Introduction
Prostate cancer is the second most common cause of
cancer-related mortality in American men. The androgen receptor
(AR) is a ligand-activated steroid hormone receptor that regulates
normal prostate development and function (1). It is also critical in the development
and progression of prostate cancer (2). Current therapeutic strategies for
prostate cancer, such as androgen ablation therapy, inhibit AR
function (3). A combination of
androgen synthesis suppression and AR inhibition may be used as a
more aggressive form therapy (4).
Therefore, identification of the chemical agents and mechanisms
that inhibit AR signaling warrant further investigation for the
development of novel prostate cancer therapeutics.
Curcumin is a non-nutritive yellow pigment found in
turmeric, a rhizome-derivative of the plant Curcuma longa
Linn. Numerous studies have demonstrated the anticancer activity of
curcumin and curcumin analogues in animal models (5–10), as well
as the effects on cell growth and apoptosis in vitro
(11–19). However, it should be noted that the
clinical efficacy of curcumin is limited, possibly due to its low
bioavailability (20–22).
In our previous study, we synthesized a series of 12
pyridine analogues of curcumin with cyclohexanone, cyclopentanone,
tetrahydropyran-4-one or tetrahydrothiopyran-4-one linkers, and
determined their anticancer activities in cultured human cancer
cells (23). It was determined that
these pyridine analogues exhibited stronger inhibitory effects than
curcumin on the growth of a number of human cancer cell lines,
including human prostate cancer PC-3 cells (23). Therefore, the aim of the present study
was to investigate the effects and mechanisms of several pyridine
curcumin analogues in CWR-22Rv1 human prostate cancer cells, as
CWR-22Rv1 cells exhibit ARs and thus were considered suitable to
investigate AR activity. Group FN showed a stronger inhibitory
effect than groups AN, BN and EN on human prostate cancer cell
growth. We also demonstrated that group FN was a greater inhibitor
of AR activity and testosterone (TT)-induced prostate-specific
antigen (PSA) expression than groups AN, BN, EN and curcumin in
CWR-22Rv1 cells.
Materials and methods
Chemistry
Twelve pyridine analogues of curcumin (Fig. 1) were synthesized by coupling the
appropriate substituted benzaldehyde with cyclohexanone,
cyclopentanone, tetrahydropyran-4-one or tetrahydrothiopyran-4-one,
as previously described (23).
Characterization of the compounds
(2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone (AN1/2/3),
(2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone (BN1/2/3),
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one
(EN1/2/3) and
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one
(FN1/2/3) was previously described in detail (23).
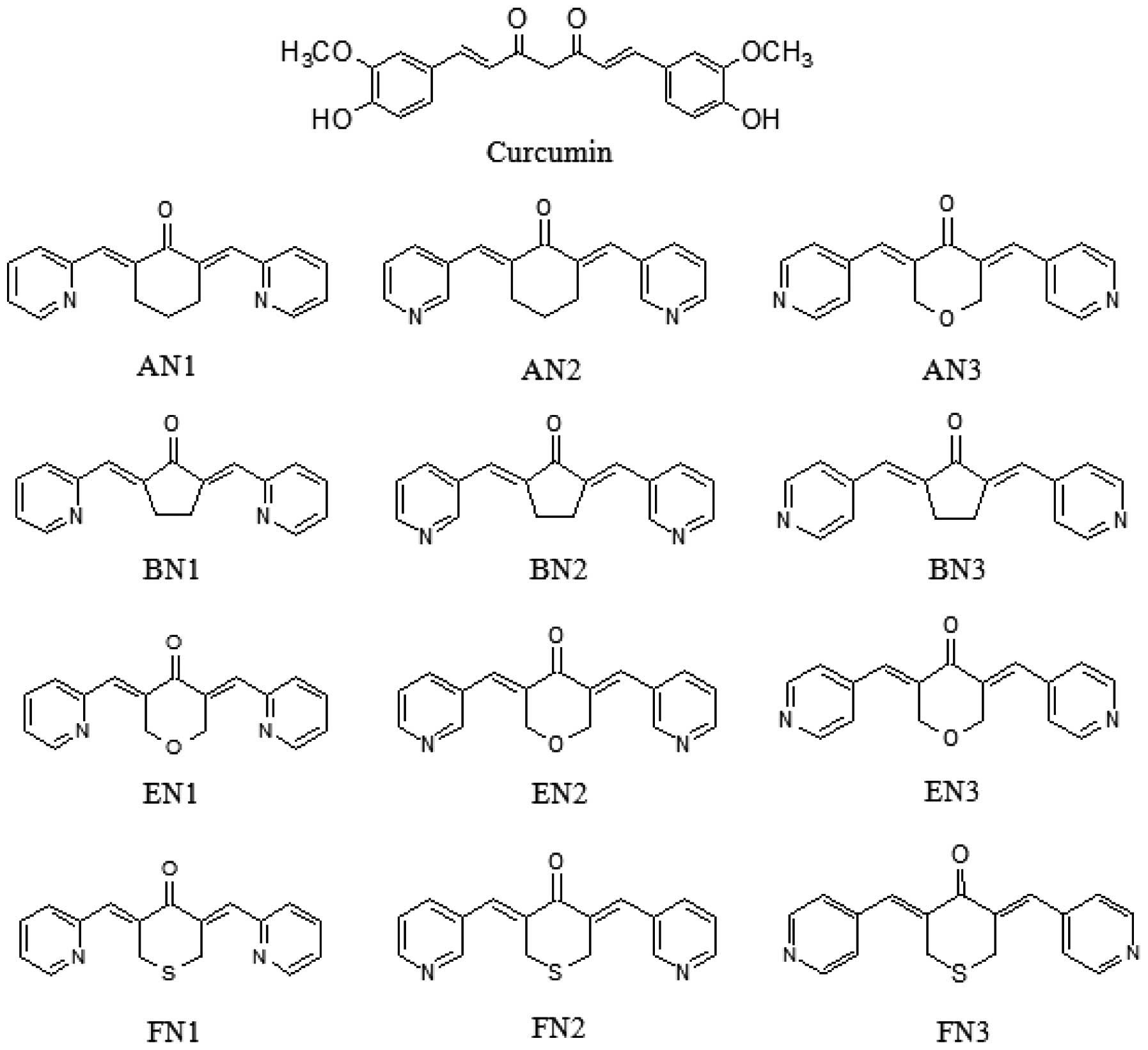 | Figure 1.Structures of curcumin and its
analogues. AN1/2/3,
(2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone; BN1/2/3,
(2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone; EN1/2/3,
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one;
FN1/2/3,
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one. |
Cell culture and reagents
CWR-22Rv1 cells were acquired from the American Type
