Introduction
Breast cancer comprises the most commonly diagnosed
type of cancer and one of the leading cause of cancer-induced
mortality in women worldwide (1).
Despite current advances in therapeutic strategies against cancer,
drug resistance remains a significant challenge; therefore, a
combination of target-specific agents may be required to
effectively eliminate these cells (2). Chemotherapy is one of the most effective
treatment strategies against cancer; however, cancer cells often
acquire a resistance to chemotherapy, therefore continuing to grow
and metastasize (3). Hirsutine, one
of the major alkaloids in Uncaria species, is known for its
cardioprotective, antihypertensive and antiarrhythmic activity
(4,5).
The present authors previously demonstrated the anti-cancer effect
of hirsutine in breast cancer cells (6,7); however
certain human breast cancer cell lines, including MCF-7, exhibited
resistance against hirsutine-induced cytotoxicity.
The present study used a chemical screening approach
and identified that the ataxia telangiectasia mutated (ATM) pathway
is key for hirsutine-resistance in human breast carcinoma MCF-7
cells. The DNA damage response was significantly amplified in MCF-7
cells following co-treatment with hirsutine and KU-60019, a
specific ATM inhibitor. While sensitization to hirsutine-induced
DNA damage response in MCF-7 cells by interfering with the ATM
pathway did not require p53, reactive oxygen species (ROS)
generation was significantly increased in hirsute and ATM
inhibitor-treated MCF-7 cells.
Materials and methods
Reagents
Hirsutine and a Cell Counting kit were purchased
from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and
KU-60019 was purchased from AdooQ Bioscience LLC (Irvine, CA, USA).
Muse™ Oxidative Stress kit was purchased from EMD Millipore
(Billerica, MA, USA). The SCADS Inhibitor kit (No. 3) was provided
by the Screening Committee of Anticancer Drugs (Tokyo, Japan). The
expression vector for the p53 dominant negative mutant
(pBABEpuro-p53DD) and pBABEpuro (control) were kindly gifted by Dr
David E. Fisher (Massachusetts General Hospital, Boston, MA,
USA).
Cell culture and stable
transfection
Human breast carcinoma MCF-7 cells were maintained
in Dulbecco's modified Eagle's medium (DMEM) containing 10% bovine
serum (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). The cells
were incubated at 37°C in a humidified atmosphere of 95% air and 5%
CO2. MCF-7 cells stably expressing dominant negative p53
were established as described previously (8). Briefly, MCF-7 cells were transfected
with pBABEpuro (control) or pBABEpuro-p53DD, which contains the p53
dominant negative mutant using Lipofectaimne 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were
selected by puromycin (100 µg/ml; Sigma-Ardrich, St. Louis, MO,
USA) for 14 days. Subsequently, puromycin-resistant clones were
isolated using sterilized cloning rings (Sigma-Ardrich) and
expanded as established MCF-7CTRL and
MCF-7p53DD cell lines. These cells were maintained in
DMEM with puromycin (100 µg/ml). Dominant negative p53 expression
was confirmed by western blot analysis.
Cell viability assay
MCF-7CTRL, MCF-7p53DD and
MCF-7 cells were plated at a final concentration of
2×104 cells/well in a 96-well plate. After a 3 h
incubation, the cells were treated with single or dual agents from
the SCADs Inhibitor kit for 24 h. For a combination assay, all
cells were pretreated with the inhibitor for 1 h. Following
treatment, 10 µl WST-1 Cell Proliferation reagent (WST-1, Dojindo,
Tokyo, Japan) was added. The 96-well plate was incubated for
another 2 h in a humidified atmosphere (37°C; 5% CO2) to
allow the formation of formazan dye and to obtain a higher
sensitivity. The absorbance was measured in a microplate reader
(Sunrise™; Tecan Group Ltd., Männedorf, Switzerland) at a
wavelength of 450/620 nm. Cell viability was determined from the
absorbance of soluble formazan dye generated by the living
cells.
Western blot analysis
MCF-7CTRL, MCF-7p53DD and
MCF-7 cells (American Type Culture Collection, Manassas, VA,
USA) were exposed to single or dual agents for 0, 3, 6 and 12
h. Treated cells were collected, washed with phosphate buffered
saline (PBS) and lysed in lysis buffer [25 mM HEPES (pH, 7.7), 0.3
M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20
mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 0.5 mM
phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 10 mg/ml
aprotinin, and 10 mg/ml leupeptin; Cell Signaling Technology,
Danvers, MA USA]. The cell lysates were separated by 5–10% sodium
dodecyl-sulfate polyacrylamide gel electrophoresis and transferred
to polyvinylidene difluoride membranes using a glycine transfer
buffer [192 mM glycine, 25 mM Tris-HCl (pH, 8.8), and 20% (v/v)
methanol; Sigma-Aldrich]. After blocking with Block Ace (DS
Biomedical, Osaka, Japan) for 4 h at room temperature, the membrane
was incubated overnight at 4°C with primary antibodies, and
subsequently for 60 min at room temperature with secondary
antibodies. Primary and secondary antibodies were used at a
dilution of 1:1,000 and 1:2,000, respectively, and the proteins
were visualized with an Amersham ECL Western Blotting Detection kit
(GE Healthcare Life Sciences, Chalfont, UK).
