, 21.2, 16.3, 32.7 and 22.1%, respectively. The
majority of patients with WHO type A, AB and B1 was observed at
Masaoka stage I and II (44/47; 93.6%). However, thymomas classified
as WHO type B2 and B3 subtype (16/19; 84.2%), which corresponded to
Masaoka stage III and IVa, exhibited more invasive behavior and
thus were considered more aggressive. The association between tumor
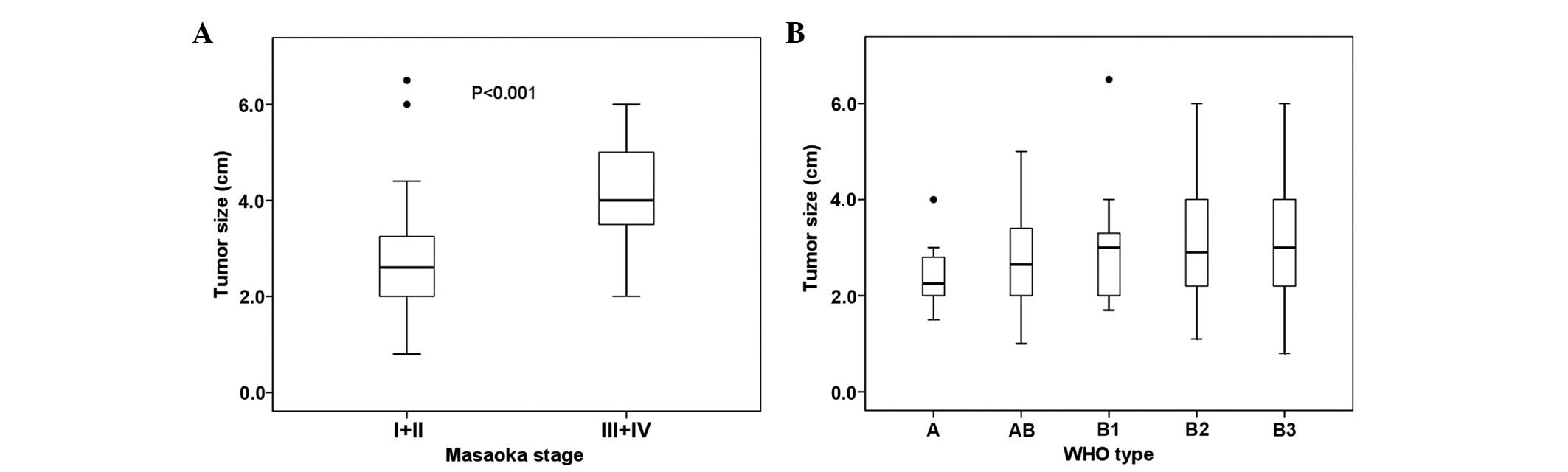
size and Masaoka stage is indicated in Fig. 1A. The mean tumor size with Masaoka
stages I and II was significantly smaller compared with tumors with
Masaoka stages III and IV (P<0.001). No significant association
was observed between tumor size and WHO classification (P=0.488)
(Fig. 1B).
 | Table II.Association between Masaoka stage and
WHO histological classification. |
Table II.
Association between Masaoka stage and
WHO histological classification.
|
|
| WHO histological
classification, n |
|---|
|
|
|
|
|---|
| Masaoka stage,
n | Total patients,
n | A | AB | B1 | B2 | B3 | Mortalities |
|---|
| Total | 104 | 8 | 22 | 17 | 34 | 23 | 11 |
| I | 46 | 4 | 9 | 6 | 17 | 8 | 1 |
| II | 42 | 4 | 11 | 10 | 6 | 10 | 3 |
| III | 14 | 0 | 2 | 1 | 10 | 4 | 6 |
| IVa | 2 | 0 | 0 | 0 | 1 | 1 | 1 |
| Mortalities | 11 | 0 | 1 | 0 | 5 | 5 | – |
Surgical procedure and
mortalities
All patients underwent ET either by sternotomy (10
patients) or VATS (94 patients). In addition, combined resection
with adjacent organs, including the lung (n=6), pericardium (n=5),
left brachiocephalic (n=3) or partial superior vena cava (n=2), was
also performed if required. There were no perioperative
mortalities, but in 7 patients perioperative myasthenic crisis
occurred in the group with MG. In addition, 18 patients (17.3%) had
postoperative complications, including 10 patients with MG that
experienced assisted breathing with a ventilator due to myasthenic
crisis. In addition, 4 patients contracted pneumonia, 2 had
pulmonary embolisms and 2 had surgical wound infections. There were
11 mortalities during the 5-year follow-up period. The
mortality-associated causes consisted of recurrence of thymoma in 3
patients and respiratory failure due to MG in 8 patients. The
mortality rate in patients with MG was significantly higher
compared with patients without MG (P=0.003).