Culture Collection (Rockville, MD, USA). RPMI-1640 tissue culture
medium, penicillin-streptomycin, L-glutamine and fetal bovine serum
(FBS) were from Gibco (Thermo Fisher Scientific, Waltham, MA, USA).
CWR-22Rv1 cells were maintained in RPMI-1640 culture medium, and
the medium was supplemented with 10% FBS, penicillin (100
U/ml)-streptomycin (100 µg/ml) and L-glutamine (300 µg/ml). Cells
were grown at 37°C in a humidified atmosphere of 5% CO2
and were passaged two times per week. Analogues were dissolved in
dimethylsulfoxide (DMSO; concentration, 100%; Sigma-Aldrich, St.
Louis, MO, USA). A final concentration of 0.1% DMSO was used in all
experiments.
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay
CWR-22Rv1 cells were seeded at a density of
2×104 cells/ml of medium in a 96-well plate (0.2
ml/well) and incubated for 24 h. The cells were then treated with
various concentrations (0.5, 1, 2 and 5µM) of curcumin analogues
for 72 h. After treatment, 5 mg/ml MTT (Sigma-Aldrich) was added to
each well of the plate and incubated for 1 h. After careful removal
of the medium, 0.1 ml DMSO was added to each well and absorbance
was recorded on a microplate reader (Infinite® 200 PRO;
Tecan, Männedorf, Switzerland) at 550 nm. The number of viable
cells after each treatment was determined using a hemacytometer
(Bright-line #1475; Thermo Fisher Scientific) under a light
microscope (BH-2; Olympus Corporation, Tokyo, Japan). Cell
viability was determined by performing a trypan blue exclusion
assay, as follows: 80 µl of cell suspension was mixed with 20 µl of
0.4% trypan blue stain solution (Sigma-Aldrich) for 2 min. Blue
cells were counted as dead cells and the cells that did not absorb
dye were counted as live cells.
AR luciferase reporter assay
AR transcriptional activity was measured by
performing an AR luciferase reporter gene expression assay. An AR
luciferase construct was stably transfected into CWR-22Rv1 cells to
generate a single stable clone, CWR-22Rv1/AR, which was used in the
present study. Briefly, CWR22-Rv-1 cells cultured in 10% FBS
RPMI-1640 medium were infected with lentivirus carrying Cignal
Lenti AR reporter (catalog no. CLS-8019L; Qiagen, Inc., Valencia,
CA, USA) in medium containing 8 µg/ml Polybrene (Sigma-Aldrich).
Following incubation for 6 h, the culture medium was replaced with
fresh 10% RPMI-1640 medium. Cells expressing stable AR luciferase
reporter were selected using puromycin (5 µg/ml) three days after
infection for 1 week. Selected cells (CWR-22Rv1/AR) were then used
for the reporter assay for AR activity.
CWR-22Rv1/AR cells were seeded at a density of
0.1×105 cells/ml of medium for 24 h. Then the medium was
changed to RPMI-1640 without FBS, and the cells were treated with
DMSO solvent as the vehicle (control) or with TT (100 nM;
Sigma-Aldrich) alone or in combination with curcumin and curcumin
analogues (1 µM) for 24 h. The luciferase activities were measured
using luciferase assay kits from Promega Corporation (Madison, WI,
USA; catalog no., E1531). Briefly, the treated cells were washed
with ice-cold phosphate-buffered saline (PBS; Gibco; Thermo Fisher
Scientific, Inc.), harvested in reporter lysis buffer (Thermo
Fisher Scientific, Inc.) and centrifuged for 5 min at 193 × g.
