Introduction
Acute promyelocytic leukemia (APL) is a distinct
subtype of myeloid leukemia with particular clinical and biological
characteristics, including a high mortality rate in newly diagnosed
patients and the presence of t(15;17)(q22;q12-21), a chromosome
reciprocal translocation between the long arms of chromosomes 15
and 17 (1,2). APL is currently considered to be a
highly curable disease. However, decreasing early mortality (EM)
prior or during induction therapy, and improvement of long-term
stabilization and cure of APL patients remain major causes of
treatment failure in APL (3). The
current risk-stratification system for APL is mainly based on the
white blood cell (WBC) and platelet counts (4). There are numerous reports about the
prognostic impact of additional molecular biomarkers, including
Fms-related tyrosine kinase 3 (FLT3), c-Kit and nucleophosmin, in
APL (5,6). FLT3 mutations have been confirmed to be
a poor prognostic molecular marker (5,7).
The Ets variant 6 (ETV6) gene, which is mapped to
12p13, is a transcription factor of the E26 transformation-specific
(ETS) family that plays a versatile role in hematological
malignancies (8). ETS family members
are essential for hematopoietic processes, including cell
proliferation, differentiation, migration and apoptosis, as well as
tissue remodeling, angiogenesis and hematopoiesis (9). High expression of members of the ETS
family of transcription factors is closely associated with poor
disease-free survival and overall survival (OS) in various
hematological malignancies (8).
Chromosomal translocations involving the ETV6 gene have been
identified in multiple hematological malignancies characterized by
the fusion of ETV6 to different partner genes, including Abelson
murine leukemia viral oncogene homolog 1 (ABL1), acute myeloid
leukemia (AML)1, myelodysplasia syndrome (MSD)1, meningioma
(disrupted in balanced translocation) 1,
1-aminocyclopropane-1-carboxylate synthase 2, Janus kinase 2,
six-twelve leukemia and neurotrophic tyrosine kinase, receptor,
type 3 (10–17).
The present study investigated the presence of ETV6
rearrangement in 258 patients with APL using split-signal
fluorescence in situ hybridization (FISH), and explored its
prognostic impact. The results identified abelson-related gene
(ARG, also known as ABL2) as an ETV6 fusion partner by reverse
transcription-polymerase chain reaction (RT-PCR) analysis in 1 case
of APL. The present study is the second to report an APL patient
with ETV6/ARG rearrangement, following the first case reported by
Iijima et al (18). To the
best of our knowledge, the present study is the first to address
the prognostic implication of ETV6 involvement in patients with
APL.
Materials and methods
Patients and samples
The present study was based on data collected from
258 patients with newly diagnosed APL at Binzhou Medical University
Hospital (Binzhou, China) from May 2000 to August 2011, who had
complete clinical data and sufficient cryopreserved bone marrow
samples for the study. The follow-up deadline was August 2014, with
a median follow-up time of 89.5 months (range, 3–199 months). The
cohort included 154 males and 104 females (median age, 36.88 years;
range, 13–72 years). Diagnosis of APL was established according to
the French-American-British Cooperative Group criteria (19) and World Health Organization
classification (1). The bone marrow
samples were collected at the time of diagnosis. A total of 30
normal marrow donors were also enrolled in the study for comparison
purposes. All patients provided informed consent for the use of
their laboratory data in the present study, which was approved by
the ethics commitee of Binzhou Medical University Hospital.
Bone marrow cell culture and
cytogenetic study
Bone marrow specimens were acquired from patients in
the absence of stimuli caused by drugs such as colony stimulating
factor, and cultivated for 16–24 h prior to harvesting the cells.
Bone marrow cell chromosomes were conventionally prepared and
analyzed by R-banding (20).
Karyotype abnormalities were identified and described according to
the International System for Human Cytogenetic Nomenclature (1995)
(21).
Split-signal FISH analysis
Split-signal FISH analysis was applied to the
chromosome samples of the aforementioned 258 APL patients,
according to the manufacturers protocol. Briefly, bacteria
artificial chromosome (BAC) clones (RP11-434C1 and RP11-525I3)
containing the ETV6 gene (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) were amplified by PCR (15), and DNA was extracted using a plasmid
DNA extraction kit (Qiagen GmbH, Hilden, Germany). Selected BAC
sequences on either side of ETV6 were used as probes, and labeled
with DIG-Nick Translation Mix (Roche Diagnostics, Basel,
Switzerland) and Biotin-Nick Translation Mix (Roche Diagnostics).
The labeled probes (termed DIG525I23 and Bio407P10, respectively)
were then purified with Quick Spin Columns (Roche Diagnostics), and
produced red and green fluorescence signals, respectively, under a
fluorescence microscope (Axio Imager.A1; Zeiss GmbH, Jena,
Germany). All subsequent hybridization procedures were performed as
previously described (15).
Flow cytometry immunophenotyping
Of the 258 patients with APL, 228 bone marrow
samples were sent to Guangzhou Jinyu Medical Science Inspection
Center (Guangzhou, China) for flow cytometry immunophenotyping
analysis, while the remaining samples were analyzed at the Central
Laboratory of Binzhou Medical University Hospital. Bone marrow
samples from APL patients were collected at the time of diagnosis
in tubes containing heparin (Taixing Biological Chemical Co., Ltd.,
Shijiazhuang, China) to avoid coagulation. Flow cytometry analysis
of the bone marrow specimens was performed with a flow cytometer
(FACSCalibur, BD Biosciences, Franklin Lakes, USA), according to
standard immunofluorescence methods (22). Briefly, fluorescein and
phycoerythrin-labeled mouse anti-human monoclonal antibodies
(LSBio; LifeSpan Biosciences, Inc., Seattle, WA, USA) against
myeloperoxidase (MPO), cluster of differentiation (CD)33, CD13,
CD117, CD34 and human leukocyte antigen-antigen D related (HLA-DR)
(5–10 µl) were mixed with heparin-anticoagulated bone marrow
samples (~50 µl) and incubated at 4℃ for 30 min, prior to the
addition of 2 ml cell lysis solution (Shanghai Weiao Biotech Ltd.,
Shanghai, China). The mixture was placed at room temperature for 10
min upon being subjected to vibration, and then washed with
distilled water and phosphate-buffered saline (PBS). Next, 0.5 ml
PBS was added to the samples, which were subsequently analyzed by
flow cytometry.
