Introduction
During 2010, renal cell carcinoma (RCC) was
estimated to account for 58,240 novel cases of the disease and
13,040 mortalities in the United States, and at present, cases are
steadily increasing at a rate of 2.5% per year across population
groups (1,2). Clear cell RCC (ccRCC) is one of the most
common subtypes of the disease, accounting for 70–80% of all RCC
cases (3). A key characteristic of
kidney cancer is its tendency to metastasize widely prior to the
appearance of any local symptoms or signs (4). In 20–30% of patients with recently
diagnosed RCC, radiological evidence of metastases exists at the
time of presentation, and 20–40% of patients undergoing a
nephrectomy to treat clinically localized RCC will develop
metastases (4). The most common
locations prone to metastases are the lungs and bones, followed in
frequency by the regional lymph nodes, liver, adrenal gland, brain,
gall bladder, pancreas and breasts (5,6).
Additionally, several studies have reported a number of rare
metastatic sites, including the ureteric stump, the ipsilateral and
contralateral ureter, and the prostatic fossa (7–9). However,
simultaneous metastases of RCC to the urinary bladder and left
retroperitoneal space have not yet been reported. To the best of
our knowledge, the current study describes the first case of RCC
presenting with simultaneous metastases to the urinary bladder and
left retroperitoneal space, occurring a short period after a
radical nephrectomy.
Case report
A 70-year-old man was referred to West China
Hospital (Chengdu, China) with chronic left flank pain that had
been present for a period of 2 months on December 15, 2014. For the
past 10 years, the patient had presented with a history of diabetes
mellitus and hypertension. Ultrasonography identified a
heterogeneous tumor, comprised of a solid component that measured
4.4×3.4×5.0 cm in size and was located in the upper pole of the
left kidney. The echo patterns of the urinary bladder were normal.
Abdominopelvic computed tomography (CT) revealed a circumscribed
and contrast-enhanced tumor located in the upper pole of the left
kidney, and no regional lymphatic metastases, or thrombi of the
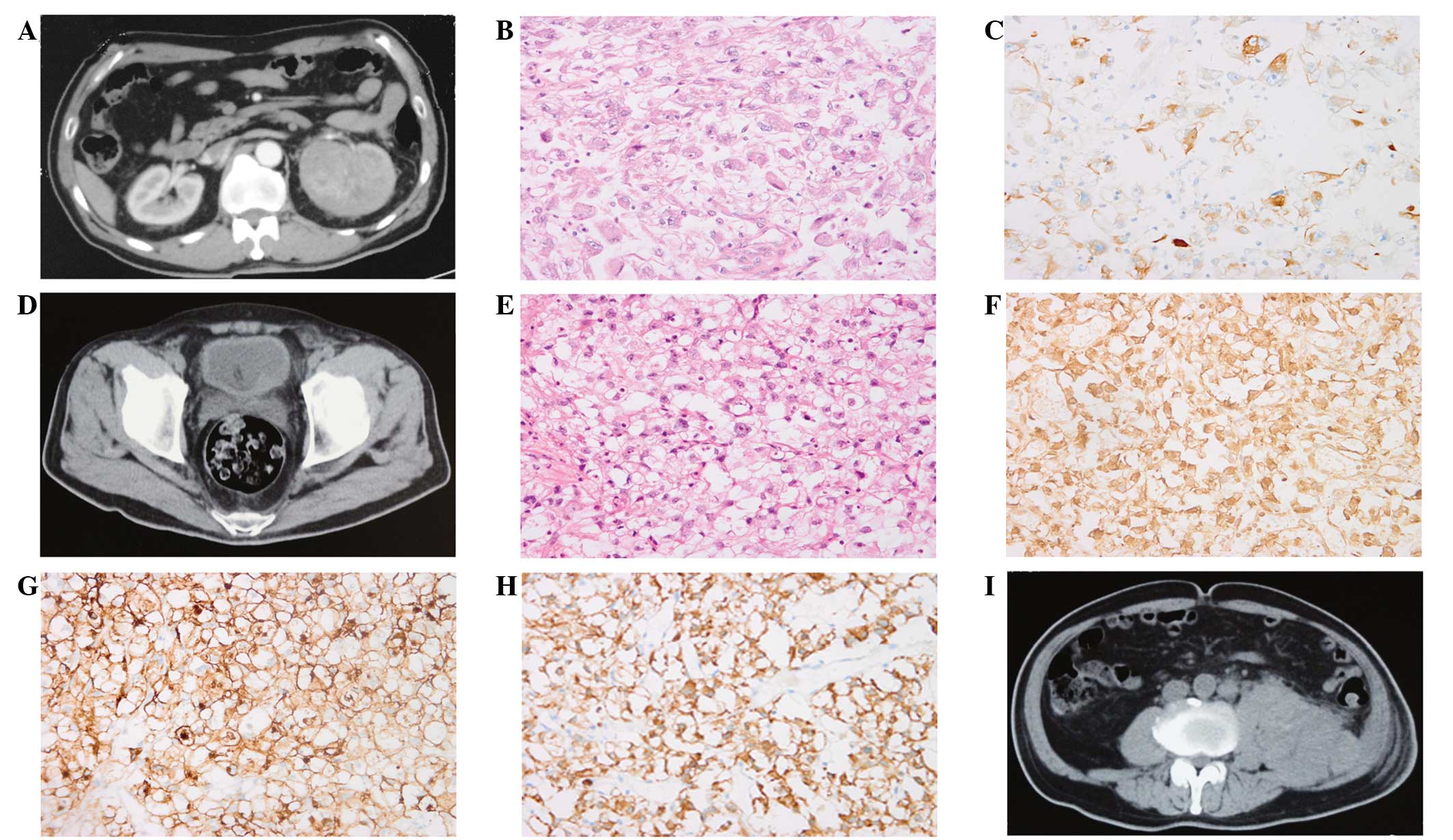
inferior vena cava and renal vein were identified (Fig. 1A). There was no radiological evidence
of metastasis to any other organs or soft tissues. Blood tests and
radiographical analysis of the chest were normal. Consequently, an
open radical nephrectomy was performed due to compression of the
renal hilum by the larger tumor. On gross examination, the tumor
was observed to be compressing the anterior branch of the renal
artery and was closely adherent to the surrounding tissue of the
