Introduction
Overweight or obese patients have become an emerging
health concern worldwide, and are associated with several diseases,
including cardiovascular disease, type 2 diabetes mellitus and
various forms of cancer (1). The
prevalence of obesity has substantially increased over the last few
decades, with the World Health Organization estimating that 500
million adults worldwide and 31% of females in the United States
are categorically obese (2,3). In Taiwan, the prevalence of obesity has
increased to 13.2% of adult women, which poses a major task in the
prevention of female cance (4). There
is accumulating evidence that being overweight carries an
established risk for renal cell cancer, colon cancer, endometrial
cancer, esophageal adenocarcinoma and postmenopausal breast cancer.
A major review by the International Agency for Research on Cancer
analyzed data regarding weight, physical activity and cancer
incidence in Europe (5). The review
concluded that obesity contributed to the cause of 39% of
endometrial cancer cases, 37% of esophageal cancer cases, 25% of
kidney cancer cases, 11% of colon cancer cases and 9% of
postmenopausal breast cancer cases (5). Data published over the last 25 years has
indicated that obesity is responsible for ~20% of cancer-associated
mortalities in women and ~14% in men (6). These rates are second only to smoking
for the number of avoidable cases of cancer (6).
In women, breast cancer is the most frequently
diagnosed form of cancer and the second highest cause of
cancer-associated mortality in the United States, with a similar
outcome reported for Asia-Pacific populations (7,8). Various
factors associated with a greater risk of breast cancer have been
identified. Among the modifiable risk factors, diet and obesity
have been evaluated for use in strategies for breast cancer
prevention. Previous studies have demonstrated that insulin
resistance and diabetes mellitus contribute to cytotoxic agent
resistance in cancer cells and poor response to chemotherapy in
patients with hepatocellular carcinoma (HCC) (9,10).
Together with insulin resistance and diabetes mellitus, weight gain
in adults is correlated with a greater risk of breast cancer and is
a poor prognostic parameter, particularly in nonsmoking women
(11–13). The mechanism underlying the
association between increased incidences of breast cancer and
obesity in women remains poorly understood, but a growing body of
evidence suggests that insulin resistance, chronic inflammation,
estrogen and adipokine interaction may be involved (14). An expression array analysis of breast
tumor tissues from postmenopausal women demonstrated that
progesterone receptor (PR) expression correlated with metabolic
upregulation of glucolysis and lipogenesis (15). Furthermore, it has been identified
that obesity is correlated with estrogen receptor (ER) and PR
expression, thus supporting their underlying associations (16).
Processes related to cancer metabolism, such as
glucolysis and lipogenesis, are part of a large body of research
that may be able to define its exact role in cancer cells. However,
the role of lipogenesis in tumor initiation remains unknown. In
cancer stem cells of the breast, ectopic expression of sterol
regulatory element-binding protein-1 (SREBP-1), which is a master
regulator of lipogenic genes, promoted cell growth and mammosphere
formation, and significantly enhanced lipogenesis in stem cells
(17). Multifunctional fatty acid
synthase (FAS) enzymes, which convert acetyl CoA into fatty acids,
is overexpressed in a wide range of human tumors (18). Under hypoxic conditions, FAS is
upregulated following activation of Akt and SREBP-1 (19). FAS expression in breast cancer confers
poor prognosis and is associated with HER2 expression in aggressive
breast cancer (20,21), whilst FAS inhibition reverses the
resistance of trastuzumab in HER2(+) breast cancer cells (22). As a long fatty acid elongase, Elovl6
contributes to de novo synthesis of fatty acids, and is
understood to modulate insulin resistance (23). As well as FAS involved in de
novo lipogenesis, the study of Elovl6 in carcinogenesis remains
limited. In nonalcoholic steatohepatitis (NASH)-associated HCC, the
expression of Elovl6 is upregulated, thus highlighting the
contribution of lipogenesis in liver carcinogenesis (24). The aim of the present study is to
nvestigate the behavior of Elovl6 in breast cancer, as it is
important to understand the molecular mechanism of lipogenesis in
mammary carcinogenesis, with ongoing efforts required to identify
novel diagnostic and therapeutic targets.