Aliquots (10 µl) of the supernatants were measured for luciferase
activity using a luminometer (Multiskan FC; Thermo Fisher
Scientific). The luciferase activity was normalized against protein
concentration and expressed as percent of luciferase activity in
the control cells. The protein concentration levels were determined
using protein assay reagents (Reagent B, catalog no., 500-0114;
Reagent A, catalog no., 500-0113; Reagent S, catalog no., 500-0115;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocol.
Western blot analysis
CWR-22Rv1 cells were seeded in 100-mm culture dishes
(10 ml/dish) at a density of 1×105 cells/ml medium and
incubated for 24 h. The medium was changed to RPMI-1640 without
FBS, and the cells were then treated with vehicle, 100 nM TT alone
or together with 1 µM curcumin, AN1, BN1, EN1 or FN1 for 24 h.
Treated CWR-22Rv1 cells were washed with ice-cold PBS and lysed
with 800 µl lysis buffer (10 mM Tris-HCl, pH 8.0, 10 mM EDTA, 150
mM sodium chloride, 1% NP-40, 0.5% SDS, in deionized water). The
resulting homogenates were centrifuged at 193 × g for 15 min at
4°C. The protein concentration of whole cell lysates was determined
using a protein assay kit (Bio-Rad Laboratories, Inc.). Equal
amounts (50 µg) of protein were then resolved on a 10% Criterion
precast gel (Bio-Rad Laboratories, Inc.) and transferred to a PVDF
membrane using a semi-dry transfer system. The membrane was then
probed with a mouse anti-human monoclonal PSA primary antibody
(dilution, 1:10,000; catalog no., CBL252; EMD Millipore, Billerica,
MA, USA). Following hybridization with primary antibody, the
membrane was washed with Tris-buffered saline (Alfa Aesar,
Haverhill, MA, USA) three times, then incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(dilution, 1:5,000; catalog no., sc-2055; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and again washed with Tris-buffered saline
three times. Final detection was performed with enhanced
chemiluminescent reagents (Thermo Fisher Scientific, Inc.). The
extent of protein loading was determined by blotting for β-actin
(mouse anti-human monoclonal antibody; dilution, 1:1,000–5,000;
catalog no., sc-8432; Santa Cruz Biotechnology, Inc.). The membrane
was incubated in stripping buffer (100 mM β-mercaptoethanol, 2%
SDS, 62.5 mM Tris-HCl, pH 6.7) at 50°C for 30 min with occasional
agitation prior to incubation in blocking buffer and re-probing
using anti-β-actin antibody (Santa Cruz Biotechnology, Inc.).
Statistical analysis
A comparative analysis of the TT-induced activation
of curcumin and its analogues was based on a repeated measurement
model. The effects of the treatments were assessed by comparing the
rates of change over time between treatment groups (comparing the
slopes between treatment groups). The analysis of variance (ANOVA)
method with the Tukey-Kramer test was used to compare effects among
the different treatment groups at study completion. All data
analyses were performed using GraphPad InState (GraphPad Software,
Inc., La Jolla, CA, USA). Data are presented as mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of curcumin analogues on
CWR-22Rv1 cell growth
The inhibitory effects of curcumin analogues on the
growth of cultured CWR-22Rv1 cells are presented in Fig. 2. All analogous compounds demonstrated
a stronger inhibitory effect than curcumin (IC50=16.99
µM) (24) as determined by MTT assay.
Among the series of four pyridine analogues of curcumin analyzed in
the present study, the FN and AN groups exhibited the most potent
inhibitory effects on the growth of cultured CWR-22Rv1 cells. The
IC50 values for the FN and AN groups were <1 µM in
CWR-22Rv1 cells, indicating that these compounds were ~20-fold more
active than curcumin (IC50=16.99 µM). The
IC50 values of the series of four pyridine analogues of
curcumin ranged between 0.49 and 4.99 µM, as indicated in Table I. Statistical analysis using ANOVA
demonstrated that the IC50 for the curcumin analogues
were significantly lower than that of curcumin (P<0.001).