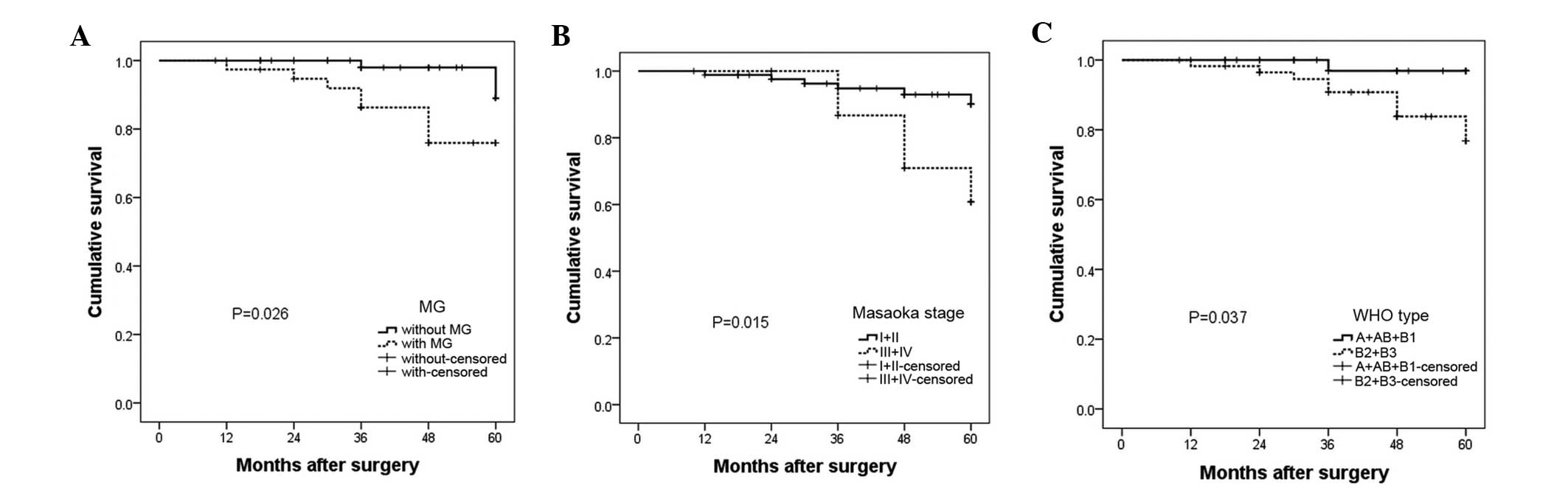
OS rates
The OS rate at 5 years was 89.1 and 76.0% in
patients without MG and with MG, respectively (Fig. 2A). The OS rate was 90.0 and 68.0% for
patients with Masaoka stages I + II and III + IV, respectively
(Fig. 2B). The OS rate was 96.9 and
76.8% in WHO types A + AB + B1 and B2 + B3, respectively (Fig. 2C). Therefore, patients with MG
(P=0.026), stages III + IV (P=0.015) and WHO type B2 + B3 (P=0.037)
had a poorer prognosis compared with patients without these
characteristics.
To determine the factors that affect patient
outcome, Cox regression analysis was performed (Table III). Univariate analysis revealed
that age (P=0.001), tumor size (P<0.001), MG association
(P=0.042), WHO type (P=0.046), Masaoka stage (P=0.016) and surgical
procedure (P=0.003) significantly affected the OS rate of patients.
Multivariate analysis revealed that age (P=0.013) and MG
association (P=0.037) significantly affected OS rate of
patients.
 | Table III.Univariate and multivariate analysis
for patients that underwent extended thymectomy for thymoma
treatment. |
Table III.
