Introduction
An enterovesical fistula (EVF) is defined as an
abnormal communication between the intestine and the bladder
(1). Enterovesical fistula (EVF) may
occur as a rare complication of diverticulitis, as well as Crohn's
disease, intestinal malignancy, radiotherapy and traumatic or
iatrogenic injuries. Patients with EVF most commonly present with
lower urinary tract symptoms, including pneumaturia, fecaluria,
frequency, urgency, suprapubic pain, recurrent urinary tract
infections and hematuria. EVF is a significant morbidity and the
associated lower urinary tract symptoms may deteriorate a patient's
quality of life (1). The standard
treatment for EVF is bowel resection with primary anastomosis, and
disease recurrence is rare (1). EVF
caused by primary small intestinal non-Hodgkin's lymphoma (NHL) has
been previously reported (2),
however, EVF developing after cytotoxic therapy or regressive
change of lymphoma is extremely rare. NHL had been classified as an
indolent and aggressive type of lymphoma. The current study
presents the case of a patient with NHL who developed EVF after
receiving cytotoxic therapy accompanied by regressive change of the
lymphoma. The study was approved by the ethics committee of Taipei
Veterans General Hospital, Taipei, Taiwan.
Case report
A cutaneous marginal zone B-cell lymphoma was
identified in the left orbital area in a 35-year-old Taiwanese man
during local excision at the Taipei Veterans General Hospital
(Taipei, Taiwan) in October 2012. Multiple neck lymphadenopathy
with B symptoms developed from January 2014. Excisional biopsy of
the neck lymph node was performed. Lymph node tissue (4-µm)
sections were formalin-fixed, paraffin embedded (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and stained with hematoxylin
and eosin (Sigma-Aldrich, St. Louis, MO, USA). Immunohistochemical
staining revealed positivity for CD20 (mouse anti-human monoclonal
antibody; clone, L-26; 1:300; cat. no. M0755; Dako, Glostrup,
Denmark), B-cell lymphoma-6 (mouse anti-human monoclonal antibody;
clone, LN22; 1:200; cat. no. NCL-L-BCL-6-564; Leica Microsystems
Ltd., Milton Keynes, UK) and MUM1 (mouse anti-human monoclonal
antibody; clone, EAU32; 1:1,000; cat. no. NCL-L-MUM1; Leica
Microsystems Ltd.). Subsequently, diffuse large B cell lymphoma, a
type of non-Hodgkin's lymphoma (NHL), was diagnosed. The clinical
stage was IV (3) with involvement of
the liver and right kidney, as well as lymphadenopathy of the
bilateral neck, terminal ileum and gastrohepatic ligament. Standard
chemotherapy with R-CHOP every 3 weeks for 6 cycles, comprising
rituximab (375 mg/m2), cyclophosphamide (750
mg/m2), doxorubicin (50 mg/m2), vincristine
(1.4 mg/m2) and prednisolone (100 mg/day for 5 days),
commenced in March 2014.
Although the neck lymphadenopathy exhibited marked
regression after the first course of chemotherapy, intermittent
nausea and vomiting, abdominal bloating and perineal pain with
dysuria developed after the second course of R-CHOP. Laboratory
examinations revealed the following results: White blood cell
count, 29,000/µl (normal range, 4,500–11,0000/µl); hemoglobin
level, 13.7 g/dl (normal range, 14–18 g/dl); platelet count,
624×103/µl (normal range, 150–350×103/µl);
blood urea nitrogen level, 4.0 mg/dl (normal range, 7.0–20.0
mg/dl); creatinine level, 1.04 mg/dl (normal range, 0.7–1.5 mg/dl);
uric acid level, 3.3 mg/dl (normal range, 2.5–7.2 mg/dl);
C-reactive protein level, 4.26 mg/dl (normal range, <0.5 mg/dl);
and lactate dehydrogenase level, 166 U/ml (normal range, 149–519
U/ml). In the urinary microscopic examination (Eclipse 50i
microscope; Nikon Corporation, Tokyo, Japan), >100 red blood
cells and >100 white blood cells per high-power field
(magnification, ×40) were identified (normal range, 0–2 per
high-power field).
Empiric antibiotic treatment with intravenous
cefuroxime (1.5 g, every 8 h) was administered to treat the urinary
tract infection, however, it was unsuccessful. The patient
continued to experience hematuria, pyuria and perineal tenderness.