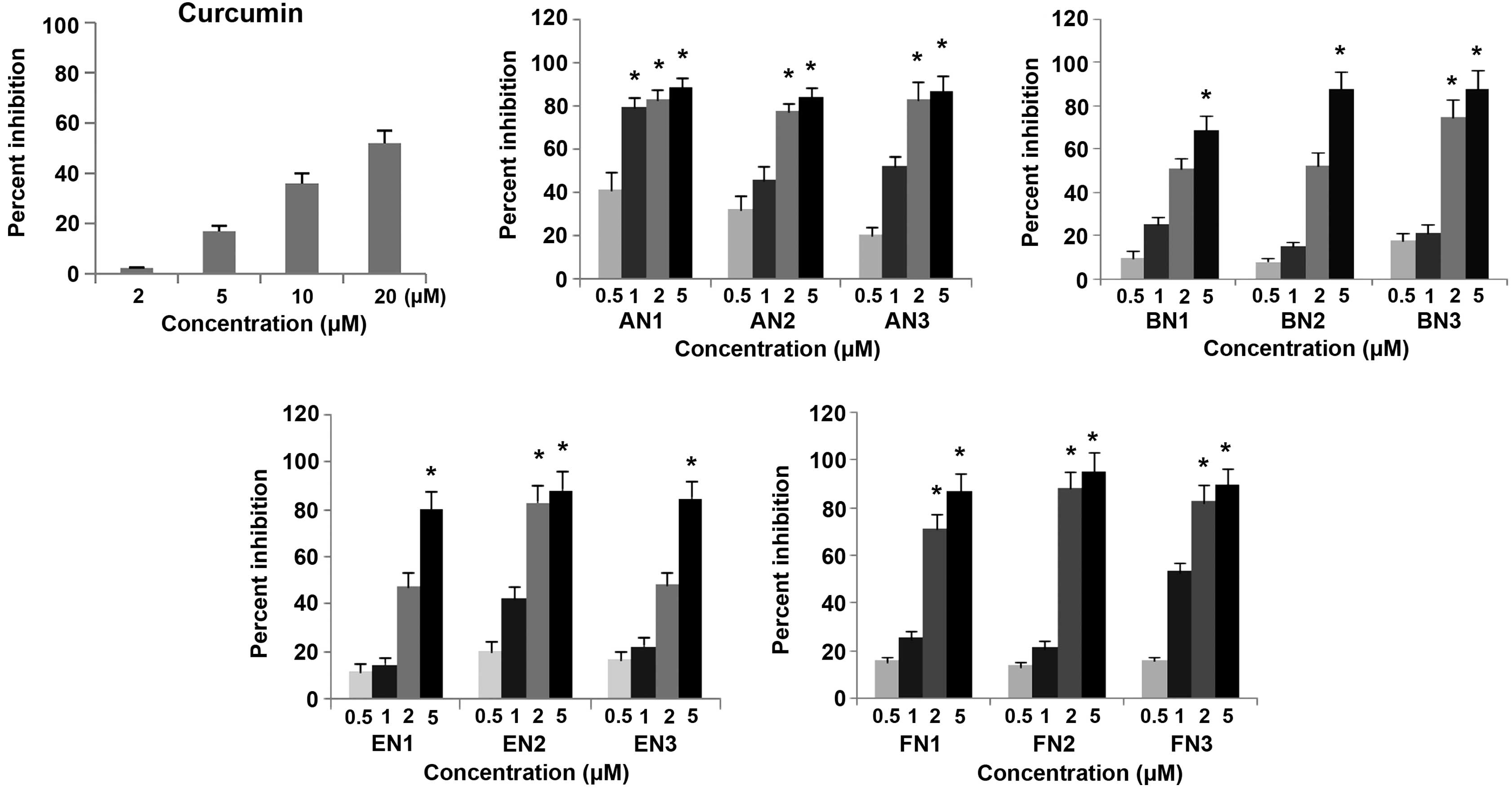 | Figure 2.Inhibitory effects of curcumin
analogues on the growth of the CWR-22Rv1 prostate cancer cell line.
CWR-22Rv1 cells were seeded at a density of 2×104
cells/ml medium in 96-well plates (0.2 ml/well) and incubated for
24 h. The cells were then treated with various concentrations (0.5,
1, 2 and 5µM) of different curcumin and its analogues for 72 h.
Effects of the different compounds on the growth of CWR-22Rv1 cells
were determined by performing a
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay.
Values are presented as the mean ± standard error of the mean from
three separate experiments. *P<0.001 for 20 µM CUR
(IC50) vs. AN, BN, EN and FN. AN1/2/3,
(2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone; BN1/2/3,
(2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone; EN1/2/3,
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one;
FN1/2/3,
(3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one. |
 | Table I.Inhibitory effects of curcumin and its
analogues on the growth of CWR-22Rv1 cells. |
Table I.
Inhibitory effects of curcumin and its
analogues on the growth of CWR-22Rv1 cells.
| Compound | IC50,
µM | P-value |
|---|
| Curcumin | 16.99±2.1 | 0.00092 |
| AN1 | 0.53±0.1 | 0.00065 |
| AN2 | 0.92±0.1 | 0.00071 |
| AN3 | 0.95±0.2 | 0.00065 |
| BN1 | 4.75±0.5 | 0.00095 |
| BN2 | 4.99±0.5 | 0.00096 |
| BN3 | 3.03±0.4 | 0.00083 |
| EN1 | 2.18±0.2 | 0.00079 |
| EN2 | 1.07±0.1 | 0.00080 |
| EN3 | 1.80±0.2 | 0.00082 |
| FN1 | 0.66±0.1 | 0.00072 |
| FN2 | 0.55±0.1 | 0.00073 |
| FN3 | 0.49±0.1 | 0.00061 |
Effects of curcumin and its analogues
on AR activity in CWR-22Rv1/AR cells
An AR-luciferase reporter gene expression assay was
performed in CWR-22Rv1/AR cells to determine the effect of curcumin
and its analogues on TT-induced activation of AR. Cultured
CWR-22Rv1/AR cells were treated with a combination of TT and a
specific curcumin analogue (1 µM) for 24 h. A marginal inhibitory
effect on the TT-induced increase in AR activity was observed in
cultured CWR-22Rv1/AR cells treated with 1 µM curcumin, or the BN
or EN groups, while greater inhibitory effects were observed in
CWR-22Rv1/AR cells treated with 1 µM of the AN or FN groups
(Fig. 3). Statistical analysis using
ANOVA with Tukey's multiple comparison tests demonstrated that AR
activity was significantly lower in cells treated with AN and FN
compounds than in cells treated with curcumin, BN and EN compounds
(P<0.001 for: TT vs. TT + AN, TT + BN, TT + EN or TT + FN; TT +
CUR vs. TT + AN, TT + BN, TT + EN or TT + FN; TT + AN vs. TT + BN,
TT + EN or TT + FN; and TT + BN vs. TT + EN or TT + FN.