pelvis and ureter. Pathological analysis indicated the final
diagnosis of ccRCC, which was confirmed as Fuhrman grade IV
(Fig. 1B) (10). On microscopic examination, the tumor
cells demonstrated a spindled shape that resembled that of sarcoma
cells, an abundant, clear cytoplasm, enlarged nuclei with a marked
irregular outline and prominently enlarged nucleoli (even at a low
magnification), with the addition of bizarre and multilobe nuclei
indicating poor differentiation. Necrosis, thick walled
vasculature, and neutrophil and lymphocyte infiltration were also
observed in the areas of the tumor lesions. Immunohistochemically,
the tumor was positive for protein kinase C (Fig. 1C), but negative for RCC and human
melanoma mark black 45.
Following radical surgery, neither targeted nor
immunological agents were administered on the basis of the RCC
treatment guidelines (11). At 1
month after the radical nephrectomy, the patient received
transurethral clot evacuation and resection of a sessile tumor
(1.0×1.4×1.0 cm) on the left bladder, due to the occurrence of
hematuria and acute urinary clot retention (Fig. 1D). Pathological analysis of the
bladder tumor indicated the presence of RCC (Fig. 1E). The primary RCC of the kidney
(Fig. 1B) presented with features
similar to those observed in the metastatic RCC to the bladder
(Fig. 1E). The metastatic tumor cells
were determined to be positive for vimentin, cluster of
differentiation (CD)10, cytokeratin (CK)8 (Fig. 1F–H), CK18 and paired box 2, but were
negative for prosaposin, prostate-specific antigen and uroplakin
III, indicating a renal origin. An abdominopelvic CT scan also
identified a large mass in the left retroperitoneal space (Fig. 1I). There was no evidence of metastasis
to the lungs, bones or other organs based on the normal results of
relevant CT scans of the bones, abdomen and thorax. Systemic
therapy, including chemotherapy and targeted therapy, for the
metastatic tumors of the bladder and the left retroperitoneal
space, was not administered, primarily due to a weakened
performance status (Karnofsky score, <40), anemia and the
unstable sugar content of the blood (11). Extensive hydrothorax and general
anasarca presented half a month after transurethral resection of
the bladder tumor (TURBT). The patient succumbed to the disease 15
days later. The mass in the left retroperitoneal space was
considered to have metastasized from the left ccRCC on the basis of
the combination of results from the consecutive abdominopelvic
enhanced CT and the late metastatic mechanisms of RCC to the
bladder and retroperitoneal space. The patient, however, had
refused to undergo a fine-needle aspiration biopsy for this
mass.