The following antibodies were purchased from Cell
Signaling Technology, Inc.: Rabbit monoclonal anti-phospho-ATM
(Ser1981; catalog no., 5883) and rabbit monoclonal
anti-phospho-histone H2A.X (Ser139; catalog no., 9947). Goat
polyclonal anti-actin (catalog no., sc-1615) and goat polyclonal
anti-α-tubulin (catalog no., sc-31779) were purchased from Santa
Cruz Biotechnology (Dallas, TX, USA). Mouse monoclonal anti-p53
(PAb421; catalog no., OP03) was purchased from
Calbiochem® (EMD Millipore).
ROS measurement
MCF-7 cells were grown in 12-well plates and
cultured overnight to allow adherence. Subsequently, the cells were
treated with single or dual agents for an additional 30 min. The
cells were collected and washed twice with PBS and resuspended in
1X Assay Buffer and 190 µl oxidative stress working solution from
the Muse™ Oxidative Stress kit were added to 10 µl cells. The cells
were incubated at 37°C for 30 min prior to analysis with a Muse™
Cell Analyzer (EMD Millipore). The assay was conducted in
triplicate and in accordance to the manufacturer's protocol.
Statistical analysis
All data are expressed as the mean ± standard
deviation of at least three independent experiments and were
analyzed for statistical significance using the Student's t-test.
Statistical analysis was performed using Microsoft Excel 2013
(Microsoft Corporation, Redmond, WA, USA) P<0.05 were considered
to indicate a statistically significant difference.
