Introduction
Osteosarcoma (OS) is the most common primary bone
malignancy, occurring predominantly in the long bones of children
and young adults. Long-term survival rates have improved from
<20% to 65–70% following the advent of multiagent chemotherapy
regimens (1). It is estimated that
the annual incidence of osteosarcoma is 4–5 cases per 1,000,000
individuals worldwide (2). Due to its
high propensity for distant metastasis (typically to the lungs), OS
is one of the leading causes of cancer-associated mortality in
adolescents (3). Metastasis, a major
cause of cancer treatment failure and mortality, is a complex
process in which various events are involved, including cell
migration, angiogenesis, adhesion and cell proliferation (4). While the molecular mechanisms underlying
cancer metastasis remain to be fully elucidated, it has been
reported that a number of inducers and suppressors have significant
roles.
Collapsin response mediator protein-1 (CRMP-1), a
member of the CRMP family, is involved in cortical neuronal
migration via reelin signaling (5).
CRMP-1 has been observed to be a cancer invasion suppressor
(6). In addition, a negative
association between CRMP-1 expression and cancer cell invasiveness
has been observed in non-small cell lung cancer (NSCLC) patients
(7). Pan et al identified a
novel isoform of CRMP family proteins in 2008, which was termed
long form CRMP-1 (LCRMP-1) (8). In
contrast to CRMP-1, which inhibits the migration and invasion of
NSCLC, LCRMP-1 promotes filopodia formation, cancer cell migration
and invasion, resulting in poor clinical outcomes in NSCLC patients
(9). Therefore, LCRMP-1 is an
invasion enhancer in NSCLC.
As OS has a high propensity for metastasis, and
LCRMP-1 is able to promote the metastasis of NSCLC, it may be
useful to identify whether LCRMP-1 is involved in the metastasis of
OS. In the present study, the expression of LCRMP-1 was
investigated and compared in OS patients and cell lines, as well as
in a normal osteoblast cell line. In addition, migration and
invasion assays were performed to investigate the migration and
invasion of OS cells.
Materials and methods
Ethical approval
All human experiments were approved by the First
Affiliated Hospital of Jinan University (Guangzhou, China). All
experiments using human specimens were performed in accordance with
the ethical standards set out in the 1964 Declaration of Helsinki
and its later amendments. Informed consent was received from all
participants prior to commencement of the study.
Specimens
Formalin-fixed, paraffin-embedded tissue samples
were obtained from 20 OS patients that received amputation at the
Department of Orthopedics, First Affiliated Hospital of Jinan
University (Guangzhou, China), between 1998 and 2013. Normal bone
tissue from the same patient was used for control. Twenty patients
with OS of the bone were selected from the files of the Department
of Clinical Pathology at the Vienna General Hospital (Vienna,
Austria). All cases were reviewed to confirm the diagnoses and to
select a paraffin wax block for immunohistochemical studies.
Cell culture and generation of small
interfering (si)RNA stable cell line
The human osteoblast hFOB 1.19 and OS cell lines
SAOS2, MG63 and U2OS were supplied by the Cell Bank of the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in Dulbecco's minimal essential
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS), penicillin
(100 U/ml), and streptomycin (100 µg/ml) at 37°C in a humidified
atmosphere with 5% CO2.
A lentiviral (LV)-008 vector (Forevergen
Biosciences, Guangzhou, China) with a U6 promoter was used to
generate the recombinant lentivirus plasmid encoding small hairpin
(sh)-LCRMP-1. The si-LCRMP-1 sequence was as follows:
5′-CAGCGAGGACACGGCCAGCGA-3′. HEK 293T cells (Forevergen
Biosciences) were cotransfected with LV-008 and packaging vectors
(Forevergen Biosciences), and the culture supernatant was harvested
following 48 and 72 h of transfection. The OS MG63 cells were
infected by sh-LCRMP-1 (shp300) and sh-scramble [negative control,
(NC)] in the presence of 5–10 µg/ml Polybrene (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), respectively. After 48 h,
the cells were grown in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin with 2 µg/ml puromycin for 12
days, to generate OS MG63 sh-LCRMP-1 stable cell lines.