Discussion
RCC is a prevalent malignancy of the kidney that is
characterized by the presence of early metastasis (4). Despite the observation of RCC
metastasizing to a number of unusual sites, including the ureteric
stump, the ipsilateral and contralateral ureter, and the prostatic
fossa (7–9), simultaneous metastases to the urinary
bladder and left retroperitoneal space have not yet been reported.
Numerous studies have indicated that the average period of time
between primary RCC diagnosis and metastasis to the bladder ranges
from 2–131 months (11). In the
present case, the metastatic masses in the left retroperitoneal
space and on the posterior wall of the urinary bladder developed
very rapidly, only 1 month after radical nephrectomy. To rule out
the possibility of metastases in the bladder and retroperitoneal
space prior to the radical nephrectomy, all of the original
radiological records were carefully reviewed, with no positive
evidence identified to suggest metastases in those sites.
Therefore, this suggests that these two sites developed metastases
following the first surgery.
The metastatic mechanisms of RCC to the bladder and
retroperitoneal space remain unclear. ccRCC is recognized for its
propensity to metastasize to unusual sites, and late metastasis,
even after ≥10 years, is not uncommon (12). Several studies have proposed a number
of possible pathways of hematogenous metastasis occurring through
the general circulation and in a retrograde manner, spreading along
the paravertebral veins, the testicular/ovarian veins, the
intrarenal veins, or by the direct intraluminal transit of tumor
cells with seeding in the distal urothelium (12–15). In
the present case, rapid metastatic RCC progression was observed in
two sites concurrently, namely the urinary bladder and left
retroperitoneal space. One of the possible reasons for the
occurrence of left RCC metastasis to the left posterior wall of the
bladder (near the left orifice of the ureter) is the direct
intraluminal transit of tumor cells and/or retrograde metastasis
along the left ureter; the current case is in accordance with the
outcomes of previous studies (16,17). A
possible explanation for the metastasis to the left retroperitoneal
space is the retrograde metastasis along the paravertebral,
testicular and intrarenal veins; this is in accordance with the
abundant, thick-walled vasculature observed pathologically in the
primary RCC. Fuhrman nuclear grade is the most commonly utilized
histological grading system in RCC (12), and is more effective than any other
parameter in predicting the development of distant metastasis
following a nephrectomy (10).
Additionally, poor differentiation of tumors corresponds with
Fuhrman grade IV, and is a very important factor for the prediction
of RCC metastasis and survival. Furthermore, an unstable sugar
content of the blood and compromised immunological responses may
also promote tumor metastasis, and decrease cancer-specificity and
over-survival, despite the early stage of the oncology (18,19). In
the present case, the unstable sugar content of the blood and a
poor diet gave rise to compromised immunological responses with
decreased resistance to cancerous metastases. No investigations
were performed to assess whether the tumor harbored any notable
genetic changes due to the lack of an appropriate control.
Additional molecular biological studies may aid the clarification
of the potential mechanisms of the rapid metastasis and the unusual
metastatic sites of RCC. The aforementioned factors are considered
as three important explanations for the rapid metastases typically
observed in ccRCC carcinogenesis.