Results
Protective role of the ATM pathway for
hirsutine-induced cytotoxicity in MCF-7 cells
As previously reported, human epidermal growth
factor (HER2)+/p53-mutated MDA-MB-453 and BT474 cell
lines exhibit a response to hirsutine-induced cytotoxicity, whereas
HER2+/p53 wild-type MCF-7 cells exhibit significant
resistance to hirsutine treatment (6,7). To
investigate the potential molecular pathway that contribute to
hirsutine resistance in MCF-7 cells, the present study evaluated
the kinase inhibitor compounds in a SCADS Inhibitor kit, which are
listed in Table I, and their effect
on the viability of MCF-7 cells in combination with hirsutine
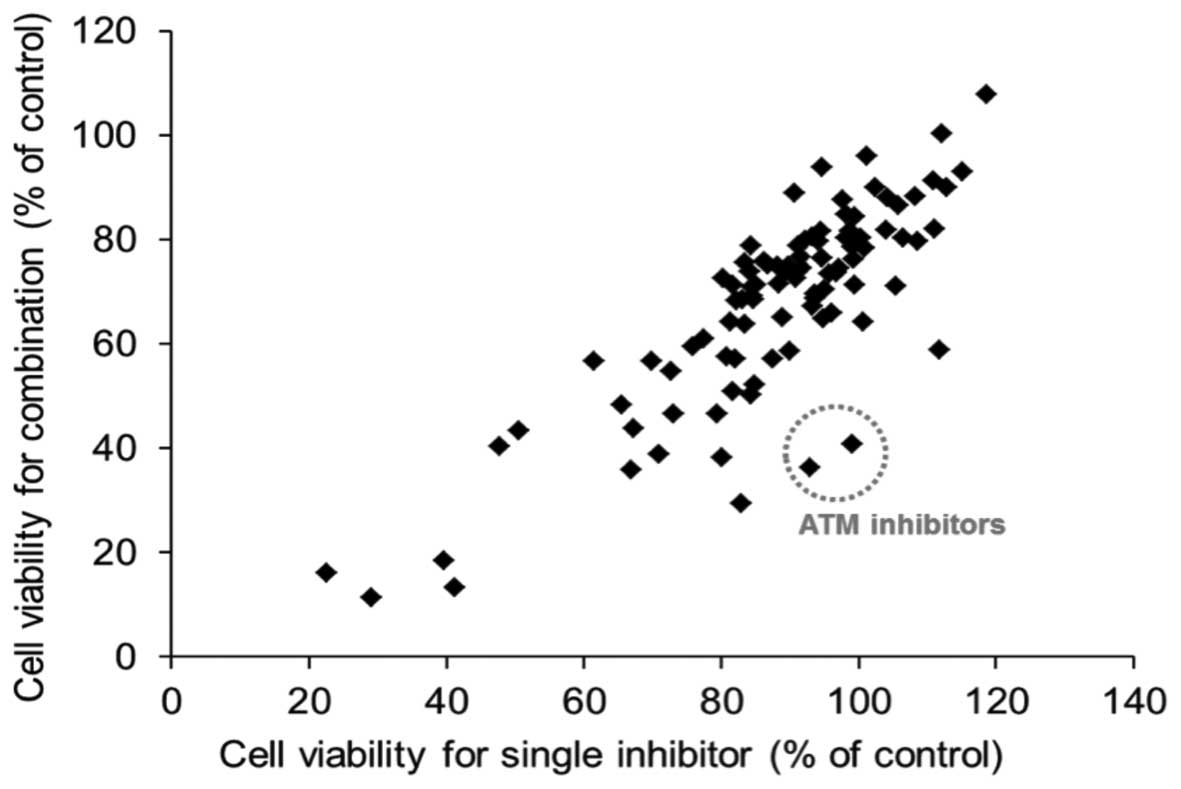
treatment. As shown in Fig. 1, ATM
inhibitors exhibited a significant effect in sensitizing
hirsutine-induced cytotoxicity in MCF-7 cells among the tested
compounds. To additionally confirm the involvement of the ATM
pathway in hirsutine resistance of MCF-7 cells, KU-60019, a second
generation specific ATM inhibitor (9–11), was
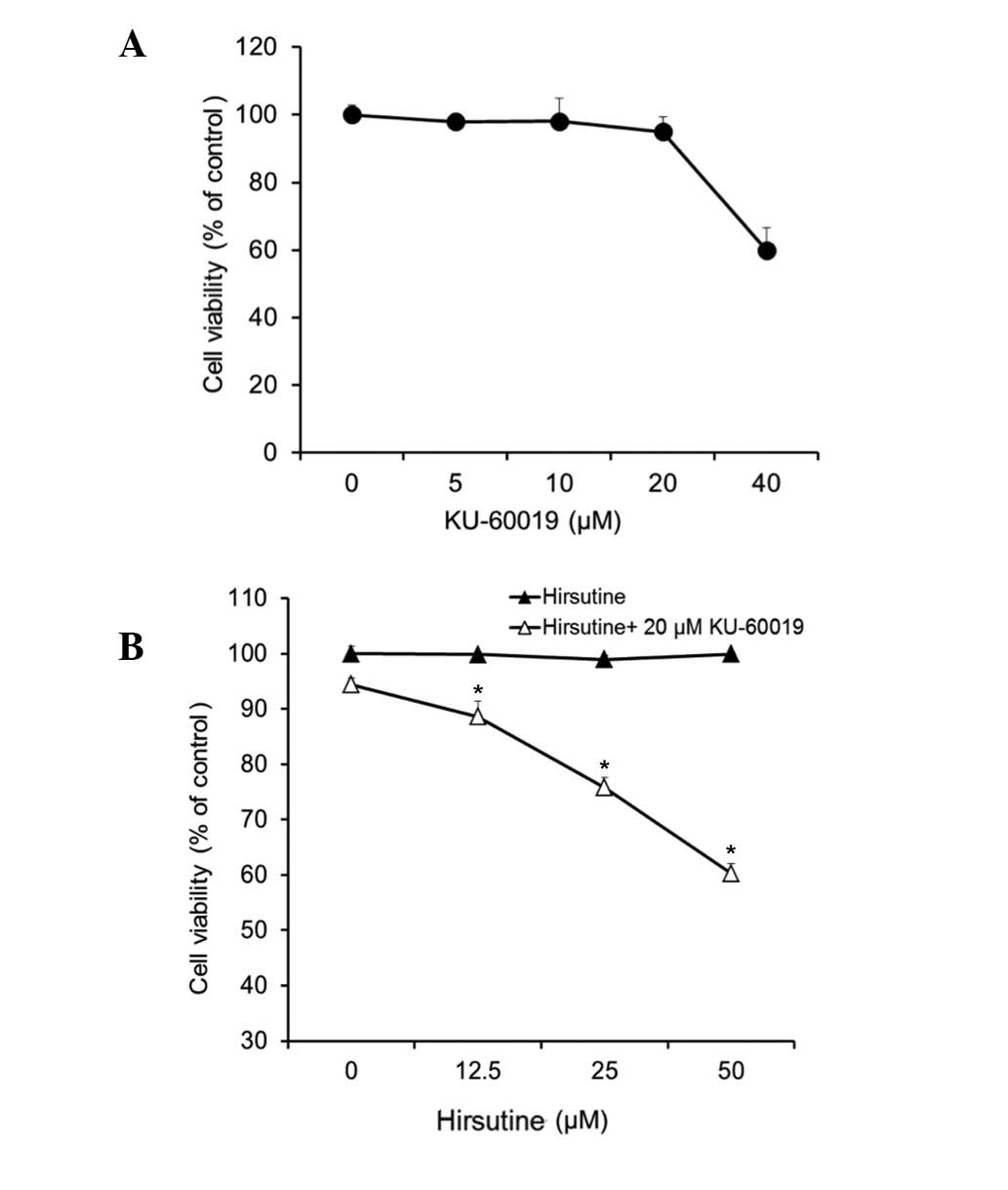
tested at a non-toxic dose (~20 mM) on its own (Fig. 2A) and in combination with hirsutine
(Fig. 2B). As shown in Fig. 2B, KU-60019 exhibited a significant
synergistic cytotoxic effect with hirsutine on MCF-7 cells
(P<0.05 hirsutine treated cells vs. hirsutine and KU-60019
cells).
 | Table I.Kinase inhibitors from the Screening
Committee of Anticancer Drug Inhibitor kit. |
Table I.