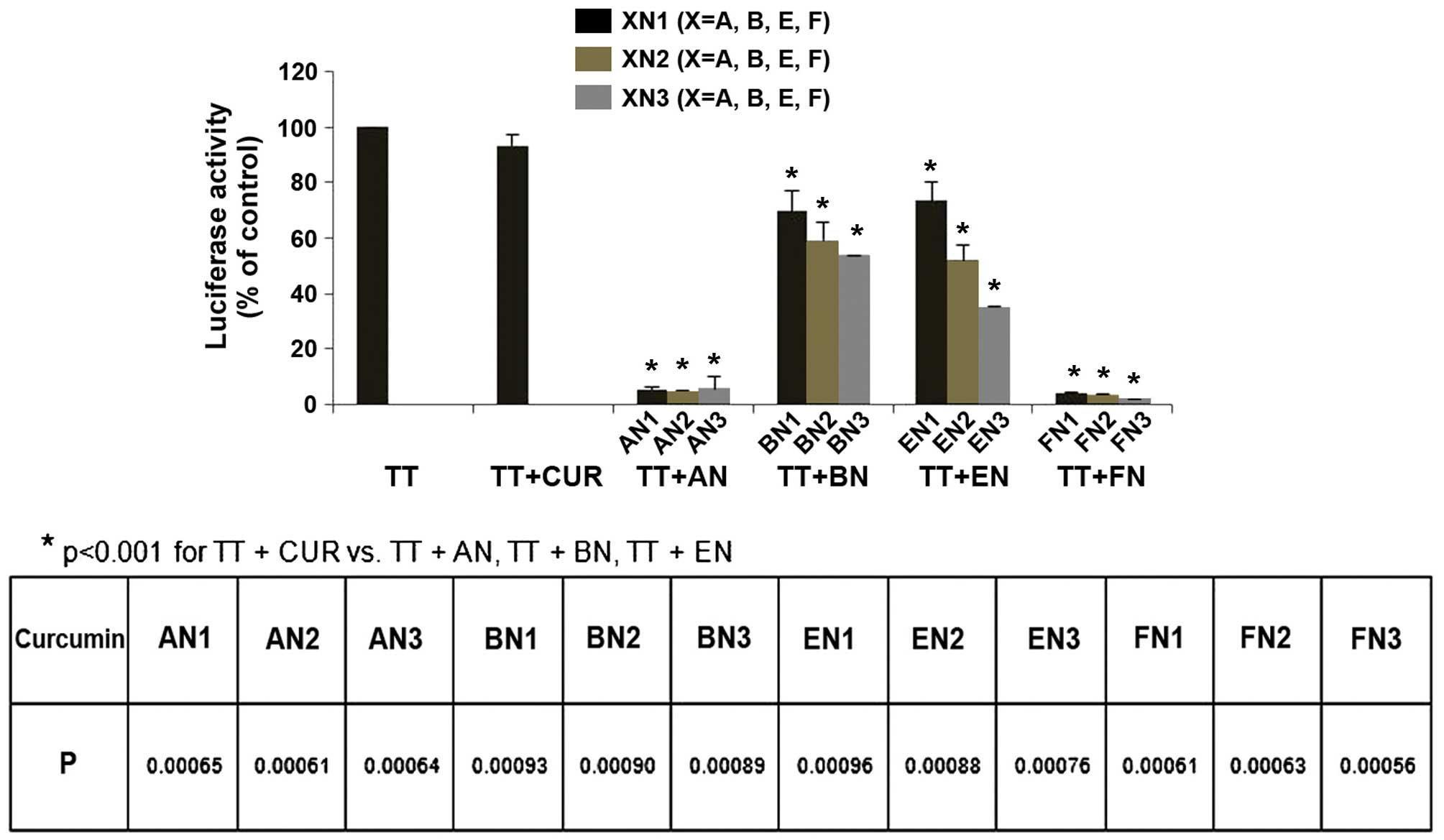 | Figure 3.Effect of CUR analogues on TT-induced
increases in androgen receptor reporter activity in CWR-22Rv1/AR
cells. CWR-22Rv1/AR cells were seeded at a density of
0.1×105 cells/ml of medium for 24 h. Then the medium was
changed to RPMI-1640 without fetal bovine serum, and the cells were
treated with vehicle (control) or TT (100 nM) alone or in
combination with CUR and CUR analogues (1 µM) for 24 h. Luciferase
activity and protein concentration were measured to determine
androgen receptor reporter activity in the CWR-22Rv1/AR cells.