Migration assay
The cell migration assay was performed using
Transwell cell culture chambers (EMD Millipore, Billerica, MA,
USA). The OS MG63 cells were cultured in serum-free DMEM for 24 h.
The cells were subsequently trypsinized and resuspended in
serum-free DMEM. The cells (5×104 per well) were seeded
into the upper chamber of the Transwell insert and incubated with
0.5% dimethyl sulfoxide (10 µM; Sigma-Aldrich, St. Louis, MO, USA)
or deguelin (15 µM; Sigma-Aldrich), and 90% DMEM medium containing
10% FBS was added to the lower chamber and incubated for 48 h.
Following incubation, the remaining cells in the upper chamber were
removed. The migrated cells on the lower surface of the filter were
fixed with 4% formaldehyde and stained with 2% crystal violet in 2%
ethanol. The cells were subsequently counted and images were
captured under a microscope (magnification, ×200; Olympus BH-2;
Olympus, Tokyo, Japan).
Invasion assay
The cell invasion assay was performed with similar
methodology to the aforementioned migration assay, but with certain
modifications. The filter membrane used in the invasion assay was
coated with Matrigel (BD Biosciences). The cells on the lower
surface of the filter were counted and images were captured under a
light microscope (magnification, ×200; Olympus BH-2). The invasive
cells were fixed with 4% formaldehyde and stained with 2% crystal
violet in 2% ethanol. Cell numbers were counted and expressed as
the mean number of cells (mean ± standard deviation). Experiments
were performed in triplicate.
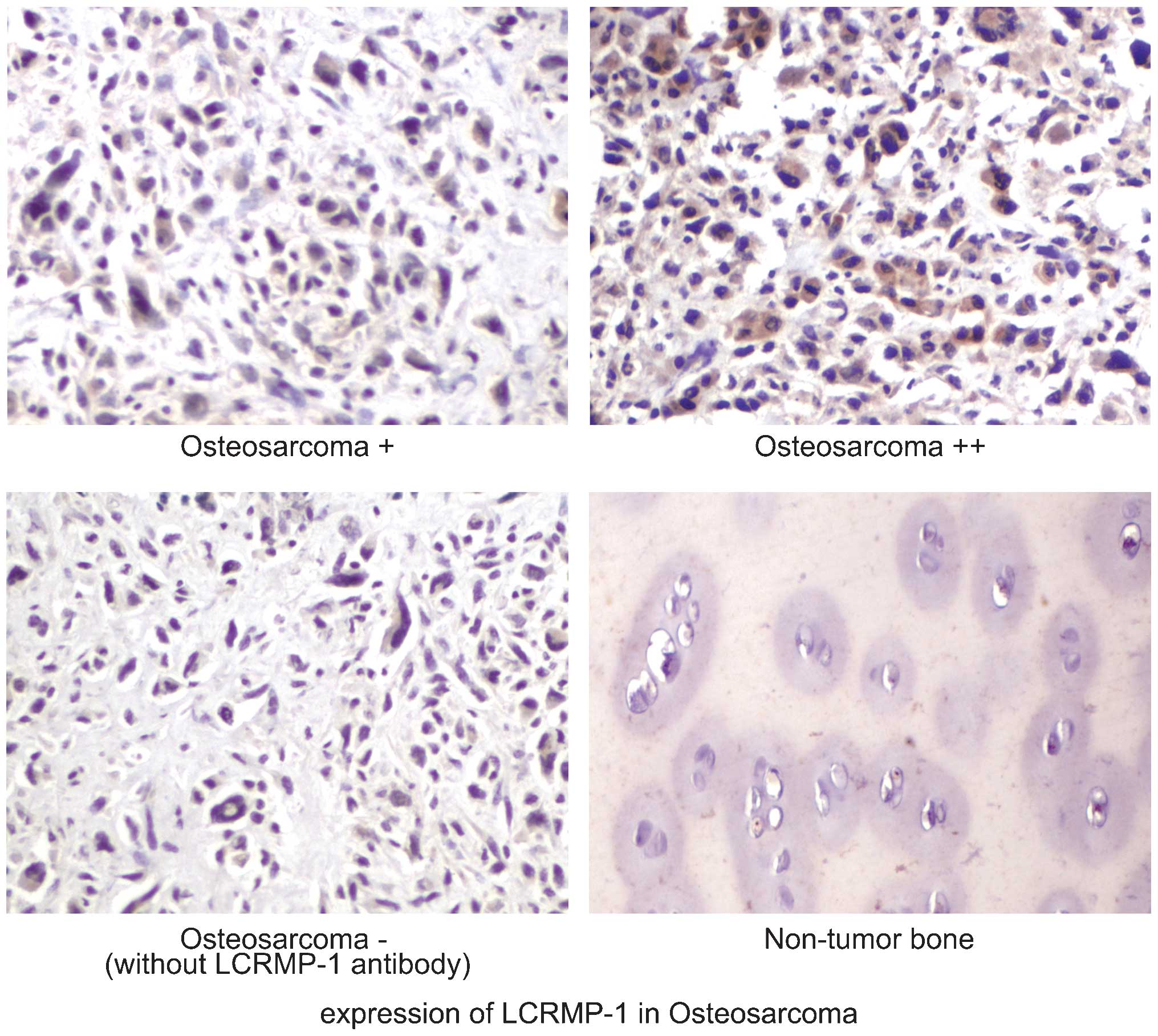
Immunohistochemistry (IHC)
The specific rabbit anti-human anti-LCRMP-1
polyclonal antibody (C2) for western blotting and IHC was produced
by the authors using a synthetic peptide derived from the unique
N-terminal region of LCRMP-1, as described previously (6). Formalin-fixed, paraffin-embedded
sections (5-µm thick) were processed for IHC. Antigen retrieval was
performed with microwave treatment in 10 mM citrate buffer (pH
6.0). The endogenous peroxidase activity was quenched by 3%