RT-PCR
The ETV6/ARG fusion gene was detected by RT-PCR in
patients with ETV6 rearrangement. Total RNA was extracted from
mononuclear cells isolated from bone marrow samples of patients
with APL using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Complementary DNA was synthesized by RT using a
reaction mixture that consisted of total RNA, random hexamer
primer, 5X RT buffer, deoxyribonucleotide triphosphates (provided
in the RT-PCR kit; Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China) and M-MLV Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) on a PCR
system. Nested PCR was performed for amplification of the ETV6/ARG
fusion gene, with specific forward primers for ETV6 (F1
5′-ATCGGGAAGACCTGGCTTACA-3′ and F2 5′-TGAAGAGCACGCCATGCCCATTG-3′)
and specific reverse primers for ARG (R1 5′-TGCCTGGGGTTCAACATCAC-3′
and R2 5′-TA-CTGCCTCCAGTCTTGTCT-3′). In the first step of the
reaction, the above primers, which were used as outer primers, may
bind to alternative, similar primer binding sites, potentially
resulting in multiple products; however, only one of those would
carry the intended sequence. In the second step of the reaction,
inter primers were used, which were designed according to the
sequences of the preceding primers. The inter primers (ETV6 F1
5′-GGGAAGACCTGGCTT-3′ and F2 5′-AGAGCACGCCATGCCCA-3′; and ARG R1
5′-CTGGGGTTCAACAT-3′ and R2 5′-TGCCTCCAGTCTTG-3′) were shorter than
the outer primers. Primers were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China), and the reaction was conducted in a 9700
Applied Biosystems thermocycler (Thermo Fisher Scientific, Inc.),
using the following cycling conditions: Incubation at 94℃ for 4
min, followed by 36 cycles of denaturation at 94℃ for 45 sec,
annealing at 58℃ for 45 sec and polymerization at 72℃ for 45 sec.
In the second step, the PCR products from the first reaction were
subjected to a second PCR with a different set of primers, using
the following cycling conditions: Incubation at 95℃ for 2 min,
followed by 36 cycles of denaturation at 95℃ for 45 sec, annealing
at 60℃ for 45 sec and polymerization at 72℃ for 30 sec. The
amplifed products were subjected to electrophoresis in 1.5% agarose
gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing
ethidium bromide (Sangon Biotech Co., Ltd.), and visualized under
ultraviolet light in a gel documentation system (Invitrogen; Thermo
Fisher Scientific, Inc.). The electrophoresis equipment, buffers
and markers used were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.).
Statistical analyses
APL samples were divided into two groups, namely
ETV6 rearrangement-positive and ETV6 rearrangement-negative groups.
Continuous and discrete patients variables were compared using
Mann-Whitney U test or Wilcoxon rank sum test and χ2
test, respectively. P<0.05 was considered to indicate a
statistically significant difference. OS was measured from the date
of first diagnosis to mortality from any cause or last follow-up.
Relapse was defined as a reappearance of t(15;17)/promyelocytic
leukemia/retinoic acid receptor alpha. Patients who succumbed to
the disease within 15 days of diagnosis were defined as early
mortality (EM). To exclude confounding influence of different
treatment regimens, all patients were included in the analysis of
the association between ETV6 expression and clinical
characteristics. By contrast, only those patients receiving
conventional standard chemotherapy [daunorubicin (Zhejiang Haizheng
Pharmaceutical Co. Ltd. (Zhejiang, China); 40 mg/m2/day
on days 1–3; and cytarabine (Zhejiang Haizheng Pharmaceutical Co.
Ltd.); 100 mg/m2/day on days 1–7] were included in the