Materials and methods
Patients and tumor samples
In 2006 and 2007, a total of 70 patients with
histologically confirmed breast cancer were treated at Chi-Mei
Medical Center (Tainan, Taiwan). All patients received standard
therapy for curative purpose of breast cancer as indicated.
Clinical information was obtained from medical records, and all
samples were obtained by mastectomy. Specimens were fixed with 10%
formalin and embedded in paraffin. The present study was approved
by the institute review board of Chi-Mei Medical Center
(institutional review board serial no. 10207-001).
Immunohistochemistry
Staining was carried out on formalin-fixed and
paraffin-embedded tissue sections using immunoperoxidase methods.
Following deparaffinization in xylene and rehydration in a graded
alcohol series, the sections were heated in a microwave with
citrate buffer for 13 min for heat-induced epitope retrieval
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The following
steps were performed using a Novolink™ Polymer Detection system
(Leica Microsystems, Ltd., Milton Keynes, UK). Novolink Peroxidase
Block was added for 5 min to neutralize the endogenous peroxidase
activity, followed by the addition of Novolink Protein Block for 5
min. Next, the sections were incubated with the primary antibody
against Elovl6 (dilution, 1:20; Atlas Antibodies, Stockholm,
Sweden) overnight at 4°C. The sections were washed and subsequently
incubated with Novolink Post Primary Block for 10 min, followed by
Novolink Polymer for 10 min. The color reaction was developed using
NovoLink DAB Substrate Buffer, and the sections were counterstained
with Mayer's hematoxylin (ScyTek Laboratories Inc., Logan, UT,
USA). The intensity of the reactions were analyzed qualitatively.
Microscopic fields with the highest degree of immunoreactivity were
selected for analysis. The intensity score represented the mean
staining intensity of the positive tumor cells, and was classified
as follows: Negative, 0; weak, 1; moderate, 2; and strong, 3, as
described previously (25,26). Intensity scores of 0 and 1 were
considered to represent negative Elov16 expression, whilst scores
of 2 and 3 were considered to represent positive Elovl6
expression.
Statistical analysis
All analyses were performed using SigmaStat version
3.1 (Systat Software, Inc., San Jose, CA, USA) and SPSS version
12.0 (SPSS, Inc., Chicago, IL, USA). The χ2 test was
used to compare categorical variables, and Kaplan-Meier survival
analysis was used to estimate survival curves. In addition,
differences between two groups were analyzed using the Wilcoxon
rank-sum test. All tests were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
A total of 70 women with invasive breast cancer were
included in the present study. All patients underwent mastectomy
and indicated adjuvant treatment. The tumor samples were harvested
from primary breast tumors. Among the 70 samples, 37.1% exhibited
positive Elovl6 expression and 62.9% were considered as negative.