Univariate and multivariate analysis
for patients that underwent extended thymectomy for thymoma
treatment.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | P-value | RR | 95% CI | P-value | RR | 95% CI |
|---|
| Gender |
0.299 | 2.020 | 0.536–7.619 | 0.512 | – | – |
| Agea |
0.001 | 1.138 | 1.055–1.228 | 0.032 | 1.097 | 1.010–1.192 |
| Tumor
sizeb | <0.001 | 2.288 | 1.491–3.510 | 0.052 | – | – |
| MG association |
0.042 | 3.974 | 1.053–14.991 | 0.042 | 0.167 | 0.037–0.940 |
| WHO
classificationc |
0.046 | 2.054 | 1.011–4.172 | 0.084 | – | – |
| Masaoka
staged |
0.009 | 2.400 | 1.239–4.648 | 0.541 | – | – |
| Surgical
proceduree |
0.003 | 0.145 | 0.041–0.510 | 0.574 | – | – |
| Radiotherapy |
0.026 | 0.175 | 0.038–0.814 | 0.322 | – | – |
Discussion
Although, thymomas are the most common adult tumors
in the anterior mediastinal compartment, it is a rare neoplasm with
an overall incidence of 0.13 cases per 100,000 individuals per year
(13–15). A recent study based on a large cohort
of patients with thymomas revealed that the 10-year OS rate was
0.73 (95% confidence interval 0.69–0.75) (16). The same study reported that Masaoka
stages III + IV, incomplete resection and non-thymoma histology (a
diagnosis of thymic carcinoma or neuroendocrine thymic tumors) had
a significant affect in increasing recurrence and in worsening
survival rates of patients (16).
Therefore, although the OS rate was relatively high, a cure for
advanced thymomas remains a challenge (17,18).
Surgical resection is considered as the primary treatment of
thymoma, with a reported operative mortality of 2% and a
complication rate of ~20% (5).
Complications may include blood vessel damage, postoperative
myasthenic crisis and pain, and wound infection (5). Although the role of surgical resection
in the treatment of thymoma is clear, the factors that affect the
outcome of patients following surgery are not fully determined. MG
is the most commonly associated paraneoplastic disease in thymoma
patients, but the affects of MG post-operatively remain unclear.
The present study compared the outcome of patients with or without
MG following surgery. The present results clearly demonstrated that
MG is an important factor that should be considered in the
management of patients with thymoma.
Thymomas usually occur in the sixth decade of life
and have no significant gender predilection (19–22). In
the present study, the median age of the patients was 54.6 years,
which was 5 years younger than in other studies (19). The ratio of men to women in the
present study was 1/1.4, which was within the range of results from
other studies (19,20). Masaoka stages are characterized by the
degree of invasion by the tumor through the capsule into
surrounding tissue structures, and is an important prognostic
factor in determining the most beneficial therapeutic method for a
patient (11). In the 104 thymoma
patients in the present study, there were 16 (15.4%) patients with
Masaoka stage III + IV. The patients all had successful surgical
resection of the entire thymus, mediastinal fat tissue between the
two phrenic nerves and adjacent organs, including the lung (n=6),
pericardium (n=5), left brachiocephalic (n=3) and partial superior
vena cava (n=2). However, there remained 3 recurrent cases at stage
III (3/14; 21.4%). Detterbeck (23)
reported that the average recurrence rate at stage III was 30%,
which is higher compared with the present results. The average
tumor size increased with the elevated Masaoka stage in the present
study, and 5-year OS rates were 90.0 and 68.0% at stages I + II and
stages III + IV, respectively, and the survival rate at stages III
+ IV was slightly lower compared with stages I + II.