analyses of treatment effects and survival rates. Kaplan-Meier
estimation was adopted to plot the corresponding survival curves,
and log-rank tests were used to examine the differences between
groups. Cox proportional hazards regression models were used to
determine independent risk factors associated with survival in
multivariate analyses. All statistical analyses were conducted with
SPSS version 16 software (SPSS, Inc., Chicago, IL, USA).
Results
Identification of ETV6
rearrangement
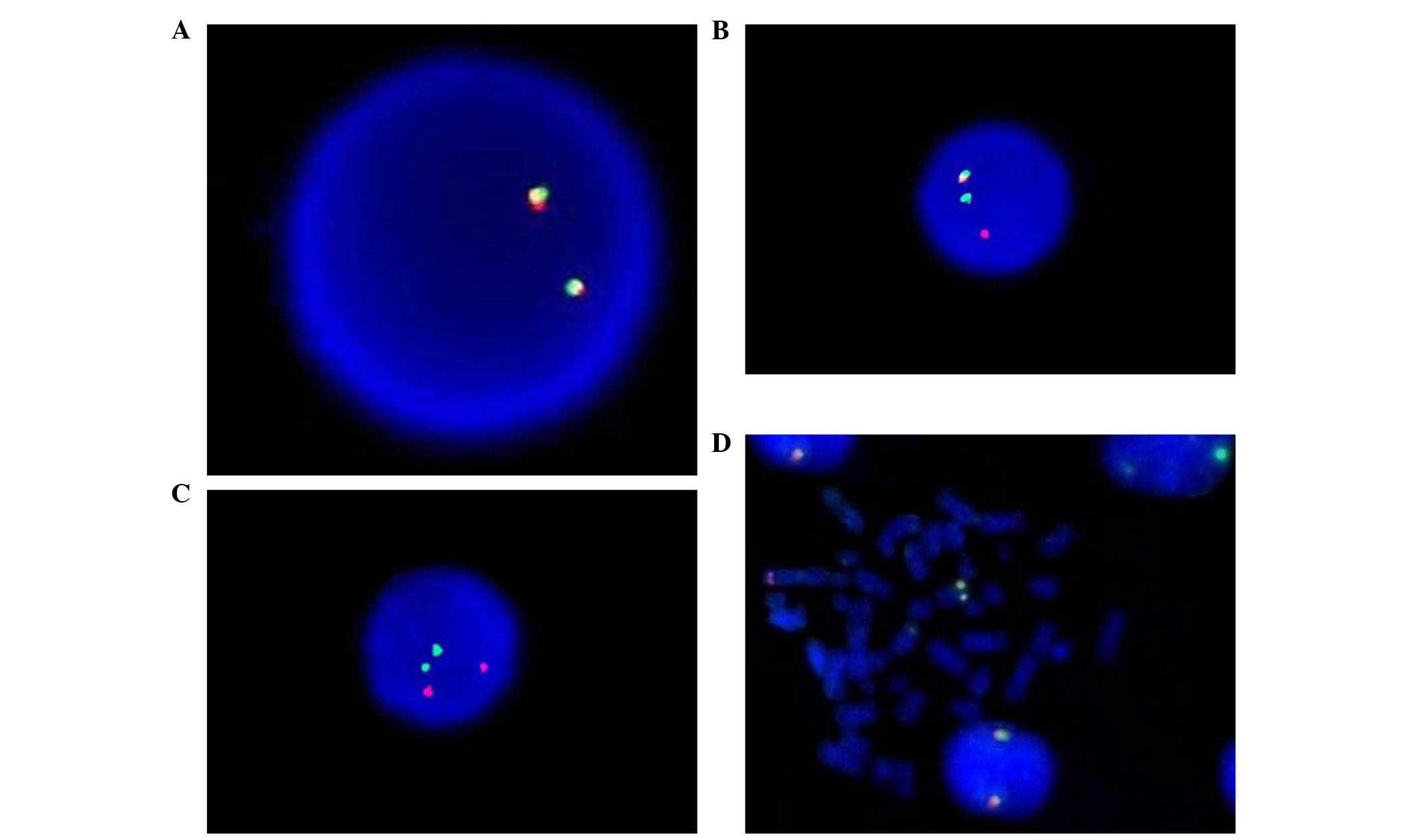
ETV6 rearrangements were detected in 8/258 (3.10%)
cases by split-signal FISH analysis (Fig.
1). Of these, 1 case exhibited abnormal karyotype with
46,XY,t(15;17)(q22;q21)t(1;12)(q25;p12). However, traditional
cytogenetic analysis did not identify any ETV6 rearrangement, with
the exception of t(15;17). ETV6 rearrangement was not detected in
any of the 30 healthy controls.
Correlation of ETV6 rearrangement with
clinical features and laboratory data in the cohort
All patients with ETV6 rearrangement displayed
higher WBC counts than patients who were negative for ETV6
rearrangement (P<0.001). The mean age of the patients was
significantly different between the two groups (P=0.017). Compared
with patients without ETV6 rearrangement, those with ETV6
rearrangement exhibited significantly more extramedullary
infiltration. Gender ratio, platelet counts and hemoglobin levels
did not differ significantly between the different subgroups
(P>0.05). The expression of CD34, CD117, MPO and HLA-DR was
higher in patients with ETV6 rearrangement than in ETV6
rearrangement-negative patients (P<0.05). The comparison of the
clinical characteristics and laboratory data of the patients with
positive and negative ETV6 rearrangement is presented in Table I.
 | Table I.Comparison of clinical features and
clinical outcomes between Ets variant 6 gene rearrangement-positive
and negative groups. |
Table I.
Comparison of clinical features and
clinical outcomes between Ets variant 6 gene rearrangement-positive
and negative groups.
| Variable | Total (n=258) | Positive
groupa (n=8) | Negative
groupa (n=250) | P-value |
|---|
| Gender, n (%) |
|
|
| 0.869 |
|
Male | 154 | 5 (3.25%) | 149 (96.75%) |
|
|
Female | 104 | 3 (2.88%) | 101 (97.12%) |
|
| Age, years
(range) | 36.88 (13–72) | 45.63±5.38 | 36.60±0.65 | 0.017 |
| Clinical
manifestation |
|
|
Hepatosplenomegaly | 52 (20.16%) | 5 (62.50%) | 47 (18.80%) | 0.010 |
|
Lymphadenectasis | 11 (4.26%) | 2 (25.00%) | 9 (3.60%) | 0.039 |
| Laboratory data
(normal range) |
|
| WBC
count, cells/µl | 4,349
(480–38,010) | 6,540±1,362 | 4,279±313 | 0.027 |
|
Hemoglobin, g/dl | 8.01
(4.40–13.10) | 6.75±0.48 | 7.86±0.11 | 0.074 |
|
Platelet count, 103
cells/µl | 32.42
(3.00–152.00) | 22.13±4.20 | 32.77±1.53 | 0.217 |
| CD34,
% | 3.03
(0.00–80.00) | 7.00±4.88 | 2.90±0.60 | 0.004 |
| CD117,
% | 7.58
(0.00–80.00) | 15.62±4.76 | 7.32±0.74 | 0.012 |
| MPO,
% | 22.31
(0.00–80.00) | 41.25±9.53 | 21.70±1.23 | 0.030 |
| CD13,
% | 75.33
(5.00–100.00) | 71.25±7.18 | 75.46±1.35 | 0.452 |
| CD33,
% | 91.98
(60.00–100.00) | 91.25±2.95 | 92.00±0.64 | 0.595 |
| HLA-DR,
% | 2.44
(0.00–80.00) | 10.62±6.16 | 2.18±0.46 | 0.018 |
| Early mortality, n
(%) | 20 (7.75) | 1 (12.50) | 19 (7.60) | >0.999 |
| CR rate, n
(%)b | 167 (88.83) | 5 (71.43) | 162 (89.50) | 0.380 |
Effect of ETV6 rearrangement on the
response to therapy and prognosis
The 258 APL patients were immediately treated with
all-trans retinoic acid [ATRA; Shandong Liangfu Pharmaceutical Co.,
Ltd. (Shandong, China); 30 mg/m2/day orally from day 1
until complete remission (CR)] as induction therapy if APL was
suspected, and the treatment lasted until achievement of CR.