In addition, pneumaturia and fecaluria occurred during
hospitalization at the Taipei Veterans General Hospital. A
cystoscopic examination revealed an irregular surface, edema and
erythematous changes of the bladder wall. An intravenous pyelogram
demonstrated no genitourinary structural abnormalities. Urine
cytology for malignancy was negative. An acid-fast stain and
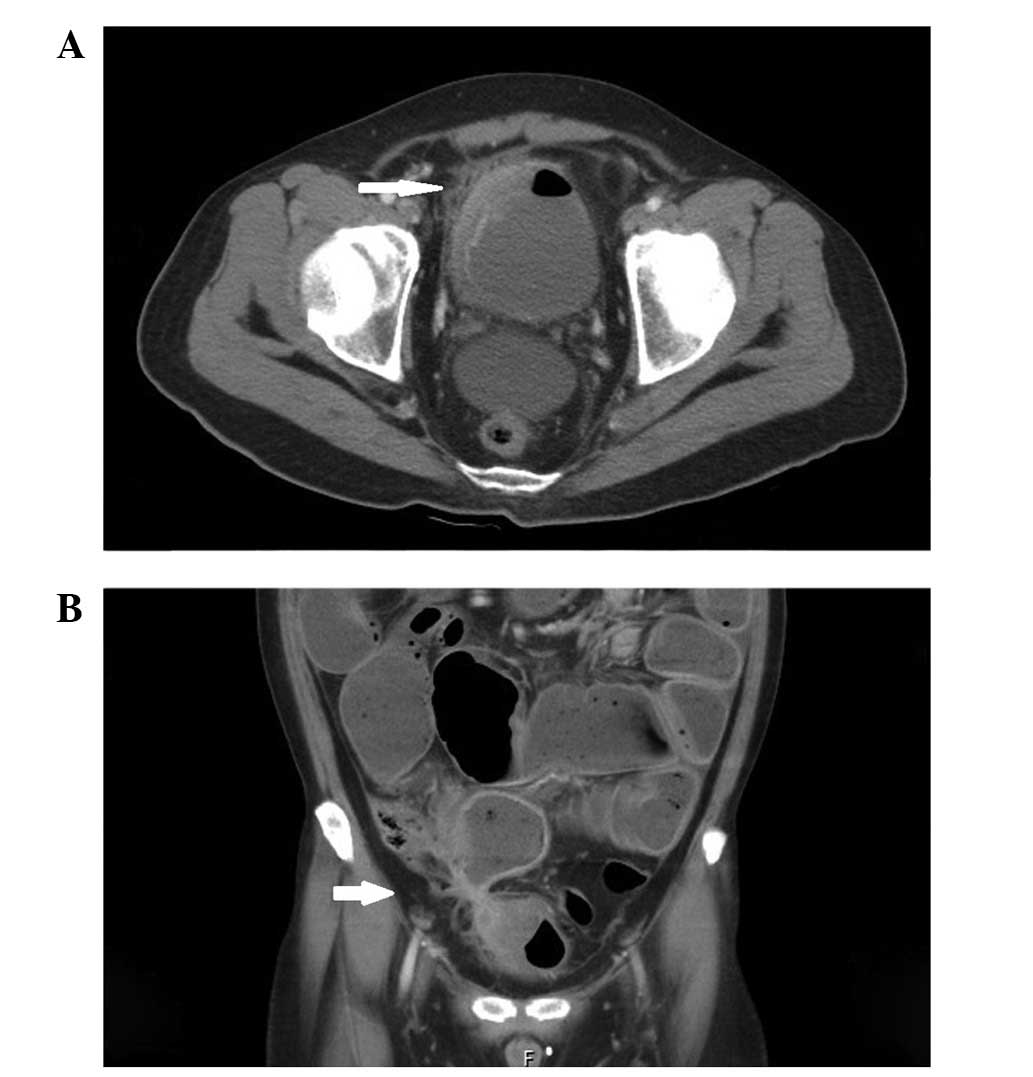
tuberculosis culture were also negative. Abdominal computed
tomography (CT) revealed adherence and communication between the
terminal ileum and bladder, with intestinal obstruction and focal
wall thickening of the urinary bladder and adjacent intestine
(Fig. 1). Furthermore, retrograde
cystourethrography was performed; however, this examination also
revealed no remarkable findings, other than inflammatory changes.
Laparotomy was performed in June 2014, and a fistula between the
terminal ileum and bladder was identified. A partial resection of
the terminal ileum, as well as partial cystectomy and ileostomy
were performed. The resected small intestine and colon, measuring
15×5 cm, contained multiple ulcers and one perforation. The
microscopic findings (magnification, ×40; Eclipse 50i microscope;
Nikon Corporation) indicated heavy acute and chronic inflammatory
cells that infiltrated the whole layer of intestinal wall, but no
evidence of lymphoma cells.
The patient experienced a good recovery, without
complication. The patient completed 6 cycles of chemotherapy with
R-CHOP, and then underwent surgery to close the ileostomy. No
recurrence of EVF was indicated during the 22-month follow-up
period.