hydrogen peroxide for 10 min. The sections were fixed using freshly
prepared 4% paraformaldehyde, followed by permeabilization with
0.1% Triton X-100 in Tris-buffered saline and blocking in 5% FBS.
The antigen was incubated with LCRMP-1 (C2) antibody overnight at
4°C. Detection of the immunoreactive staining was performed using
the Super Sensitive™ Polymer HRP Detection system (BioGenex,
Fremont, CA, USA) according to the manufacturer's protocols. The
intensity of anti LCRMP-1 staining was semiquantitatively analyzed.
Briefly, the number of positively stained cells and total number of
cells in a given area were evaluated by two pathologists in a
blinded manner. If the amount of positively stained cells was ≤5%
the tissue was considered to be negative, 6–25% was weak expression
(+); and 26–50% was moderate expression (++). A total of at least
400 cells from five areas of individual tissue samples were
evaluated. The non-tumor bone was stained with anti-LCRMP-1
antibody.
Western blot analysis
Samples were lysed in sample buffer [(50 mM
Tris-HCl, pH 6.8; 10% glycerol, 2% sodium dodecyl sulfate (SDS),
0.000125% bromophenol blue and 5% β-mercaptoethanol] and subjected
to SDS-polyacrylamide gel electrophoresis (10–40 µg protein of
protein/lane), followed by incubation with rabbit anti-human
anti-LCRMP-1 polyclonal antibody (C2), N-Cadherin (dilution,
1:1,000; catalog no., ab18203; Abcam, Cambridge, MA, USA), matrix
metalloproteinase (MMP)-2 (dilution, 1:1,000; catalog no., ab37150;
Abcam) and MMP-9 antibody (dilution, 1:1,000; catalog no., ab38898;
Abcam). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; dilution,
1:2,500; ab9485, Abcam) was used as a loading control. Subsequent
to being washed five times with phosphate-buffered saline
containing 0.1% Tween 20, the membrane was incubated with the goat
anti rabbit IgG (H+L) secondary antibodies (catalog no., G-21234;
GE Healthcare Life Sciences, Uppsala, Sweden) for 1 h, and bands
were detected by enhanced chemiluminescence (Forevergen
Biosciences).