Kinase inhibitors from the Screening
Committee of Anticancer Drug Inhibitor kit.
| No. | Category | Compound |
|---|
| 1 | Control | Dimethyl
sulfoxide |
| 2 | AK | ABT-702 |
| 3 | AKT | Akt inhibitor IV |
| 4 | AKT | Akt inhibitor
VIII |
| 5 | AKT | Akt inhibitor XI |
| 6 | AMPK | Compound C |
| 7 | ATM | ATM/ataxia
telangiectasia kinase inhibitor |
| 8 | ATM | ATM kinase
inhibitor |
| 9 | Aurora | Aurora kinase/Cdk
inhibitor |
| 10 | Aurora | Aurora kinase
inhibitor II |
| 11 | Aurora | Aurora kinase
inhibitor III |
| 12 | Bcr-Abl | AG957 |
| 13 | BTK | LFM-A13 |
| 14 | BTK | Terreic acid |
| 15 | CAMKII | KN-93 |
| 16 | CAMKII | KN-62 |
| 17 | CAMKII | Lavendustin C |
| 18 | CDK | Kenpaullone |
| 19 | CDK | Purvalanol A |
| 20 | CDK | Olomoucine |
| 21 | CDK | Alsterpaullone,
2-cyanoethyl |
| 22 | CDK | Cdk1/2 inhibitor
III |
| 23 | CDK | Cdk2/9 inhibitor |
| 24 | CDK | NU6102 |
| 25 | CDK | Cdk4 inhibitor |
| 26 | CDK | NSC625987 |
| 27 | Chk | SB218078 |
| 28 | Chk | Isogranulatimide |
| 29 | Chk | Chk2 inhibitor |
| 30 | Chk | Chk2 inhibitor
II |
| 31 | CK | Ellagic acid |
| 32 | CK | TBB |
| 33 | CK | DMAT |
| 34 | CK | D4476 |
| 35 | Clk | TG003 |
| 36 | DGK | DGK inhibitor
II |
| 37 | DNA-PK | IC60211 |
| 38 | eEF2 | TX-1918 |
| 39 | EGFR | BPIQ-II |
| 40 | EGFR | AG1478 |
| 41 | EGFR | AG490 |
| 42 | FGFR | SU4984 |
| 43 | FGFR | SU5402 |
| 44 | Flt-3 | Flt-3
inhibitor |
| 45 | FMS | cFMS receptor
tyrosine kinase inhibitor |
| 46 | Fyn | SU6656 |
| 47 | GSK | GSK-3 inhibitor
IX |
| 48 | GSK |
1-Azakenpaullone |
| 49 | GSK |
Indirubin-3′-monoxime |
| 50 | HER2 | AG825 |
| 51 | IGF-IR | AG1024 |
| 52 | IGF-IR | AGL 2263 |
| 53 | IKK | BMS-345541 |
| 54 | IKK | IKK-2 inhibitor
VI |
| 55 | IRAK | IRAK-1/4
inhibitor |
| 56 | Jak | JAK inhibitor
I |
| 57 | Jak | JAK3 inhibitor
VI |
| 58 | JNK | SP600125 |
| 59 | JNK | JNK inhibitor
VIII |
| 60 | Lck | Damnacanthal |
| 61 | Lck | PP2 |
| 62 | MAPK | ERK inhibitor
II |
| 63 | MEK | PD98059 |
| 64 | MEK | U-0126 |