Conventional standard chemotherapy with daunorubicin and
cytarabine, in addition to arsenic trioxide
[As2O3; Beijing Shuanglu Pharmaceutical Co.,
Ltd. (Beijing, China); 10 mg/day, intravenous drip on days 1–8],
were administered as soon as ATRA had exerted an effect. With the
exception of 1 case, who succumbed to disease within 15 days (EM),
the other 7 patients with ETV6 rearrangement received traditional
standard chemotherapy based on ATRA, of whom, 5 cases experienced
CR. In the ETV6 rearrangement-negative group, 19 patients succumbed
to disease within 15 days (EM) and 181 cases received traditional
standard chemotherapy, including 162 cases of CR. There was no
obvious difference in EM rates between the two groups (12.5 vs.
7.6%; P>0.999). CR rates did not exhibit significant differences
between the two groups receiving standard chemotherapy or ATRA
(71.43 vs. 89.50%; P=0.380). However, it is worth mentioning that 4
patients with ETV6 rearrangement relapsed within 1 year following
CR. The relapse rate within 1 year was markedly different between
the two groups once CR was achieved (80.00 vs. 11.11%; P<0.001).
The association between ETV6 rearrangement and clinical outcome is
presented in Table I. The
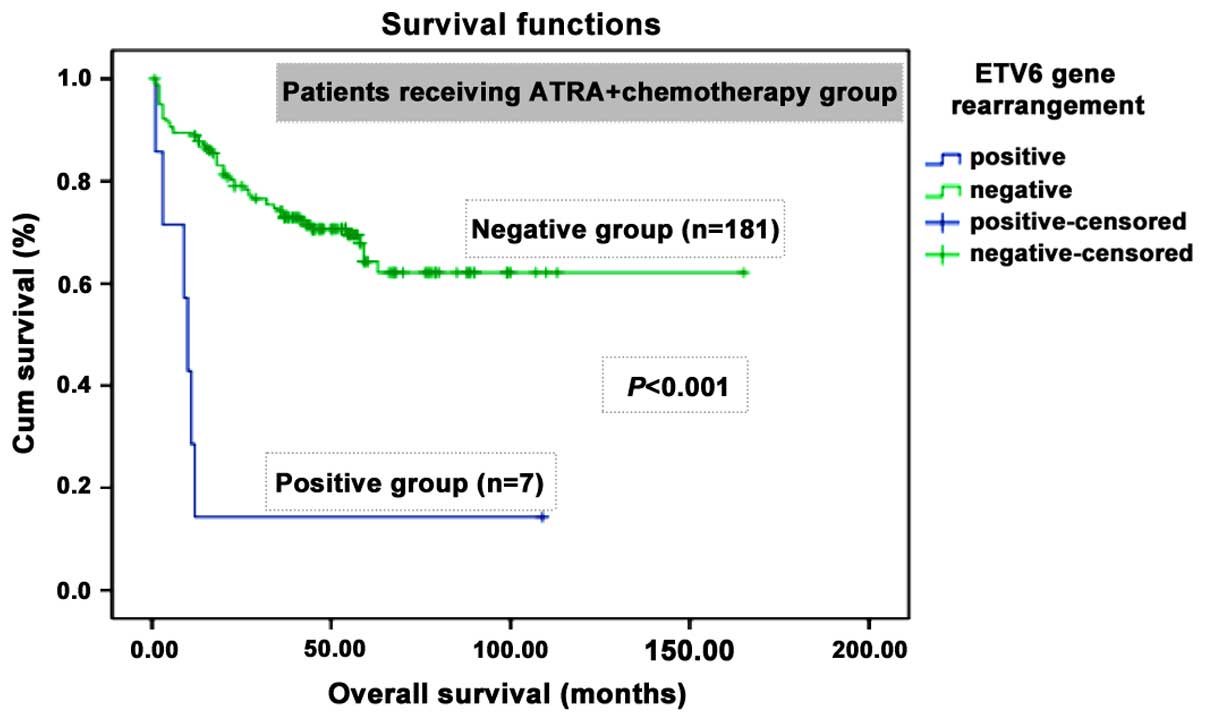
Kaplan-Meier curve for OS revealed that the patients with ETV6
rearrangement had a significant shorter OS (P<0.001) than those
without ETV6 rearrangement after a median follow-up time of 89.5
months (Fig. 2). Cox proportional
hazards regression models revealed that WBC and platelet counts, in
addition to ETV6 rearrangement, were independent prognostic factors
for OS in APL patients (Table II).
These findings indicated that ETV6 rearrangement was an independent
unfavorable prognostic factor for APL patients (P=0.044).
 | Table II.Multivariate analysis (Cox
proportional hazards regression model) of overall survival in
patients with acute promyelocytic leukemia. |
Table II.
Multivariate analysis (Cox
proportional hazards regression model) of overall survival in
patients with acute promyelocytic leukemia.
| Variables | Wald
χ2 | Exponential
coefficient (β) | 95% CI for β | P-value |
|---|
| WBC
counta | 10.081 | 1.127 | 1.047–1.213 | 0.001 |
| Platelet
counta |
5.454 | 0.980 | 0.963–0.967 | 0.020 |
| ETV6
rearrangementa |
4.068 | 0.381 | 0.149–0.973 | 0.044 |
| Hemoglobin
levels | – | – | – | 0.077 |
| Age | – | – | – | 0.113 |
Identification of ETV6/ARG fusion gene
by RT-PCR and clinical implication
The fusion partner of ETV, ARG, was identified by
RT-PCR in 1 of 8 cases. RT-PCR detected ETV6/ARG fusion gene
products, 2 of which were the result of an alternative splicing
event in the ARG gene (Fig. 3A).