Values are presented as the mean ± standard error of the mean from
three separate experiments. TT, testosterone; CUR, curcumin; AN,
(2E,6E)-2,6-bis(pyridin-n-methylene)cyclohexanone; BN,
(2E,5E)-2,5-bis(pyridin-n-methylene)cyclopentanone; EN,
(3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydropyran-4-one; FN,
(3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydrothiopyran-4-one. |
Effects of curcumin and its analogues
on protein expression of PSA in CWR-22Rv1 cells
PSA protein levels were evaluated by western blot
analysis using an anti-PSA antibody on cultured CWR-22Rv1 cells
treated with TT, curcumin and curcumin analogues AN1, BN1, EN1 or
FN1 for 24 h. Treatment of CWR-22Rv1 cells with AN1 and FN1
resulted in a strong decrease in the level of PSA compared with TT
treatment alone, while the other compounds (curcumin, BN1 and EN1)
were less active (Fig. 4). The
current results indicate that the effects of AN1 and FN1 on
CWR-22Rv1 cells are associated with a decrease in PSA protein
expression in TT-induced cells.
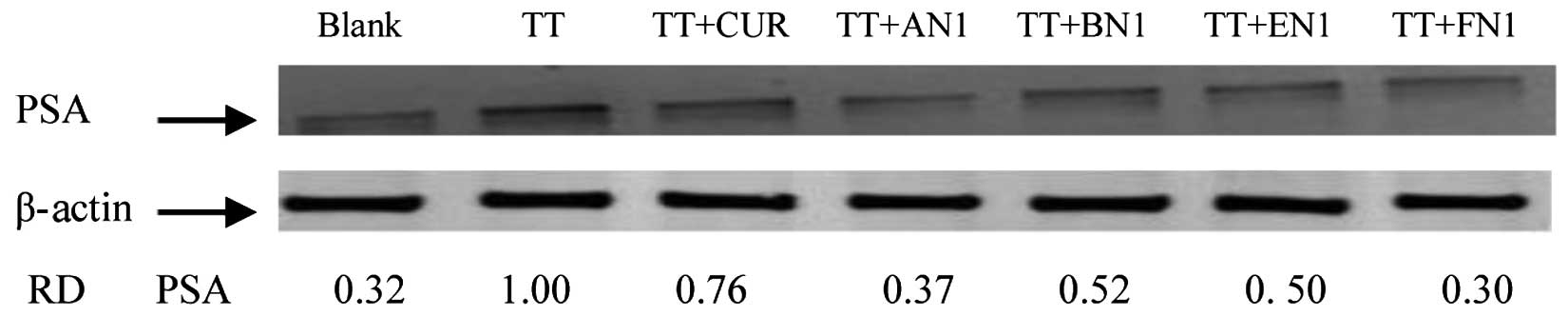 | Figure 4.Effect of curcumin and its analogues
on TT-induced increases in PSA formation in CWR-22Rv1 cells.
CWR-22Rv1 cells were seeded at a density of 1×105
cells/ml medium in 100-mm culture dishes (10 ml/dish) and incubated
for 24 h. The medium was changed to RPMI-1640 without fetal bovine
serum, and the cells were then treated with vehicle, or 100 nM TT
alone or in combination with 1 µM curcumin, AN1, BN1, EN1 or FN1
for 24 h. PSA expression was determined by western blot analysis
with anti-PSA antibody. The extent of protein loading was
determined by blotting for β-actin, and the levels of PSA in
western blots were analyzed by optical density measurements and
normalized for β-actin to obtain the RD for the various samples. RD
values represent individual selected bands. Representative blots
from three experiments are shown. PSA, prostate-specific antigen;
TT, testosterone; AN,
(2E,6E)-2,6-bis(pyridin-n-methylene)cyclohexanone; BN,
(2E,5E)-2,5-bis(pyridin-n-methylene)cyclopentanone; EN,
(3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydropyran-4-one; FN,
(3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydrothiopyran-4-one; RD,
relative optical density. |
Discussion
Our previous study reported the synthesis and
evaluation of five series of curcumin-related compounds (a total of
61 compounds) for their inhibitory effects on cultured prostate,
pancreatic and colon cancer cells (23). The present study focused on pyridine
analogues of curcumin, which are a subset of active compounds from
our previous study. The present study provides the first evidence
that pyridine analogues of curcumin inhibit AR activity in prostate
cancer cells cultured in vitro.
The twelve pyridine analogues of curcumin (AN, BN,
EN and FN groups) exhibited stronger anticancer activities than
curcumin in cultured CWR-22Rv1 human prostate cancer cells in the
current study. Among the curcumin analogues, the FN group
demonstrated the strongest inhibitory effect CWR-22Rv1 cell growth
compared with the other curcumin analogues. In addition, the
present study observed that all curcumin analogues analyzed were
more potent inhibitors of AR in CWR-22Rv1 cells than curcumin.
Group FN was the most potent of the four curcumin analogues for
inhibiting the activation of AR.
The natural product curcumin (diferuloylmethane) has
been demonstrated to inhibit various targets in prostate epithelial
cells, particularly in cancer formation and progression. Among
these targets are transcription factors, receptors, intracellular
kinases, cytokines and growth factors (25). The effect of curcumin on AR and its
target PSA, using both endogenously expressed AR in LNCaP cells and
ectopically expressed AR in PC-3 cells, has been demonstrated by
several independent studies (26–27).