| 65 | MEK | MEK inhibitor
I |
| 66 | Met | SU11274 |
| 67 | MLCK | ML-7 |
| 68 | p38 | SB202190 |
| 69 | p38 | SB239063 |
| 70 | PDGFR | AG1296 |
| 71 | PDGFR | SU11652 |
| 72 | PDGFR | PDGF receptor
tyrosine kinase inhibitor V |
| 73 | PDGFR | PDGF receptor
tyrosine kinase inhibitor IV |
| 74 | PI3K | LY-294002 |
| 75 | PI3K | Wortmannin |
| 76 | PKA | H-89 |
| 77 | PKA |
4-Cyano-3-methylisoquinoline |
| 78 | PKC | Bisindolylmaleimide
I, HCl |
| 79 | PKC | Go7874 |
| 80 | PKG | Rp-8-CPT-cGMPS |
| 81 | PKG | KT5823 |
| 82 | PKR | PKR inhibitor |
| 83 | Raf | RAF1 kinase
inhibitor I |
| 84 | Raf | ZM 336372 |
| 85 | ROCK | H-1152 |
| 86 | ROCK | Y-27632 |
| 87 | Hsp90 | Radicicol |
| 88 | Src | PP1 analog |
| 89 | Syk | Syk inhibitor |
| 90 | TGF-βRI | SB431542 |
| 91 | TGF-βRI | TGF-β RI kinase
inhibitor II |
| 92 | Tpl2 | Tpl2 kinase
inhibitor |
| 93 | TrKA | TrkA inhibitor |
| 94 | VEGFR | VEGFR receptor
tyrosine kinase inhibitor II |
| 95 | VEGFR | VEGF recptor 2
kinase inhibitor I |
| 96 | VEGFR | SU1498 |
Involvement of the ATM pathway in
hirsutine-induced cytotoxicity through modulation of the DNA damage
response
Considering the DNA damage response was one of the
mechanisms of hirsutine-induced cytotoxicity, the present study
investigated whether a co-administration of hirsutine with KU-60019
also induces a DNA damage response in hirsutine-resistant MCF-7
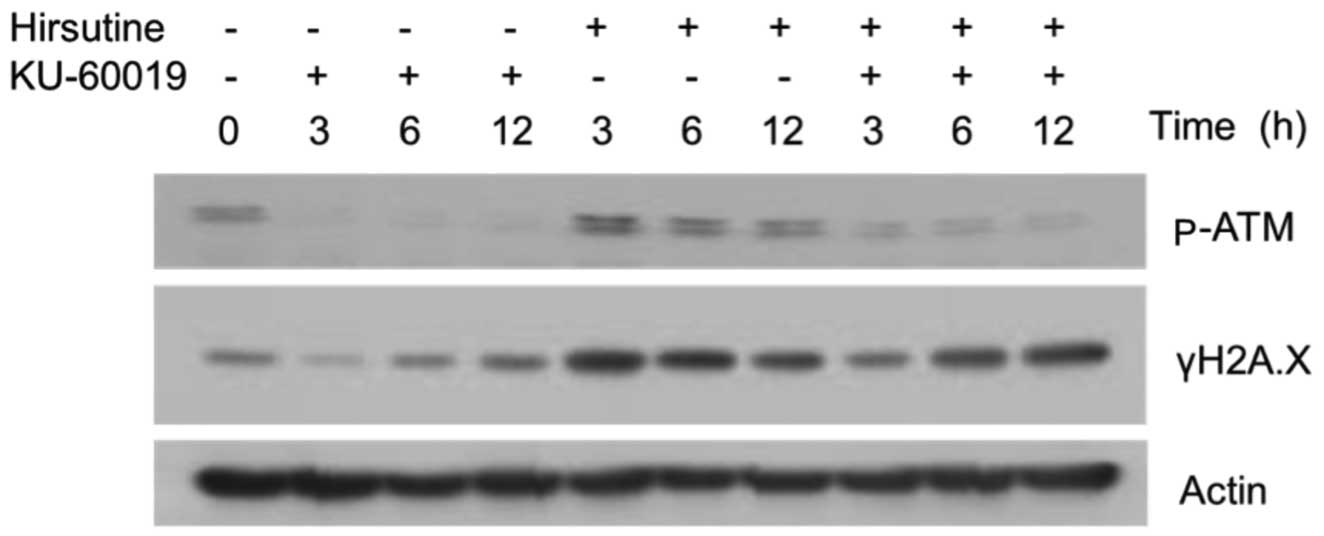
cells. As shown in Fig. 3, hirsutine
did not induce persistent activation of the DNA damage response, as
observed by the expression of γH2A.X in MCF-7 cell. Notably,
treatment with KU-60019 alone did have an affect; combination of
hirsutine and KU-60019 significantly induced the persistent DNA
damage response along with the suppression of ATM activation. Taken
together with the cytotoxicity data, the present study concludes
that interference of the ATM pathway is an important mechanism for
hirsutine-induced cytotoxicity by modulation of the DNA damage
response.