MG occurs in 15–60% of patients with thymoma,
according to various studies (24–26). In
the present results, thymoma patients with MG accounted for 36.5%
of the patients. In the majority of studies, the incidence of MG in
thymoma patients is the highest in WHO type B2 (21,27,28). In
the present study, the incidence of MG in type A, AB, B1, B2 and B3
was 2.6, 13.2, 13.2, 52.6 and 18.4%, respectively. The prevalence
of MG was significantly different in type B2 thymoma compared with
that in other types of thymoma. In the 38 patients that had thymoma
with MG, muscle weakness relief following surgery was 77.8%, which
is similar to the 81.1% reported by Yu et al (29). However, 1 out of 66 (1.5%) patients
without preoperative MG developed postoperative MG in the present
study. This percentage was significantly lower compared with the
4.8% reported by Sun et al (30). During the surgery that the present
patients underwent, ET was performed and the ectopic thymus was
removed. In the MG group, the paraneoplastic thymus in 31.5%
patients was hyperplastic, while it was only hyperplastic in 6.1%
of patients in the group without MG. This suggests that MG
development does not always result from thymoma. Therefore, the
paraneoplastic thymus or ectopic thymic tissue may be important in
MG development, which should be addressed in future studies.
There is controversy regarding whether or not MG
affects the prognosis of thymomas (26,31). This
debate is critical to guide future management of patients with
thymoma. In the present study, survival analysis revealed that the
5-year survival rate of thymoma patients with MG (76.0%) was
reduced compared with patients without MG (89.1%) (P=0.026). In
addition, multivariate analysis by Cox regression demonstrated that
MG (P=0.042) and age (P=0.032) were independent prognostic factors
for survival. However, in a previous study performed in 228
patients, it was revealed that the prognosis was similar between
patients with (90.0%) and without MG (89.3%) (31). The variance in the present and
previous results are probably due to varying severities of MG, and
different Masaoka stage, diagnosis methods and treatment
procedures.
In summary, the present results clearly demonstrate
that ET is a reliable method for the treatment of thymoma.
Similarly to previous reports, the incidence of MG was
significantly different in WHO type B2 thymoma compared with other
types of thymoma. In addition, it is also possible that MG in
certain thymoma patients was not caused by thymoma, but by the
paraneoplastic thymus. Long-term survival may be expected not only
for patients at early Masaoka stages, but also for patients without
MG, and the prognosis of patients with thymoma with MG is poorer
compared with patients without MG. Therefore, the present findings
provide useful information for the future management of patients
with thymomas.
Glossary
Abbreviations
Abbreviations:
|
MG
|
myasthenia gravis
|
|
ET
|
extended thymectomy
|
|
WHO
|
World Health Organization
|
|
VATS
|
video-assisted thoracoscopic
surgery
|
|
OS
|
overall survival
|
References
|
1
|
Ruffini E and Venuta F: Management of
thymic tumors: A European perspective. J Thorac Dis. 6(Suppl 2):
S228–S237. 2014.PubMed/NCBI
|
|
2
|
Engels EA: Epidemiology of thymoma and
associated malignancies. J Thorac Oncol. 5(10 Suppl 4): S260–S265.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baas P and Rhodius R: Thymoma update 2011.
Eur J Cancer. 47(Suppl 3): S315–S316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koppitz H, Rockstroh JK, Schüller H,
Standop J, Skowasch D, Müller-Hermelink HK and Schmidt-Wolf IG:
State-of-the-art classification and multimodality treatment of
malignant thymoma. Cancer Treat Rev. 38:540–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Detterbeck FC and Parsons AM: Management
of stage I and II thymoma. Thorac Surg Clin. 21:59–67, vi–vii.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaiser LR: Surgical treatment of thymic
epithelial neoplasms. Hematol Oncol Clin North Am. 22:475–488.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suster S and Moran CA: Thymoma
classification: Current status and future trends. Am J Clin Pathol.
125:542–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas CR, Wright CD and Loehrer PJ:
Thymoma: State of the art. J Clin Oncol. 17:2280–2289.
1999.PubMed/NCBI
|
|
9
|
Fujii Y: Thymus, thymoma and myasthenia
gravis. Surg Today. 43:461–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Regnard JF, Magdeleinat P, Dromer C,
Dulmet E, de Montpreville V, Levi JF and Levasseur P: Prognostic
factors and long-term results after thymoma resection: A series of
307 patients. J Thorac Cardiovasc Surg. 112:376–384. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masaoka A: Staging system of thymoma. J
Thorac Oncol. 5(10 Suppl 4): S304–S312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ströbel P, Marx A, Zettl A and
Müller-Hermelink HK: Thymoma and thymic carcinoma: An update of the
WHO Classification 2004. Surg Today. 35:805–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bushan K, Sharma S and Verma H: A review
of thymic tumors. Indian J Surg Oncol. 4:112–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelly RJ: Thymoma versus thymic carcinoma:
Differences in biology impacting treatment. J Natl Compr Canc Netw.