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from 5×106 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Initially, RNA was reverse transcribed
using M-MLV Reverse Transcriptase (M1705; Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. qPCR
was performed on a MiniOpticon™ Real-Time PCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using 20 µl reaction
mixture, according to the manufacturer's protocol. The reagent used
in qPCR was GoTaq® qPCR Master Mix (A6002; Promega
Corporation). GAPDH was the internal control (Gene ID, 2597). The
oligonucleotide primers used were as follows: LCRMP-1 forward,
5′-GGAGGCAGCGAGGACAC-3′ and reverse, 5′-CGTCAGCATAAAGGGATTGG-3′;
and GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′GACAAGCTTCCCGTTCTCAG-3′, provided by Forevergen Bioscience. The
product signals were detected using SYBR Green. The PCR
amplification program consisted of denaturation at 95°C in advance
for 2 min, followed by 40 cycles, each cycle consisting of
denaturation at 95°C for 15 sec, annealing and extension at 60°C
for 30 sec. The expression level of LCRMP-1 relative to that of
GAPDH was defined as 2−ΔΔCq = 2−[(ΔCq) LCRMP-1 -
(ΔCq) GAPDH] (10). All
experiments were repeated thrice.
Statistical analysis
All statistical analyses were performed using the
SPSS 19.0 statistical software package (IBM SPSS, Armonk, NY, USA)
Data are expressed as the mean ± standard deviation. Statistical
significance was determined by an analysis of variance. P-values of
<0.05 were considered to indicated a statistically significant
difference.
Results
Upregulation of LCRMP-1 in
osteosarcoma specimens and cell lines
The expression of LCRMP-1 in OS and normal tissues
was evaluated using IHC. The expression of LCRMP-1 in the OS
specimens was significantly increased compared with that in normal
bone tissues (Fig. 1). Western blot
and RT-qPCR analyses were subsequently performed to evaluate the
expression of LCRMP-1 at the protein and mRNA levels in the OS
SAOS2, MG63 and U2OS cell lines. The results of the present study
showed that LCRMP-1 mRNA and protein levels were significantly