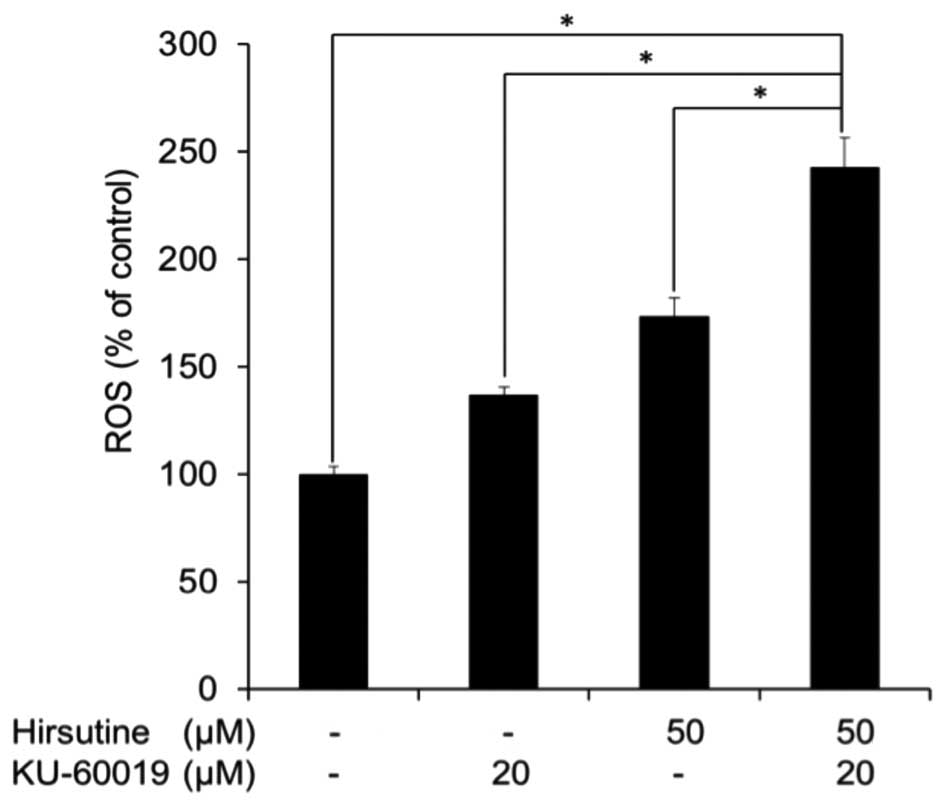
Hirsutine induces p53-independent DNA
damage response and ROS generation in MCF-7 cells
Since p53 is a well known product of the DNA damage
response, which induces cell death or repair and is expressed in
hirsutine-resistant cell lines (12),
the importance of p53 in ATM-dependent hirsutine-resistance of
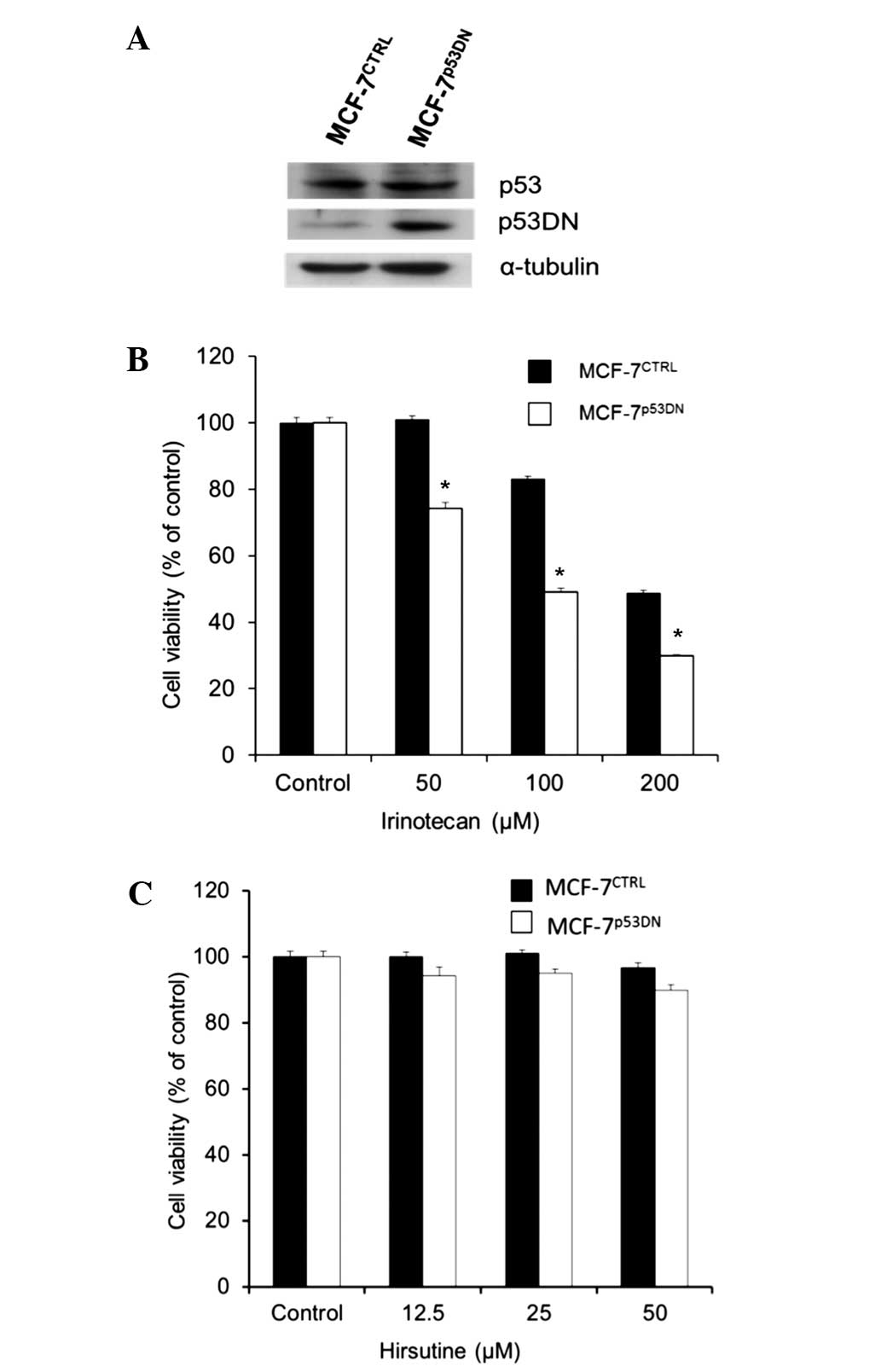
MCF-7 cells was examined by the present study using MCF-7 cells
that overexpressed dominant-negative p53 (MCF-7p53DN
cells; Fig. 4A). While
MCF-7p53DN cells exhibited a higher sensitivity to
irinotecan (Fig. 4B), which is a
typical DNA damage-inducing agent, no difference was observed in
the response between hirsutine-treated MCF-7p53DN and
MCF-7CTRL cells (Fig. 4C).