ETV6/ARG fusion gene products were not detected in the other 7
cases or healthy donors. The patient exhibiting ETV6/ARG fusion
gene was a 56-year-old man, who was hospitalized at the Department
of Hematology of Binzhou Medical University Hospital for ‘fever,
weakness’ in August 2009. Physical examination on admission
revealed multiple superficial lymphadenopathies and
hepatosplenomegaly. Blood assay demonstrated markedly increased
leukocytes levels, including WBC count of 14, 860 cells/µl (normal
range, 4,000–10,000 cells/µl), hemoglobin levels of 10.8 g/dl
(normal range, 11.0–15.0 g/dl) and platelet count of
5×103 cells/µl (normal range, 100–300 cells/µl), which
was accompanied by 40% abnormal promyelocytes and an obviously
abnormal coagulation index, including a prothrombin time of 15.6
sec (normal range, 10.0–14.0 sec), fibrinogen content of 0.8 g/l
(normal range, 2.0–4.0 g/l) and D-dimer content of 3.7 mg/l (normal
range, 0.0–0.7 mg/l). with 40% abnormal promyelocytes and an
obviously abnormal coagulation index. Marrow aspiration disclosed
that blasts comprised 95% of the myeloid cells, and the blasts were
of median size and full of fine particles in the cytoplasm. Flow
cytometry analysis of the bone marrow revealed the following
cellular characteristics: CD34+, HLA-DR+
(Fig. 3B), CD33+,
CD13+ and CD117−. Cytogenetic analysis
revealed an abnormal karyotype with
46,XY,t(15;17)(q22;q21)t(1;12)(q25;p12) by FISH. Diagnosis of M3v
AML was considered. The patient was treated with ATRA upon
diagnosis, while hydroxycarbamide [Shandong Qilu King-Phar
Pharmaceutical Co., Ltd. (Shandong, China); 2 g/day, orally from
day 1 until WBC count <10,000 cells/µl] and dexamethasone
[Shanghai Tongyong Pharmaceutical Co., Ltd. (Shanghai, China); 5
mg/day, intravenous drip on days 1–10]. were used to prevent
differentiation syndrome. However, his WBC count rapidly increased
following oral administration of the drugs, and the patient
succumbed to hematencephalon within 10 days of admission to
hospital.
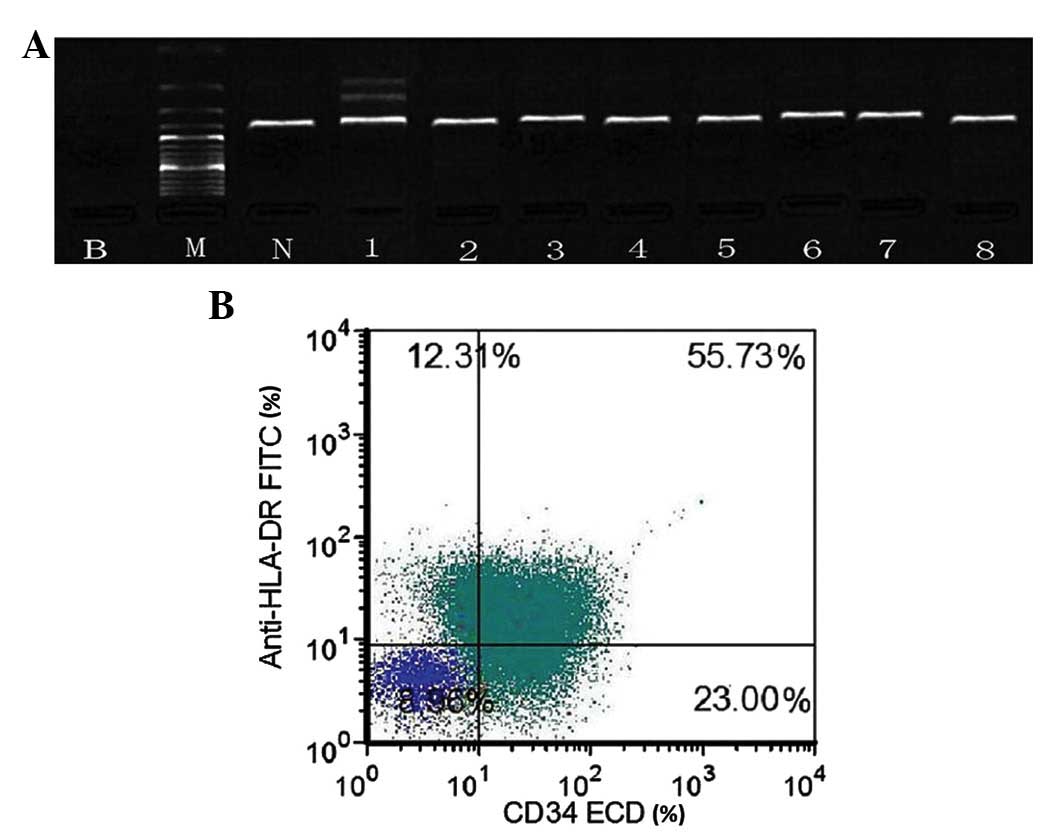 | Figure 3.(A) Reverse transcription polymerase
chain reaction analysis of ETV6/ARG fusion products. Patient
samples (1–8) exhibited ETV6 rearrangement. In case 1,
two specific bands corresponding to different ETV6/ARG fusion
products were detected, which were the result of an alternative
splicing event in the ARG gene. (B) Flow cytometry analysis of bone
marrow specimens revealed abnormal promyelocytic cells positive for
cluster of differentiation 34 and human leukocyte antigen-antigen D
related. B, blank control using double distilled H2O; M,
DNA marker DL2000; N, normal healthy donor; HLA-DR, human leukocyte
antigen-antigen D related FITC, fluorescein isothiocyanate; CD,
cluster of differentiation; ECD, electron coupled dye; ETV6, Ets
variant 6; ARG, abelson-related gene. |
Discussion
The ETV6 gene (also known as TEL), which is located
on chromosome 12p13 and possesses a total length of 300 kb,
encoding for 452 amino acids, is a member of the ETS family of
transcription regulators, and is important in hematopoietic
processes and hematological malignancies (23). ETV6 participates in the regulation of
cell growth and differentiation, and stimulates erythroid
differentiation of murine erythroid leukemia cells (24). Previous studies reported that chimeric
mice with ETV6 knockout embryonic stem cells exhibited defective
hematopoiesis within the first week of birth (25). ETV6 contains an ETS DNA-binding domain
near its carboxy-terminus and a helix-loop-lelix (HLH) domain near
its amino-terminus, which mediates homotypic or heterotypic
oligomerization with other ETV6 molecules or transcription factors,
respectively (23,26). Fusions between ETV6 and a number of
different partner genes, mainly those coding for tyrosine kinases
or transcription factors that are important for the initiation,
progress and prognosis of disease, have been observed in various
hematological malignancies (11–13).