increased in the OS cell lines compared with the levels in
osteoblast cells (P<0.05), and that the MG63 cells demonstrated
the highest expression of LCRMP-1 (Fig.
2A and B). This suggested that LCRMP-1 was overexpressed in OS,
and that it may have a significant role in OS development and
progression.
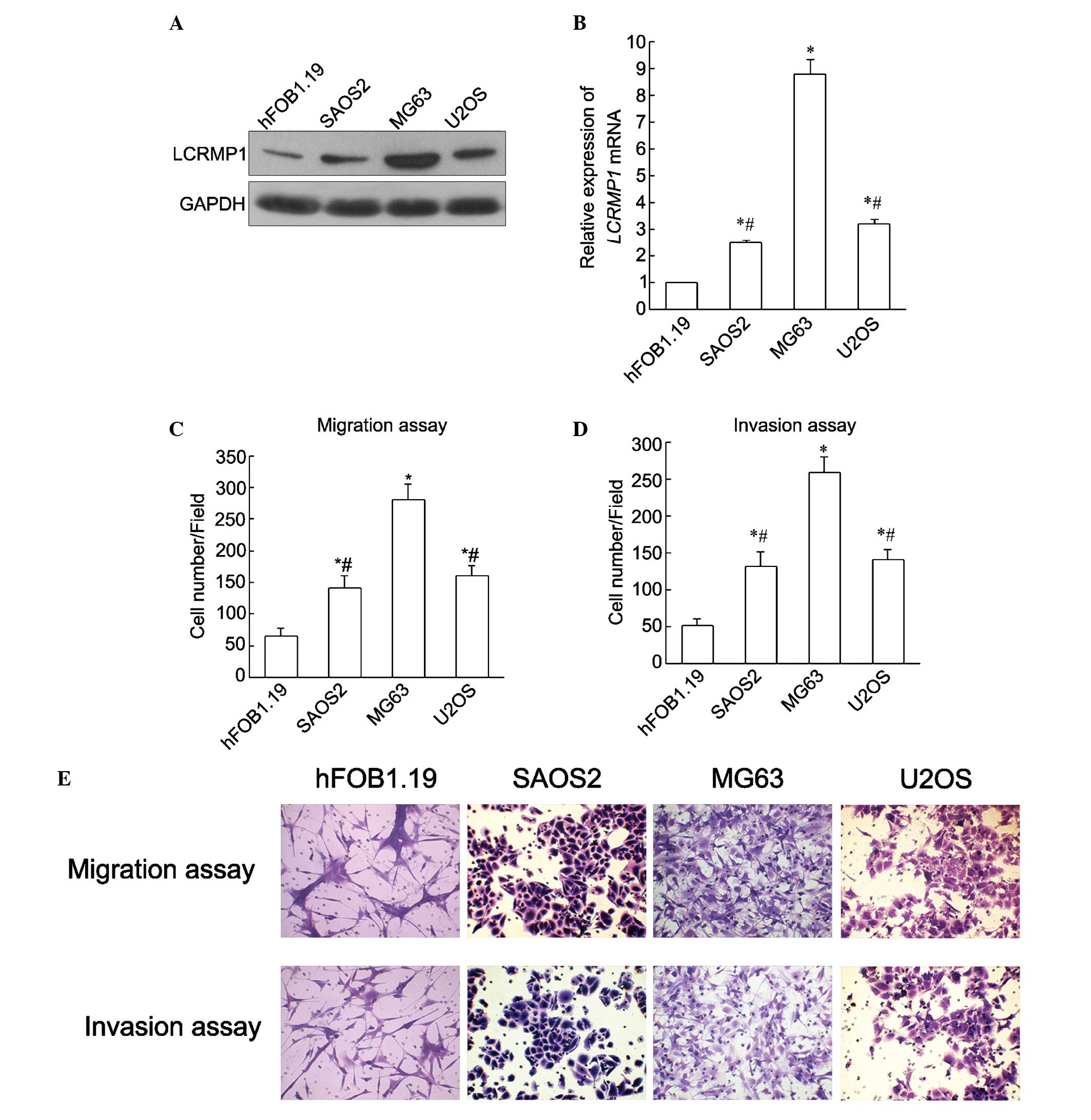 | Figure 2.Invasion and migration ability in OS
cell lines with varying expression levels of LCRMP-1. LCRMP-1 was
upregulated in OS cells at the (A) protein and (B) mRNA levels.
mRNA levels were confirmed by reverse transcription-quantitative
polymerase chain reaction and protein levels were confirmed by
western blotting. (C) Migration and (D) invasion ability in three
OS cell lines, SAOS2, MG63 and U2OS, compared with the human
osteoblast hFOB1.19 cell line. (E) Immunohistochemistry results are
presented. Increased migration and invasion abilities were observed
in the three OS cell lines. In addition, migration and invasion
abilities were consistent with LCRMP-1 expression in each cell
line. Among the three OS cell lines, the MG63 cells demonstrated
the highest migration and invasion abilities. *P<0.05 vs.
hFOB1.19. #P<0.05 vs. MG63. All experiments were
performed in triplicate and the results are expressed as the mean ±
standard deviation, analyzed by analysis of variance. OS,
osteosarcoma; LCRMP-1, long form collapsin response mediator
protein-1; mRNA, messenger RNA; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
To investigate the potential role of LCRMP-1 in the
migration and invasion of OS cells, migration and invasion assays
were performed in the OS SAOS2, MG63 and U2OS cell lines. As shown
in Fig. 2, the migrated and invasive
cells were stained, and the number of stained cells represented the
migratory and invasive ability of the OS cells. Increased migration
and invasion abilities were observed in the three OS cell lines. In
addition, migration and invasion abilities were consistent with
LCRMP-1 expression in each cell line. Among the three OS cell
lines, the MG63 cells demonstrated the highest migration and
invasion abilities (P<0.05; Fig.