At present, systemic therapy for the treatment of
metastatic RCC includes palliative excision and/or use of targeted
agents, such as sunitinib, sorafenib and temsirolimus. The
therapeutic options available for RCC metastasis to the bladder are
a TURBT or a local excision of the bladder. In the present case,
TURBT was performed. Considering the weakened performance status of
the patient, which includes the symptoms of anemia, an unstable
sugar content of the blood and a Karnofsky score of <40, the
patient refused systemic treatment or mass exploration in the
retroperitoneal space. Subsequently, hydrothorax and general
anasarca developed half a month after TURBT, and the patient
succumbed to the disease shortly after.
In conclusion, the current study describes an
extremely rare case of RCC metastasizing to the bladder and left
retroperitoneal space over a short period of time. Despite this
case being extremely rare, urologists and oncologists should be
aware of the possibility of RCC metastasizing to the urinary
bladder and retroperitoneal space in patients at the early stage of
the disease, particularly when the patient presents with hematuria
and clot retention.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant nos. 81200551 and 81270841).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mustafa A, Gupta S, Hudes GR, Egleston BL,
Uzzo RG and Kruger WD: Serum amino acid levels as a biomarker for
renal cell carcinoma. J Urol. 186:1206–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cronin RE, Kaehny WD, Miller PD, Stables
DP, Gabow PA, Ostroy PR and Schrier RW: Renal cell carcinoma:
Unusual systemic manifestations. Medicine (Baltimore). 55:291–311.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai TH, Tang SH, Chuang FP, Wu ST, Sun
GH, Yu DS, Chang SY and Cha TL: Ipsilateral synchronous neoplasms
of kidney presenting as acute pyelonephritis and bladder
metastasis. Urology. 73:1163.e9–1163.e11. 2009. View Article : Google Scholar
|
|
7
|
Gelister JS, Falzon M, Crawford R, Chapple
CR and Hendry WF: Urinary tract metastasis from renal carcinoma. Br
J Urol. 69:250–252. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang DG, Nam JG, Kang JS, Yang HT, Jung HH
and Choi NG: Metastases to ureteral stump and bladder from renal
cell carcinoma. Korean J Urol. 42:875–878. 2001.
|
|
9
|
Kruck S, Scharpf M, Stenzl A and Bedke J:
A rare case of synchronous renal cell carcinoma of the bladder
presenting with gross hematuria. Rare Tumors. 5:72–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiraishi K, Mohri J, Inoue R and Kamiryo
Y: Metastatic renal cell carcinoma to the bladder 12 years after
radical nephrectomy. Int J Urol. 10:453–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebele JN, Sauter G, Epstein JI and
Sesterhenn IA: Clear Cell Renal Carcinoma. World Health
Organization Classification of Tumours. Pathology and Genetics of
Tumours of the Urinary System and Male Genital Organs. (IARC,
Lyon). 23–25. 2004.
|
|
13
|
Gulati M, Gore JL, Pantuck AJ, Kim Y,
Barajas L and Rajfer J: Ureteral tumor thrombus from renal cell
carcinoma extending into bladder. Urol Oncol. 25:393–395. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saitoh H: Distant metastasis of renal
adenocarcinoma. Cancer. 48:1487–1491. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remis RE and Halverstadt DB: Metastatic
renal cell carcinoma to the bladder: Case report and review of the
literature. J Urol. 136:1294–1296. 1986.PubMed/NCBI
|
|
16
|
Shaw RE: Metastasis to the bladder from
carcinoma of the kidney. Br J Surg. 48:420–422. 1961. View Article : Google Scholar
|
|
17
|
Abeshouse BS: Metastasis to ureters and
urinary bladder from renal carcinoma; report of two cases. J Int
Coll Surg. 25:117–126. 1956.PubMed/NCBI
|
|
18
|
Psutka SP, Stewart SB, Boorjian SA, Lohse
CM, Tollefson MK, Cheville JC, Leibovich BC and Thompson RH:
Diabetes mellitus is independently associated with an increased
risk of mortality in patients with clear cell renal cell carcinoma.
J Urol. 192:1620–1627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel HD, Kates M, Pierorazio PM, Gorin
MA, Jayram G, Ball MW, Hyams ES and Allaf ME: Comorbidities and
causes of death in the management of localized T1a kidney cancer.
Int J Urol. 21:1086–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|