However, little is known about its prognostic implication in
APL.
APL is the most common type of adult leukemia, and
is currently considered to be a highly curable disease (4). Since the application of
As2O3 and ATRA in the treatment of APL, the
prognosis has improved greatly (27).
However, improving the relapse rate and EM incidence remain the
greatest challenges for the future management of APL (28,29). The
current risk-stratification system used for APL is mainly based on
the WBC and platelet counts (4).
Previous studies have indicated that certain chromosome
abnormalities may be considered as important indicators for
prognosis (30). Novel molecular
biomarkers may aid to improve the risk stratification of APL and to
identify those patients with a particularly poor prognosis
(31,32). In the present study, a high expression
of myeloid blast-associated antigens was identified in the ETV6
rearrangement-positive group, whereas the levels of
promyelocyte-specific antigens were not significantly different
between the ETV6 rearrangement-positive and the ETV6
rearrangement-negative groups. Although the EM and CR rates between
the two groups were not significantly different, the rate of
relapse within 1 year was significantly higher in the ETV6
rearrangement-positive group than in the ETV6
rearrangement-negative group. Therefore, ETV6 rearrangement
expression in the bone marrow may serve as a novel prognostic
biomarker for risk stratification in patients newly diagnosed with
APL.
The occurrence of ETV6 rearrangement is common in
childhood lymphocytic malignancies (33); however, only a few rare or sporadic
case reports exist concerning adult AML (34,35). The
expression of ETV6 mutations in myeloid malignancies has been
widely reported (36,37). However, little is known about the
clinical significance of ETV6 rearrangement in APL. In the present
study, ETV6 rearrangement was detected in a large cohort of 258 APL
patients, of which 8 cases were positive for ETV6 rearrangement.
ETV6 rearrangement is an independent unfavorable prognostic factor
for OS in APL, which is secondary only to the white blood cell and
platelet counts. Despite the lower overall expression rate of ETV6
rearrangement in APL, compared with other leukemia subtypes such as
acute lymphoblastic leukemia (ALL), MDS or non-M3 AML, ETV6 fusion
was highly expressed (5/57 patients, 8.77%) in those patients with
a WBC >5,000 cells/µl. The detection of ETV6 mutation is
important in patients with a WBC >5,000 cells/µl, since the loss
of tumor suppressor activity is possibly the pathogenic mechanism
of the mutation. ETV6 rearrangement may inhibit the growth and
normal differentiation of hematopoietic progenitor cells; thus,
cells remain in a primitive stage (38).
The present study also confirmed the existence of
ETV6/ARG in 1 of 8 APL patients. The patient, who exhibited a high
leukocyte count, significant extramedullary infiltration features
and was not sensitive to chemotherapy, succumbed to cerebral
hemorrhage shortly after diagnosis. The present study is the second
report of an APL patient with ETV6/ARG rearrangement. Previously,
Griesinger et al (39)
reported ETV6/ARG rearrangement in a case of T-cell ALL. The ARG
gene is a non-receptor tyrosine kinase that, together with ABL1, is
characterized by a high homology with ABL1 in its protein tyrosine
kinase (PTK), Src homology (SH)2 and SH3 domains (18). Aberrant activation of ABL and ARG
downstream of several oncogenic growth factor receptors are
associated with cancer progression, metastasis, drug resistance and
poor prognosis (40). Imatinib, as a
tyrosine kinase inhibitor specifically targeting kinases of the ABL
family, is able to inhibit cell growth via inhibition of ARG in the
APL cell line HT93A (41). As an
oncogene, the ETV6/ARG fusion gene may depend on the oncogenic
activity of chimeric PTK proteins by forming HLH domain-dependent
homo-oligomers that result in the activation of tyrosine kinases in
a ligand-independent manner (8). The
ETV6/ARG fusion protein may induce progenitor cells to
differentiate into myeloid cells rather than lymphoid cells
(42). A previous in vitro
study demonstrated that the ETV6/ARG oncoprotein contributes to
autonomous cell growth through activating phosphorylated signal
transducer and activator of transcription, leading to the
upregulation of c-Myc oncogene expression (43). The present authors have also detected
ETV6 rearrangements in other hematological malignancies such as AML
(non M3), lymphadenoma and multiple myeloma by split-signal FISH
(44–47).
In conclusion, ETV6 rearrangement is a reproducible
event in hematological malignancies, which has been detected by
different investigators under different conditions in various
hematological malignancies, and is closely associated with poor
patient prognosis. Thus, ETV6 rearrangement may become a molecular
target in the treatment of hematological malignancies. In future
studies, gene sequencing, transfection, RNA interference technology
and animal models could be applied to reveal the pathogenic
mechanism of ETV6 gene rearrangement.
Acknowledgements
The authors would like to thank Guangzhou Jinyu
Medical Science Inspection Center for performing the majority of
flow cytometry immunophenotyping analyses. The present study was
partly supported by the Provincial Key Clinical Specialist Fund of
Shandong (Jinan, China; grant no. 20130415).
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 2:(4th). IARC
Press. Lyon: 112–114. 2008.
|
|
2
|
Tallman MS and Kwaan HC: Reassessing the
hemostatic disorder associated with acute promyelocytic leukemia.
Blood. 79:543–553. 1992.PubMed/NCBI
|
|
3
|
Cull EH and Altman JK: Contemporary
treatment of APL. Curr Hematol Malig Rep. 9:193–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jun M: Chinese Society of Hematology,
Chinese Medical Association & Chinese Society of Hematologist,
Chinese Medical Doctor Association: Guidelines for diagnosis and
treatment of acute promyelocytic leukemia (2014). Zhonghua Xue Ye
Xue Za Zhi. 35:475–477. 2014.(In Chinese). PubMed/NCBI
|
|
5
|
Schnittger S, Bacher U, Haferlach C, Kern
W, Alpermann T and Haferlach T: Clinical impact of FLT3 mutation
load in acute promyelocytic leukemia with t(15;17)/PML-RARA.