2). Therefore, the MG63 cell line was used in follow-up
experiments to further investigate the role of LCRMP-1 in the
migration and invasion of OS cells by knockdown of LCRMP-1.
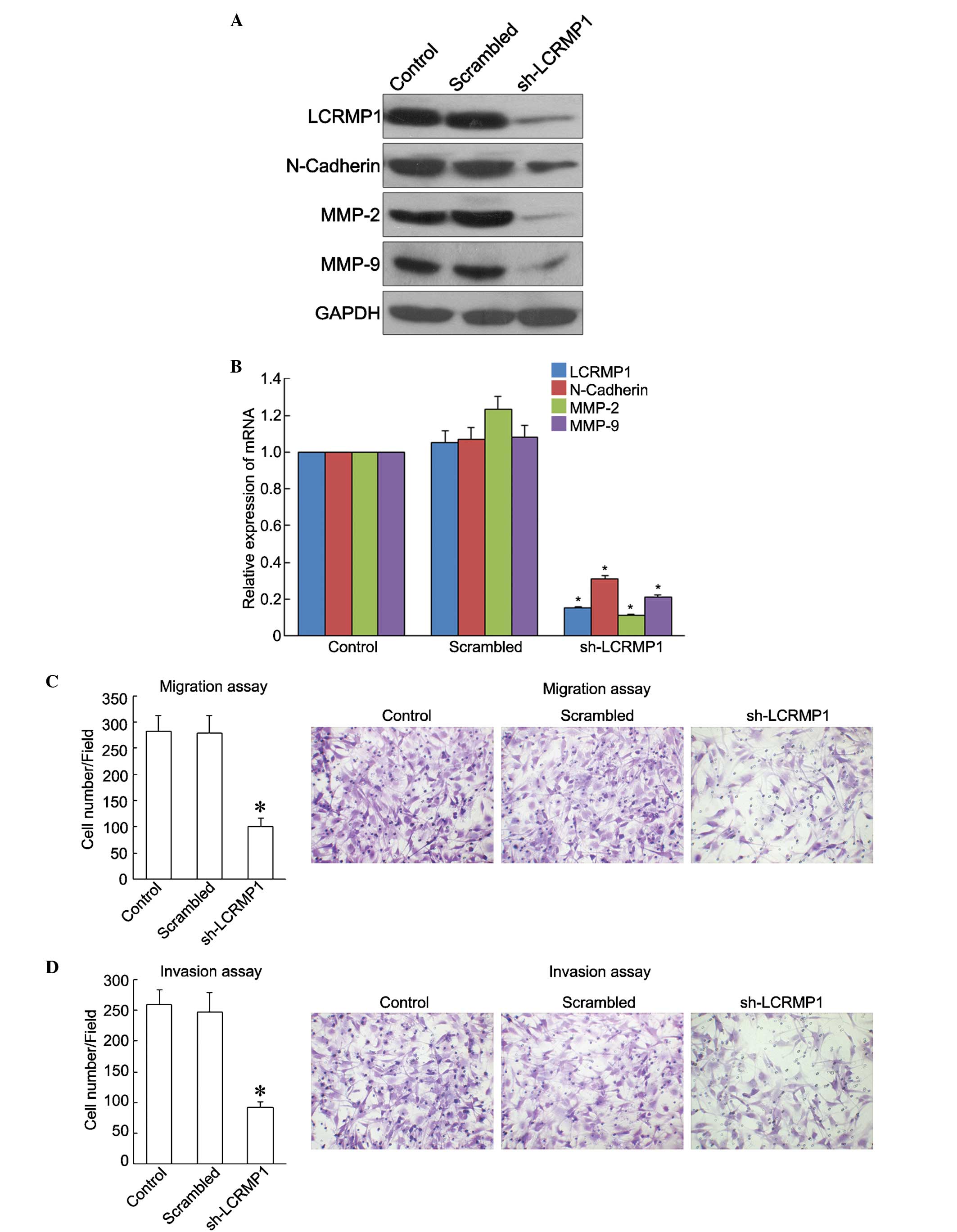
Knockdown of LCRMP-1 suppresses OS
cell migration and invasion
A stable LCRMP-1-knockdown cell line (sh-LCRMP-1)
was established to further investigate the role of LCRMP-1 in OS
metastasis. The role of LCRMP-1 in tumor cell migration and
invasion was examined in cells that constitutively expressed
si-LCRMP-1 and in control cells. Western blot and RT-qPCR analyses
showed that LCRMP-1 expression was significantly decreased in the
sh-LCRMP-1 cells compared with that in the control and negative
control cells (scramble) (P<0.05). In addition, reduced
expression of migration and invasive factors, including N-Cadherin,
MMP-2 and MMP-9, at the protein and mRNA levels was observed in the
sh-LCRMP-1 cells (P<0.05; Fig. 3A and
B). Migration and invasion assays were subsequently performed
to evaluate the effects of LCRMP-1 downregulation on OS metastasis.