Therefore, the present study concludes that inhibition of the ATM
pathway did not require p53 to confer hirsutine-resistance of MCF-7
cells. By contrast, it is known that mitochondrial activity and ROS
generation are major contributors for the p53-independent DNA
damage response (13). Consequently,
the ROS expression level in MCF-7 cells treated with hirsutine and
KU-60019 was evaluated. As shown in Fig.
5, the ROS expression level was significantly increased
following co-treatment with hirsute and KU-60019 compared with
cells treated with hirsutine or KU-60019 alone. Collectively, the
present data indicate the potential utility of interfering with the
ATM pathway to overcome hirsutine resistance by inducing
p53-independent DNA damage response and ROS generation in MCF-7
cells.
Discussion
The DNA damage response is one molecular event that
results in apoptosis, and consequently numerous anti-cancer agents
induce a DNA damage response (14–18).
Hirsutine, one of the major alkaloids in Uncaria species,
exhibits an anti-metastatic effect in a murine breast cancer model
(6) and has an anti-tumor effect on
HER2+ breast cancer cells by inducing DNA damage
(7). However, certain breast cancer
cell lines, primarily hormone receptor (estrogen or progesterone
receptor) positive breast cancer MCF-7 and ZR-75-1 cells, have
exhibited resistance to hirsutine-induced cytotoxicity and the DNA
damage response in previous studies (6,7). The
present study used a chemical screening approach, which identified
that the ATM pathway is key for hirsutine-resistance in MCF-7
cells, and the DNA damage response is significantly amplified
following co-treatment of hirsutine and KU-60019, a specific ATM
inhibitor, in MCF-7 cells.
It has been widely recognized that the consequences
resulting from the DNA damage response to induce cell death is
counter regulated by the DNA repair response (12,19,20). ATM
kinases, key protein kinases for the DNA damage response, are known
to regulate double-strand break repair (21,22). In
response to low levels of DNA damage, ATM kinases activate p53 to
induce cell cycle arrest leading to successful DNA repair (19). In the present study, no difference was
observed in the hirsutine response between p53
MCF-7p53DN and control cells. Therefore, the present
study concludes that the sensitization to hirsutine-induced DNA
damage response in MCF-7 cells by interfering with the ATM pathway
is independent of p53. In addition to the p53-dependent DNA repair
response, the ATM-ROS pathway has been previously reported to
amplify a DNA-damaging response following genotoxic stress
(23). In the present study, the
level of ROS generation was significantly increased in MCF-7 cells
treated with a combination of ATM inhibitor and hirsute.
Considering p38 mitogen-activated protein kinase (MAPK) is known to
be important in the DNA damage response induced by genotoxic stress
with DNA-damaging chemotherapeutic agents (24) and a loss of ATM impairs the
proliferation of stem cells through oxidative stress-mediated p38
MAPK signaling (25,26), the present study hypothesizes that p38
MAPK stress signaling pathway possibly contributes to the
sensitization of MCF-7 cells to the hirsutine-induced DNA damage
response by interfering with the ATM pathway.
The present results indicate the potential utility
of interfering with the ATM pathway to overcome hirsutine
resistance in breast cancer cells, which induces a p53-independent
DNA damage response and ROS generation.
Acknowledgements
This work is partly supported by a grant-in-aid for
the Cooperative Research Project from the Institute of Natural
Medicine, University of Toyama. The authors would like to thank the
Screening Committee of Anticancer Drugs supported by a grant-in-aid
for Scientific Research on Innovative Areas, Scientific Support
Programs for Cancer Research (The Ministry of Education, Culture,
Sports, Science and Technology; Tokyo, Japan) for the provision of
the SCADS Inhibitor kit and Dr David E. Fisher for providing the
expression vector for p53 dominant negative mutant. Mr. Chenghua
Lou is supported by the Campus Asian Program of the University of
Toyama.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu LX, Gu XF, Zhu YC and Zhu YZ:
Protective effects of novel single compound, Hirsutine on hypoxic
neonatal rat cardiomyocytes. Eur J Pharmacol. 650:290–297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horie S, Yano S, Aimi N, Sakai S and
Watanabe K: Effects of hirsutine, an antihypertensive indole
alkaloid from Uncaria rhynchophylla, on intracellular calcium in
rat thoracic aorta. Life Sci. 50:491–498. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lou C, Takahashi K, Irimura T, Saiki I and
Hayakawa Y: Identification of Hirsutine as an anti-metastatic
phytochemical by targeting NF-κB activation. Int J Oncol.