Haematologica. 96:1799–1807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin J, Sun AN, Tian XP, Tian H, Wang RX,
Yang Z, Wang XL, Wu DP, Qiu HY, Pan JL, et al: Clinical
significance of common leukemia gene mutations in patients with
acute promyelocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
21:39–44. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Liu J, Zou XL, Liu TT, Jiang M and Niu T:
FLT3 mutation in acute promyelocytic leukemia patients with
extramedullary relapse. Sichuan Da Xue Xue Bao Yi Xue Ban. (45):
670–674. 2014.(In Chinese). PubMed/NCBI
|
|
8
|
Maki K, Arai H, Waga K, Sasaki K, Nakamura
F, Imai Y, Kurokawa M, Hirai H and Mitani K: Leukemia-related
transcription factor TEL is negatively regulated through
extracellular signal-regulated kinase-induced phosphorylation. Mol
Cell Biol. 24:3227–3237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akagi T, Kuure S, Uranishi K, Koide H,
Costantini F and Yokota T: ETS-related transcription factors ETV4
and ETV5 are involved in proliferation and induction of
differentiation-associated genes in embryonic stem (ES) cells. J
Biol Chem. 290:22460–22473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto K, Yakushijin K, Nakamachi Y,
Miyata Y, Sanada Y, Tanaka Y, Okamura A, Kawano S, Hayashi Y,
Matsuoka H and Minami H: Extramedullary T-lymphoid blast crisis of
an ETV6/ABL1-positive myeloproliferative neoplasm with
t(9;12)(q34;p13) and t(7;14)(p13;q11.2). Ann Hematol. 93:1435–1438.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuneo A, Agostini P, Vitale A, Foà R and
Castoldi G: Frequency of ETV6/AML1 fusion in adult acute
lymphoblastic leukemia. Leukemia. 17:476–477. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Achkar WA, Aljapawe A, Liehr T and Wafa A:
De novo acute myeloid leukemia subtype-M4 with initial
trisomy 8 and later acquired t(3;12)(q26;p12) leading to
ETV6/MDS1/EVI1 fusion transcript expression: A case report. Oncol
Lett. 7:787–790. 2014.PubMed/NCBI
|
|
13
|
Nofrini V, Berchicci L, La Starza R,
Gorello P, Di Giacomo D, Arcioni F, Pierini V, Crescenzi B, Romoli
S, Matteucci C and Mecucci C: MN1-ETV6 fusion gene arising from MDS
with 5q-. Leuk Res. 35:e123–e126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yagasaki F, Jinnai I, Yoshida S, Yokoyama
Y, Matsuda A, Kusumoto S, Kobayashi H, Terasaki H, Ohyashiki K,
Asou N, et al: Fusion of TEL/ETV6 to a novel ACS2 in
myelodysplastic syndrome and acute myelogenous leukemia with
t(5;12)(q31;p13). Genes Chromosomes Cancer. 26:192–202. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding BT, Guo NJ, Sun JZ, Gao HM, Wang YS
and Chen Y: Analysis and clinical significance of ETV6
rearrangement in myelodysplastic syndromes patients. Zhonghua Xue
Ye Xue Za Zhi. 28:804–807. 2007.(In Chinese). PubMed/NCBI
|
|
16
|
Suto Y, Sato Y, Smith SD, Rowley JD and
Bohlander SK: A t(6;12)(q23;p13) results in the fusion of ETV6 to a
novel gene, STL, in a B-cell ALL cell line. Genes Chromosomes
Cancer. 18:254–268. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lambros MB, Tan DS, Jones RL, Vatcheva R,
Savage K, Tamber N, Fenwick K, Mackay A, Ashworth A and Reis-Filho
JS: Genomic profile of a secretory breast cancer with an ETV6-NTRK3
duplication. J Clin Pathol. 62:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iijima Y, Ito T, Oikawa T, Eguchi M,
Eguchi-Ishimae M, Kamada N, Kishi K, Asano S, Sakaki Y and Sato Y:
A new ETV6/TEL partner gene, ARG (ABL-related gene or ABL2),
identified in an AML-M3 cell line with a t(1;12)(q25;p13)
translocation. Blood. 95:2126–2131. 2000.PubMed/NCBI
|
|
19
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposed revised
criteria for the classification of acute myeloid leukemia. A report
of the French-American-British Cooperative Group. Ann Intern Med.
103:620–625. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schreck RR, Warburton D, Miller OJ, Beiser
SM and Erlanger BF: Chromosome structure as revealed by a combined
chemical and immunochemical procedure. Proc Natl Acad Sci USA.
70:804–807. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitelman F: ISCN 1995: An International
System for Human Cytogenetic Nomenclature. Karger, Basel: 1–114.
1995.
|
|
22
|
Chen SS: Flowcytometric-immunophenotyping
for leukemia needs to be standardized. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 10:1–5. 2002.(In Chinese). PubMed/NCBI
|
|
23
|
Bohlander SK: ETV6: A versatile player in
leukemogenesis. Semin Cancer Biol. 15:162–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi W, Sasaki K, Kvomatsu N and
Mitani K: TEL/ETV6 accelerates erythroid differentiation and
inhibits megakaryocytic maturation in a human leukemia cell line
UT-7/GM. Cancer Sci. 96:340–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang LC, Swat W, Fujiwara Y, Davidson L,
Visvader J, Kuo F, Alt FW, Gilliland DG, Golub TR and Orkin SH: The
TEL/ETV6 gene is required specifically for hematopoiesis in the
bone marrow. Genes Dev. 12:2392–2402. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hart SM and Foroni L: Core binding factor
genes and human leukemia. Haematologica. 87:1307–1323.