As shown in Fig. 3C and D,
LCRMP-1-knockdown significantly inhibited the migration and
invasion of the MG63 cells compared with the control and scrambled
cells (P<0.05). The results of the present study suggested that
LCRMP-1 promotes the migration and invasion of OS cells.
Discussion
Osteosarcoma is the most common primary bone
malignancy in children and adolescents, accounting for ~20% of all
bone cancer cases. In total, 15–25% of OS cases metastasize
(primarily to the pulmonary system), resulting in failure of
anticancer treatments in OS patients. It has been reported that the
5-year disease-free survival of OS patients with metastasis is
10–20%, while in patients without metastasis, the rate is 60–70%
(11,12). However, the molecular mechanisms
underlying OS metastasis largely remain to be elucidated,
therefore, the identification of novel factors involved in OS
metastasis has significant and potential applications in anticancer
treatments for OS patients.
LCRMP-1 is a newly identified tumor enhancer in
NSCLC patients. A previous study showed that LCRMP-1 overexpression
promoted cancer cell invasion in vitro and in vivo,
and that it was associated with poor overall and disease-free
survival in NSCLC patients (8). It
has also been shown that LCRMP-1 enhances filopodia formation and
is phosphoregulated by glycogen synthase kinase 3β in NSCLC cells
(9,13). The results of these previous studies
led the present study to investigate the role of LCRMP1 in OS
progression. In the present study, the results suggested that
LCRMP-1 may promote the migration and invasion of OS cells.
The diagnosis of OS depends largely on biopsy and
imaging examinations, and a failed early diagnosis of OS leads to
delayed treatment (14–16). In addition, certain patients still
suffer from metastases and other complications following aggressive
treatments, including ablation and chemotherapy, suggesting that OS
is highly resistant to chemotherapy and has a high potential to
metastasize (17,18). Therefore, novel approaches for
diagnosis and novel drug targets are required. It has been reported
that LCRMP-1 promotes the migration and invasion of NSCLC cells
(8,9).
In addition, increased expression of N-cadherin and MMPs following
LCRMP-1 upregulation has confirmed that LCRMP-1 has a significant
role in the migration, invasion and metastasis of tumor cells, as
N-cadherin and MMPs are involved in epithelial-mesenchymal
transition and remodelling of the extracellular matrix (19,20). In
the present study, overexpression of LCRMP-1 was detected in OS
cell lines. Furthermore, knockdown of LCRMP-1 resulted in
significant downregulation of N-cadherin and MMPs (MMP-2 and
MMP-9), which was consistent with the significantly reduced number
of OS cells with migratory and invasive properties. The results of
the present study suggest that LCRMP-1 may be able to promote OS
metastasis, possibly via activation of N-cadherin and MMPs.