However, in these reports, curcumin was used at relatively high
concentrations, typically at >20 µM. It has previously been
reported that curcumin has poor bioavailability in animal models
and humans (28). This limitation has
led researchers to generate a variety of synthetic analogues of
curcumin, and investigate their capability to affect a number of
molecular pathways implicated in tumorigenesis and cancer
progression (29–32). Typical structure modifications include
the introduction of substituents on the phenyl rings and
modifications of the length of the linker between the phenyl rings.
The addition of two bulky side chains on the phenyl rings of
curcumin analogues has been shown to inhibit AR function (33), and diketone or enol-ketone
modification of the linker also inhibits the expression of AR
(34). The present study demonstrated
that replacing the phenyl ring with pyridine significantly enhanced
the inhibitory effect of curcumin analogues on the AR. Furthermore,
curcumin analogues with pyridine rings and cyclohexanone or
tetrahydrothiopyran-4-one linkers exhibited a more potent
inhibitory effect on AR than analogues with cyclopentanone or
tetrahydropyran-4-one linkers. These results indicate that both the
distal rings and the linker are important for the inhibitory effect
of curcumin analogues on AR activity.
During the past decade, numerous curcumin analogues
have been synthesized to improve the stability and anticancer
activity of curcumin. Our previous study and another previous study
indicated that cyclohexanone-containing analogues of curcumin have
more potent anticancer activities than curcumin (23,24).
Further modification of cyclohexanone-containing curcumin analogues
by replacing the ortho-hydroxylmethoxyl benzene with a pyridine
ring strongly enhanced the anticancer activities of these curcumin
analogues (23). In the present
study, pyridine analogues of curcumin composed of a five-carbon
linker with a cyclohexanone, cyclopentanone, tetrahydropyran-4-one
or tetrahydrothiopyran-4-one moiety were synthesized, and evaluated
for their effects on growth inhibition in CWR-22Rv1 cells. The
present study identified that analogues with
tetrahydrothiopyran-4-one (FN) as the linker were more potent
inhibitors than those with tetrahydropyran-4-one (EN),
cyclohexanone (AN) or cyclopentanone (BN). Earlier studies revealed
that the six-membered cyclohexanone ring system is, in general,
superior to the five-membered cyclopentanone system for inhibiting
the growth of several cancer cell lines (23,24). The
current results confirmed this structure activity association, as
pyridine cyclohexanone curcumin analogues displayed stronger
inhibitory activities than pyridine cyclopentanone curcumin
analogues. The present study also demonstrated that the replacement
of the cyclohexanone core with tetrahydrothiopyran-4-one further
increased the anticancer activities, while replacement of
cyclohexanone with tetrahydropyran-4-one did not. This indicates
that the introduction of sulfur in the linker may enhance the
anticancer activities of pyridine curcumin analogues. Additionally,
compounds with a nitrogen heteroatom in the ortho position of the
pyridine ring typically exhibited activities than compounds with a
nitrogen heteroatom in the meta or para position of the pyridine
ring.
In conclusion, the results of the present study
demonstrated that pyridine analogues of curcumin with
tetrahydrothiopyran-4-one as the linker (FN group compounds)
possessed more potent inhibitory effects on cultured prostate
cancer cells compared with those using cyclohexanone,
cyclopentanone or tetrahydropyran-4-one as the linker. The strong
effects of the FN group compounds on prostate cancer cells were
associated with inhibition of AR activity. The present identified
FN group compounds as leading compounds for the further development
of novel anti-prostate cancer drugs that target AR signaling, which
is important for the survival of prostate cancer cells. Thus, FN
compounds warrant additional studies using suitable animal
models.
Acknowledgements
The present study was supported by the China
National Science Foundation Grants (grant no. 81272452), the PhD
Start-up Fund of Natural Science Foundation of Guangdong Province
(grant no. 2014A030310329) and the Medical Scientific Research
Foundation of Guangdong Province (grant no. B2014072).
References
|
1
|
Gao W, Bohl CE and Dalton JT: Chemistry
and structural biology of androgen receptor. Chem Rev.
105:3352–3370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klotz L: Hormone therapy for patients with
prostate carcinoma. Cancer. 88(12 Suppl): S3009–S3014. 2000.
View Article : Google Scholar
|
|
4
|
Simmons MN and Klein EA: Combined androgen
blockade revisited: Emerging options for the treatment of
castration-resistant prostate cancer. Urology. 73:697–705. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuttan R, Bhanumathy P, Nirmala K and
George MC: Potential anticancer activity of turmeric (Curcuma
longa). Cancer Lett. 29:197–202. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang MT, Smart RC, Wong CQ and Conney AH:
Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and
ferulic acid on tumor promotion in mouse skin by
12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 48:5941–5946.
1988.PubMed/NCBI
|
|
7
|
Huang MT, Wang ZY, Georgiadis CA, Laskin
JD and Conney AH: Inhibitory effects of curcumin on tumor
initiation by benzo[a]pyrene and 7, 12-dimethylbenz[a]anthracene.