2002.PubMed/NCBI
|
|
27
|
Tomita A, Kiyoi H and Naoe T: Mechanisms
of action and resistance to all-trans retinoic acid (ATRA) and
arsenic trioxide (As2O3) in acute
promyelocytic leukemia. Int J Hematol. 97:717–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He B, Hu S, Qiu G and Gu W: Clinical
characteristics of acute promyelocytic leukemia manifesting as
early death. Mol Clin Oncol. 1:908–910. 2013.PubMed/NCBI
|
|
29
|
Xu F, Yin CX, Wang CL, Jiang XJ, Jiang L,
Wang ZX, Yi ZS, Huang KK and Meng FY: Immunophenotypes and immune
markers associated with acute promyelocytic leukemia prognosis. Dis
Markers. 2014:4219062014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lazarevic V, Hörstedt AS, Johansson B,
Antunovic P, Billström R, Derolf A, Hulegårdh E, Lehmann S,
Möllgård L, Nilsson C, et al: Incidence and prognostic significance
of karyotypic subgroups in older patients with acute myeloid
leukemia: The Swedish population-based experience. Blood Cancer J.
4:e1882014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won D, Shin SY, Park CJ, Jang S, Chi HS,
Lee KH, Lee JO and Seo EJ: OBFC2A/RARA: A novel fusion gene in
variant acute promyelocytic leukemia. Blood. 121:1432–1435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albano F, Zagaria A, Anelli L, Orsini P,
Minervini CF, Impera L, Casieri P, Coccaro N, Tota G, Brunetti C,
et al: Lymphoid enhancer binding factor-1 (LEF1) expression as a
prognostic factor in adult acute promyelocytic leukemia.
Oncotarget. 5:649–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eguchi-Ishimae M, Eguchi M, Ishii E,
Miyazaki S, Ueda K, Kamada N and Mizutani S: Breakage and fusion of
the TEL (ETV6) gene in immature B lymphocytes induced by
apoptogenic signals. Blood. 97:737–743. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kralik JM, Kranewitter W, Boesmueller H,
Marschon R, Tschurtschenthaler G, Rumpold H, Wiesinger K, Erdel M,
Petzer AL and Webersinke G: Characterization of a newly identified
ETV6-NTRK3 fusion transcript in acute myeloid leukemia. Diagn
Pathol. 6:192011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cazzaniga G, Tosi S, Aloisi A, Giudici G,
Daniotti M, Pioltelli P, Kearney L and Biondi A: The tyrosine
kinase abl-related gene ARG is fused to ETV6 in an AML-M4Eo patient
with a t(1;12)(q25;p13): Molecular cloning of both reciprocal
transcripts. Blood. 94:4370–4373. 1999.PubMed/NCBI
|
|
36
|
Murati A, Brecqueville M, Devillier R,
Mozziconacci MJ, Gelsi-Boyer V and Birnbaum D: Myeloid
malignancies: Mutations, models and management. BMC Cancer.
12:3042012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walz C, Erben P, Ritter M, Bloor A,
Metzgeroth G, Telford N, Haferlach C, Haferlach T, Gesk S, Score J,
et al: Response of ETV6-FLT3-positive myeloid/lymphoid neoplasm
with eosinophilia to inhibitors of FMS-like tyrosine kinase 3.
Blood. 118:2239–2242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Vlierberghe P, Ambesi-Impiombato A,
Perez-Garcia A, Haydu JE, Rigo I, Hadler M, Tosello V, Della Gatta
G, Paietta E, Racevskis J, et al: ETV6 mutations in early immature
human T cell leukemias. J Exp Med. 208:2571–2579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Griesinger F, Janke A, Podleschny M and
Bohlander SK: Identification of an ETV6-ABL2 fusion transcript in
combination with an ETV6 point mutation in a T-cell acute
lymphoblastic leukaemia cell line. Br J Haematol. 119:454–458.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hossain S, Dubielecka PM, Sikorski AF,
Birge RB and Kotula L: Crk and ABI1: Binary molecular switches that
regulate abl tyrosine kinase and signaling to the cytoskeleton.
Genes Cancer. 3:402–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishimura N, Furukawa Y, Sutheesophon K,
Nakamura M, Kishi K, Okuda K, Sato Y and Kano Y: Suppression of ARG
kinase activity by STI571 induces cell cycle arrest through
up-regulation of CDK inhibitor p18/INK4c. Oncogene. 22:4074–4082.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iijima Y, Okuda K, Tojo A, Tri NK,
Setoyama M, Sakaki Y, Asano S, Tokunaga K, Kruh GD and Sato Y:
Transformation of Ba/F3 cells and Rat-1 cells by ETV6/ARG.
Oncogene. 21:4374–4383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Iriyama N, Hatta Y and Takei M: ETV6/ARG
oncoprotein confers autonomous cell growth by enhancing c-Myc
expression via signal transducer and activator of transcription 5
activation in the acute promyelocytic leukemia cell line HT93A.
Leuk Lymphoma. 56:2416–2423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Naiel A, Vetter M, Plekhanova O,
Fleischman E, Sokova O, Tsaur G, Harbott J and Tosi S: A novel
three-colour fluorescence in situ hybridization approach for the
detection of t(7;12)(q36;p13) in acute myeloid leukaemia reveals
new cryptic three way translocation t(7;12;16). Cancers (Basel).
5:281–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Otsubo K, Kanegane H, Eguchi M,
Eguchi-Ishimae M, Tamura K, Nomura K, Abe A, Ishii E and Miyawaki
T: ETV6-ARNT fusion in a patient with childhood T lymphoblastic
leukemia. Cancer Genet Cytogenet. 202:22–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Najfeld V, Cozza A, Berkofsy-Fessler W,
Prchal J and Scalise A: Numerical gain and structural
rearrangements of JAK2, identified by FISH, characterize both
JAK2617V>F-positive and -negative patients with Ph-negative MPD,
myelodysplasia, and B-lymphoid neoplasms. Exp Hematol.
35:1668–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kwon WK, Lee JY, Mun YC, Seong CM, Chung
WS and Huh J: Clinical utility of FISH analysis in addition to
G-banded karyotype in hematologic malignancies and proposal of a
practical approach. Korean J Hematol. 45:171–176. 2010. View Article : Google Scholar : PubMed/NCBI
|