Discussion
EVF is an abnormal communication between the
intestine and the bladder. Depending on the area of the bowel
involved, EVF is classified as colovesical, rectovesical,
ileovesical or appendicovesical fistulae. The prevalence is ~1 in
every 3,000 surgical hospital admissions (4). The symptoms of EVF include pneumaturia,
fecaluria, frequency, urgency, suprapubic pain, recurrent urinary
tract infections and hematuria (1).
Inflammatory changes of the bowel wall with
adherence to the bladder wall are considered to result in EVF
formation (1). EVF is not only a
complication of primary small intestine lymphoma but also occurs in
patients undergoing treatment of lymphoma. In the current patient,
lymphoma with terminal ileum involvement was noted initially.
However, it was subsequently identified that a large number of
inflammatory cells had infiltrated the bowel wall following
chemotherapy. The regressive change of lymphoma cells may have
resulted in focal intestinal inflammation and adherence, and led to
fistula and intestinal obstruction. Another potential mechanism is
a cytotoxic agent associated with regional enteritis following EVF
formation (5).
The diagnosis of EVF may be challenging for
clinicians and radiologists. Various diagnostic tools and
procedures are used to detect EVF. The Taipei Veterans General
Hospital prefers to use CT and magnetic resonance imaging
techniques to identify the fistula tract, as they provide a higher
detection rate compared with endoscopy or radiographic examination.
The accuracy of these diagnostic tests may be associated with the
location and characteristics of EVF (1,6). For
example, cystourethrography was arranged for the present patient,
however, no contrast outside the bladder was shown. When the
location of EVF is higher than the contrast agent can reach,
contrast cannot be used to track the fistula.
Management of EVF is dependent on the patient's
status. As spontaneous closure of the fistula is typically rare,
surgical resection is preferred for the majority patients with EVF
(1,6).
The outcome of surgical resection is good and recurrence is
uncommon for patients with benign disease or nonradiation-induced
fistulae (1,6), as well as primary intestinal NHL
(2). However, patients with NHL may
have to decide between surgical repair and postponing the scheduled
chemotherapy, or delaying the surgery and risk developing sepsis.
Thus far, the optimal timing of surgical treatment has not been
established. In the present case, surgical repair was arranged and
the scheduled chemotherapy was postponed, as the patient was
aggravated by the symptoms and the lymphoma was under control.
Ansari et al also reported a patient with NHL who developed
EVF following chemotherapy and and had an uneventful recovery
following a surgical resection (5).
In conclusion, the appearance of EVF is a rare
complication, but may develop in patients following cytotoxic
treatment. When suspicious of EVF formation, appropriate diagnostic
investigation should be used to aid clinicians in the early
recognition of EVF and avoid any delay in treatment. Surgical
resection of the fistula is feasible.
References
|
1
|
Golabek T, Szymanska A, Szopinski T,
Bukowczan J, Furmanek M, Powroznik J and Chlosta P: Enterovesical
fistulae: Aetiology, imaging, and management. Gastroenterol Res
Pract. 2013:6179672013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shinji S, Akimaru K, Tsuchiya Y, Shimizu
T, Kawamoto M, Iwamoto M, Yamaguchi N, Suzuki H, Yamada T, Nikaido
T and Uchida E: Enterovesical fistula caused by non-Hodgkin's
lymphoma of the ileum: Report of a case. Surg Today. 42:1005–1009.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Alliance,
Australasian Leukaemia and Lymphoma Group; Eastern Cooperative
Oncology Group; European Mantle Cell Lymphoma Consortium; Italian
Lymphoma Foundation; European Organisation for Research; Treatment
of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula
Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's
Study Group; Japanese Lymphorra Study Group; Lymphoma Study
Association; NCIC Clinical Trials Group; Nordic Lymphoma Study
Group; Southwest Oncology Group; United Kingdom National Cancer
Research Institute: Recommendations for initial evaluation,
staging, and response assessment of Hodgkin and non-Hodgkin
lymphoma: The Lugano classification. J Clin Oncol. 32:3059–3068.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pugh JI: On the pathology and behaviour of
acquired non-traumatic vesico-intestinal fistula. Br J Surg.
51:644–657. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ansari MS, Nabi G, Singh I, Hemal AK and
Pandey G: Colovesical fistula an unusual complication of cytotoxic
therapy in a case of non-Hodgkin's lymphoma. Int Urol Nephrol.
33:373–374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scozzari G, Arezzo A and Morino M:
Enterovesical fistulas: Diagnosis and management. Tech Coloproctol.
14:293–300. 2010. View Article : Google Scholar : PubMed/NCBI
|