Previously, cancer cell lines derived from OS,
including SAOS2, MG63 and U2OS, have been used as important models
for studying the metastatic mechanisms of OS, through evaluating
morphological behaviors and gene expression alterations (21). In the present study, the results
indicated that LCRMP-1 may promote the migration and invasion of OS
cells. However, the downstream and upstream events of LCRMP-1
overexpression in OS require investigation in future studies to
further understand the underlying molecular mechanisms.
In summary, the present study showed that LCRMP-1
was overexpressed in OS specimens and cell lines. LCRMP-1 promoted
OS metastasis, possibly through the activation of N-cadherin and
MMPs (MMP-2 and MMP-9). The results of the present study suggest
that LCRMP-1 may be a potential target for the effective treatment
of metastatic OS.
References
|
1
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamashita N, Uchida Y, Ohshima T, Hirai S,
Nakamura F, Taniguchi M, Mikoshiba K, Honnorat J, Kolattukudy P,
Thomasset N, et al: Collapsin response mediator protein 1 mediates
reelin signaling in cortical neuronal migration. J Neurosci.
26:13357–13362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shih JY, Lee YC, Yang SC, Hong TM, Huang
CY and Yang PC: Collapsin response mediator protein-1: A novel
invasion-suppressor gene. Clin Exp Metastasis. 20:69–76. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih JY, Yang SC, Hong TM, Yuan A, Chen
JJ, Yu CJ, Chang YL, Lee YC, Peck K, Wu CW and Yang PC: Collapsin
response mediator protein-1 and the invasion and metastasis of
cancer cells. J Natl Cancer Inst. 93:1392–1400. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan SH, Chao YC, Chen HY, Hung PF, Lin PY,
Lin CW, Chang YL, Wu CT, Lee YC, Yang SC, et al: Long form
collapsin response mediator protein-1 (LCRMP-1) expression is
associated with clinical outcome and lymph node metastasis in
non-small cell lung cancer patients. Lung Cancer. 67:93–100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan SH, Chao YC, Hung PF, Chen HY, Yang
SC, Chang YL, Wu CT, Chang CC, Wang WL, Chan WK, et al: The ability
of LCRMP-1 to promote cancer invasion by enhancing filopodia
formation is antagonized by CRMP-1. J Clin Invest. 121:3189–3205.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meyers PA and Gorlick R: Osteosarcoma.
Pediatr Clin North Am. 44:973–989. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WL, Hong TM, Chang YL, Wu CT, Pan SH
and Yang PC: Phosphorylation of LCRMP-1 by GSK3β promotes filopoda
formation, migration and invasion abilities in lung cancer cells.
PLoS One. 7:e316892012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klein MJ and Siegal GP: Osteosarcoma:
Anatomic and histologic variants. Am J Clin Pathol. 125:555–581.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harada S, Wei S and Siegal GP: Molecular
pathology of osteosarcoma. In: Bone Cancer. Primary Bone Cancers
and Bone Metastases. Heymann D: (2nd). Academic Press. (Cambridge,
MA). 213–222. 2014.
|
|
16
|
Tirino V, Paino F, Papaccio F, La Noce M,
Papaccio G and Desiderio V: Stemness markers of osteosarcoma. In:
Bone Cancer. Primary Bone Cancers and Bone Metastases. Heymann D:
(2nd). Academic Press. (Cambridge, MA). 205–211. 2014.
|
|
17
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Janeway KA and Grier HE: Sequelae of
osteosarcoma medical therapy: A review of rare acute toxicities and
late effects. Lancet Oncol. 11:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bryan RT and Tselepis C: Cadherin
switching and bladder cancer. J Urol. 184:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Patel S, Sumitra G, Koner BC and Saxena A:
Role of serum matrix metalloproteinase-2 and −9 to predict breast
cancer progression. Clin Biochem. 44:869–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walkley CR: Modeling osteosarcoma: In
vitro and in vivo approaches. In: Bone Cancer. Primary
Bone Cancers and Bone Metastases. Heymann D: (2nd). Academic Press.
(Cambridge, MA). 195–204. 2014.
|