11:577–583. 2013.PubMed/NCBI
|
|
15
|
Lamarca A, Moreno V and Feliu J: Thymoma
and thymic carcinoma in the target therapies era. Cancer Treat Rev.
39:413–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruffini E, Detterbeck F, Van Raemdonck D,
Rocco G, Thomas P, Weder W, Brunelli A, Evangelista A and Venuta F:
European Association of Thoracic Surgeons (ESTS) Thymic Working
Group: Tumours of the thymus: A cohort study of prognostic factors
from the European Society of Thoracic Surgeons database. Eur J
Cardiothorac Surg. 46:361–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lucchi M and Mussi A: Surgical treatment
of recurrent thymomas. J Thorac Oncol. 5(10 Suppl 4): S348–S351.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riely GJ and Huang J: Induction therapy
for locally advanced thymoma. J Thorac Oncol. 5(10 Suppl 4):
S323–S326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gadalla SM, Rajan A, Pfeiffer R,
Kristinsson SY, Björkholm M, Landgren O and Giaccone G: A
population-based assessment of mortality and morbidity patterns
among patients with thymoma. Int J Cancer. 128:2688–2694. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagawa K, Asamura H, Matsuno Y, Suzuki
K, Kondo H, Maeshima A, Miyaoka E and Tsuchiya R: Thymoma: A
clinicopathologic study based on the new World Health Organization
classification. J Thorac Cardiovasc Surg. 126:1134–1140. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel S, Macdonald OK, Nagda S, Bittner N
and Suntharalingam M: Evaluation of the role of radiation therapy
in the management of malignant thymoma. Int J Radiat Oncol Biol
Phys. 82:1797–1801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rena O, Papalia E, Oliaro A, Ruffini E,
Filosso P, Novero D, Maggi G and Casadio C: Does adjuvant radiation
therapy improve disease-free survival in completely resected
Masaoka stage II thymoma? Eur J Cardiothorac Surg. 31:109–113.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Detterbeck FC: Evaluation and treatment of
stage I and II thymoma. J Thorac Oncol. 5(10 Suppl 4): S318–S322.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo K and Monden Y: Thymoma and
myasthenia gravis: A clinical study of 1,089 patients from Japan.
Ann Thorac Surg. 79:219–224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
López-Cano M, Ponseti-Bosch JM,
Espin-Basany E, Sánchez-Garcia JL and Armengol-Carrasco M: Clinical
and pathologic predictors of outcome in thymoma-associated
myasthenia gravis. Ann Thorac Surg. 76:1643–1649, disc 1649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Margaritora S, Cesario A, Cusumano G,
Meacci E, D'Angelillo R, Bonassi S, Carnassale G, Porziella V,
Tessitore A, Vita ML, et al: Thirty-five-year follow-up analysis of
clinical and pathologic outcomes of thymoma surgery. Ann Thorac
Surg. 89:245–252; discussion 252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kondo K, Yoshizawa K, Tsuyuguchi M, Kimura
S, Sumitomo M, Morita J, Miyoshi T, Sakiyama S, Mukai K and Monden
Y: WHO histologic classification is a prognostic indicator in
thymoma. Ann Thorac Surg. 77:1183–1188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okumura M, Ohta M, Tateyama H, Nakagawa K,
Matsumura A, Maeda H, Tada H, Eimoto T, Matsuda H and Masaoka A:
The World Health Organization histologic classification system
reflects the oncologic behavior of thymoma: A clinical study of 273
patients. Cancer. 94:624–632. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu S, Li F, Chen B, Lin J, Yang M, Fu X,
Li J and Bu B: Eight-year follow-up of patients with myasthenia
gravis after thymectomy. Acta Neurol Scand. 131:94–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun XG, Wang YL, Liu YH, Zhang N, Yin XL
and Zhang WJ: Myasthenia gravis appearing after thymectomy. J Clin
Neurosci. 18:57–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu L, Zhang XJ, Ma S, Jing Y, Li F and
Krasna MJ: Different characteristics of thymomas with and without
myasthenia gravis. Ann Surg Oncol. 19:94–98. 2012. View Article : Google Scholar : PubMed/NCBI
|