45:2085–2091. 2014.PubMed/NCBI
|
|
7
|
Lou C, Yokoyama S, Saiki I and Hayakawa Y:
Selective anticancer activity of hirsutine against HER2-positive
breast cancer cells by inducing DNA damage. Oncol Rep.
33:2072–2076. 2015.PubMed/NCBI
|
|
8
|
Garraway LA, Widlund HR, Rubin MA, Getz G,
Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J,
et al: Integrative genomic analyses identify MITF as a lineage
survival oncogene amplified in malignant melanoma. Nature.
436:117–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Golding SE, Rosenberg E, Valerie N,
Hussaini I, Frigerio M, Cockcroft XF, Chong WY, Hummersone M,
Rigoreau L, Menear KA, et al: Improved ATM kinase inhibitor
KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and
ERK prosurvival signaling, and inhibits migration and invasion. Mol
Cancer Ther. 8:2894–2902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Golding SE, Rosenberg E, Adams BR,
Wignarajah S, Beckta JM, O'Connor MJ and Valerie K: Dynamic
inhibition of ATM kinase provides a strategy for glioblastoma
multiforme radiosensitization and growth control. Cell Cycle.
11:1167–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hickson I, Zhao Y, Richardson CJ, Green
SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ and Smith
GC: Identification and characterization of a novel and specific
inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer
Res. 64:9152–9159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norbury CJ and Zhivotovsky B: DNA
damage-induced apoptosis. Oncogene. 23:2797–2808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nair RR, Bagheri M and Saini DK:
Temporally distinct roles of ATM and ROS in
genotoxic-stress-dependent induction and maintenance of cellular
senescence. J Cell Sci. 128:342–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Huang M, Yang F, Chen Y, Miao ZH,
Qian XH, Xu YF, Qin YX, Luo HB, Shen X, et al: R16, a novel
amonafide analogue, induces apoptosis and G2-M arrest via poisoning
topoisomerase II. Mol Cancer Ther. 6:484–495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Y, Lu J, Miao Z, Lin L and Ding J:
Reactive oxygen species contribute to cell killing and
P-glycoprotein downregulation by salvicine in multidrug resistant
K562/A02 cells. Cancer Biol Ther. 6:1794–1799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai B, Lyu H, Huang J, Wang S, Lee CK, Gao
C and Liu B: Combination of bendamustine and entinostat
synergistically inhibits proliferation of multiple myeloma cells
via induction of apoptosis and DNA damage response. Cancer Lett.
335:343–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kudoh T, Kimura J, Lu ZG, Miki Y and
Yoshida K: D4S234E, a novel p53-responsive gene, induces apoptosis
in response to DNA damage. Exp Cell Res. 316:2849–2858. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rudolf E, Kralova V, Rudolf K and John S:
The role of p38 in irinotecan-induced DNA damage and apoptosis of
colon cancer cells. Mutat Res. 741–742:27–34. 2013. View Article : Google Scholar
|
|
19
|
Ljungman M: The DNA damage response-repair
or despair? Environ Mol Mutagen. 51:879–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roos WP and Kaina B: DNA damage-induced
cell death by apoptosis. Trends Mol Med. 12:440–450. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valerie K and Povirk LF: Regulation and
mechanisms of mammalian double-strand break repair. Oncogene.
22:5792–5812. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lavin MF: Ataxia-telangiectasia: From a
rare disorder to a paradigm for cell signalling and cancer. Nat Rev
Mol Cell Biol. 9:759–769. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito K, Takubo K, Arai F, Satoh H, Matsuoka
S, Ohmura M, Naka K, Azuma M, Miyamoto K, Hosokawa K, et al:
Regulation of reactive oxygen species by Atm is essential for
proper response to DNA double-strand breaks in lymphocytes. J
Immunol. 178:103–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sanchez-Prieto R, Rojas JM, Taya Y and
Gutkind JS: A role for the p38 mitogen-activated protein kinase
pathway in the transcriptional activation of p53 on genotoxic
stress by chemotherapeutic agents. Cancer Res. 60:2464–2472.
2000.PubMed/NCBI
|
|
25
|
Kim J and Wong PK: Loss of ATM impairs
proliferation of neural stem cells through oxidative
stress-mediated p38 MAPK signaling. Stem Cells. 27:1987–1998. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ito K, Hirao A, Arai F, Takubo K, Matsuoka
S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y and Suda T:
Reactive oxygen species act through p38 MAPK to limit the lifespan
of hematopoietic stem cells. Nat Med. 12:446–451. 2006. View Article : Google Scholar : PubMed/NCBI
|