Carcinogenesis. 13:2183–2186. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang MT, Lou YR, Ma W, Newmark HL, Reuhl
KR and Conney AH: Inhibitory effects of dietary curcumin on
forestomach, duodenal, and colon carcinogenesis in mice. Cancer
Res. 54:5841–5847. 1994.PubMed/NCBI
|
|
9
|
Rao CV, Rivenson A, Simi B and Reddy BS:
Chemoprevention of colon carcinogenesis by dietary curcumin, a
naturally occurring plant phenolic compound. Cancer Res.
55:259–266. 1995.PubMed/NCBI
|
|
10
|
Leite KR, Chade DC, Sanudo A, Sakiyama BY,
Batocchio G and Srougi M: Effects of curcumin in an orthotopic
murine bladder tumor model. Int Braz J Urol. 35:599–606; discussion
606–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: Potential anticancer agents. Med Res Rev. 30:818–860.
2010.PubMed/NCBI
|
|
12
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teiten MH, Gaascht F, Eifes S, Dicato M
and Diederich M: Chemopreventive potential of curcumin in prostate
cancer. Genes Nutr. 5:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bill MA, Bakan C, Benson DM Jr, Fuchs J,
Young G and Lesinski GB: Curcumin induces proapoptotic effects
against human melanoma cells and modulates the cellular response to
immunotherapeutic cytokines. Mol Cancer Ther. 8:2726–2735. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johnson SM, Gulhati P, Arrieta I, Wang X,
Uchida T, Gao T and Evers BM: Curcumin inhibits proliferation of
colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer
Res. 29:3185–3190. 2009.PubMed/NCBI
|
|
16
|
Piantino CB, Salvadori FA, Ayres PP, Kato
RB, Srougi V, Leite KR and Srougi M: An evaluation of the
anti-neoplastic activity of curcumin in prostate cancer cell lines.
Int Braz J Urol. 35:354–360; discussion 361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo CT, Chen BC, Yu CC, Weng CM, Hsu MJ,
Chen CC, Chen MC, Teng CM, Pan SL, Bien MY, et al: Apoptosis
signal-regulating kinase 1 mediates denbinobin-induced apoptosis in
human lung adenocarcinoma cells. J Biomed Sci. 16:432009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sahu RP, Batra S and Srivastava SK:
Activation of ATM/Chk1 by curcumin causes cell cycle arrest and
apoptosis in human pancreatic cancer cells. Br J Cancer.
100:1425–1433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thangapazham RL, Sharma A and Maheshwari
RK: Multiple molecular targets in cancer chemoprevention by
curcumin. AAPS J. 8:E443–E449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
23
|
Wei X, Du ZY, Zheng X, Cui XX, Conney AH
and Zhang K: Synthesis and evaluation of curcumin-related compounds
for anticancer activity. Eur J Med Chem. 53:235–245. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou DY, Ding N, Van Doren J, Wei XC, Du
ZY, Conney AH, Zhang K and Zheng X: Effects of curcumin analogues
for inhibiting human prostate cancer cells and the growth of human
PC-3 prostate xenografts in immunodeficient mice. Biol Pharm Bull.
37:1029–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB: Prostate cancer and curcumin:
Add spice to your life. Cancer Biol Ther. 7:1436–1440. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura K, Yasunaga Y, Segawa T, Ko D,
Moul JW, Srivastava S and Rhim JS: Curcumin down-regulates AR gene
expression and activation in prostate cancer cell lines. Int J
Oncol. 21:825–830. 2002.PubMed/NCBI
|
|
27
|
Tsui KH, Feng TH, Lin CM, Chang PL and
Juang HH: Curcumin blocks the activation of androgen and
interlukin-6 on prostate specific antigen expression in human
prostatic carcinoma cells. J Androl. 29:661–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adams BK, Ferstl EM, Davis MC, Herold M,
Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA,
Rickles FR, et al: Synthesis and biological evaluation of novel
curcumin analogs as anti-cancer and anti-angiogenesis agents.
Bioorg Med Chem. 12:3871–3883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Basile V, Ferrari E, Lazzari S, Belluti S,
Pignedoli F and Imbriano C: Curcumin derivatives: Molecular basis
of their anti-cancer activity. Biochem Pharmacol. 78:1305–1315.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishida J, Ohtsu H, Tachibana Y, Nakanishi
Y, Bastow KF, Nagai M, Wang HK, Itokawa H and Lee KH: Antitumor
agents. Part 214: Synthesis and evaluation of curcumin analogues as
cytotoxic agents. Bioorg Med Chem. 10:3481–3487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohori H, Yamakoshi H, Tomizawa M, Shibuya
M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C,
et al: Synthesis and biological analysis of new curcumin analogues
bearing an enhanced potential for the medicinal treatment of
cancer. Mol Cancer Ther. 5:2563–2571. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou J, Geng G, Shi Q, Sauriol F and Wu
JH: Design and synthesis of androgen receptor antagonists with
bulky side chains for overcoming antiandrogen resistance. J Med
Chem. 52:5546–5550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi Q, Shih CC and Lee KH: Novel
anti-prostate cancer curcumin analogues that enhance androgen
receptor degradation activity. Anticancer Agents Med Chem.
9:904–912. 2009. View Article : Google Scholar : PubMed/NCBI
|


