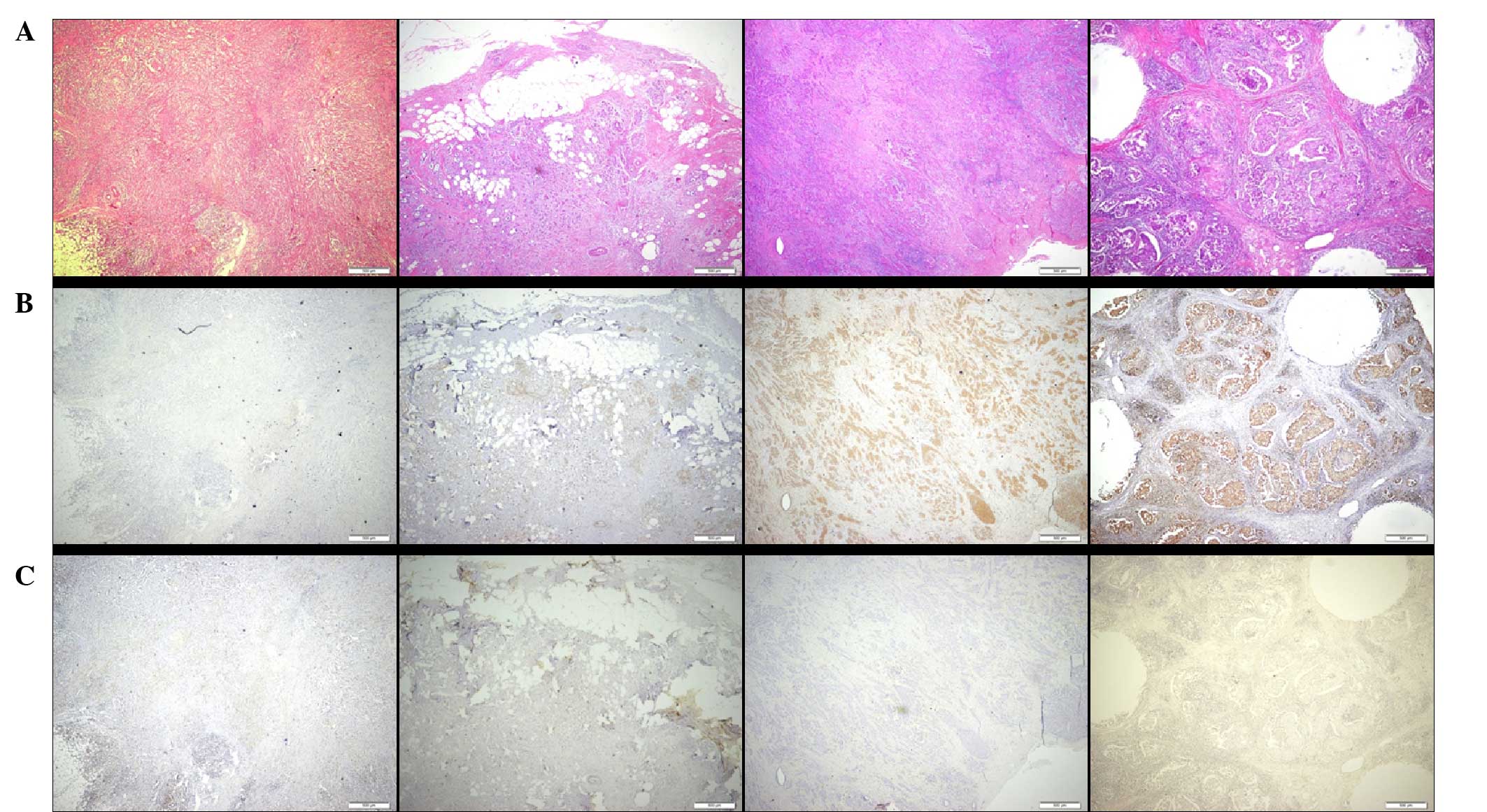
Positive Elovl6 expression was defined as moderate to strong
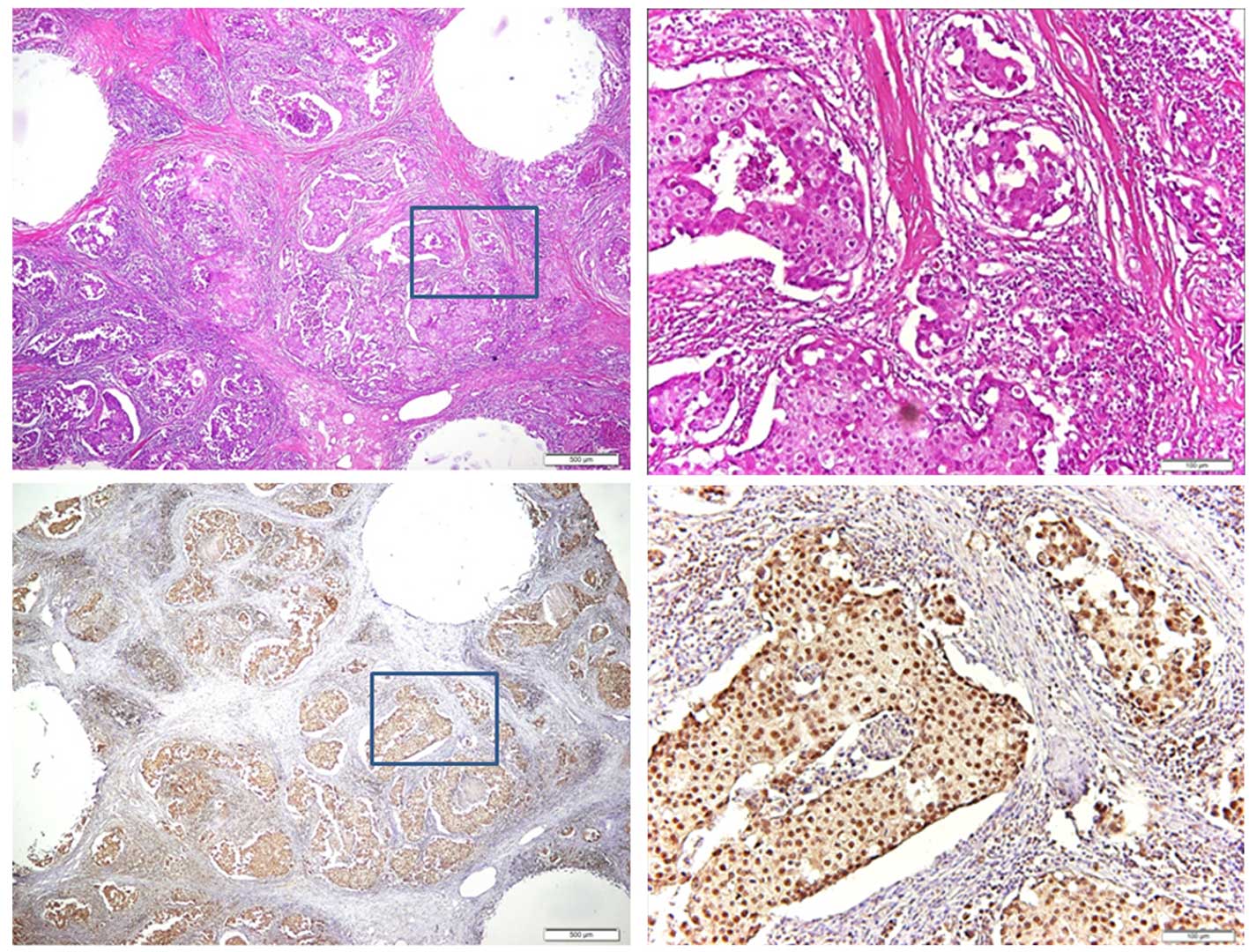
intensity from immunohistochemistry staining (Fig. 1). In high-power field microscopic
analysis, the breast cancer cells exhibited positive expression of
Elovl6 with cytoplasmic and nuclear distribution (Fig. 2). In order to analyze the contribution
of Elovl6 to the development of breast cancer, the present study
evaluated the association between Elovl6 expression in clinical
breast tumor tissues and clinicopathological parameters, including
primary tumor size, lymph node metastasis, stage, grade, ER, PR,
HER2 and age. The results demonstrated that positive Elov16
expression was correlated with positive lymph node involvement
(P=0.018)(Table I). Positive lymph
node involvement is known to function as an important predictor of
breast cancer patient survival. In the present study, 8/70 patients
experienced recurrence within 5 years of breast cancer resection. A
total of 4/26 (15.4%) patients with positive Elovl6 expression
experienced recurrence compared with 4 out of 44 (9.1%) patients
with negative Elovl6 expression (Table
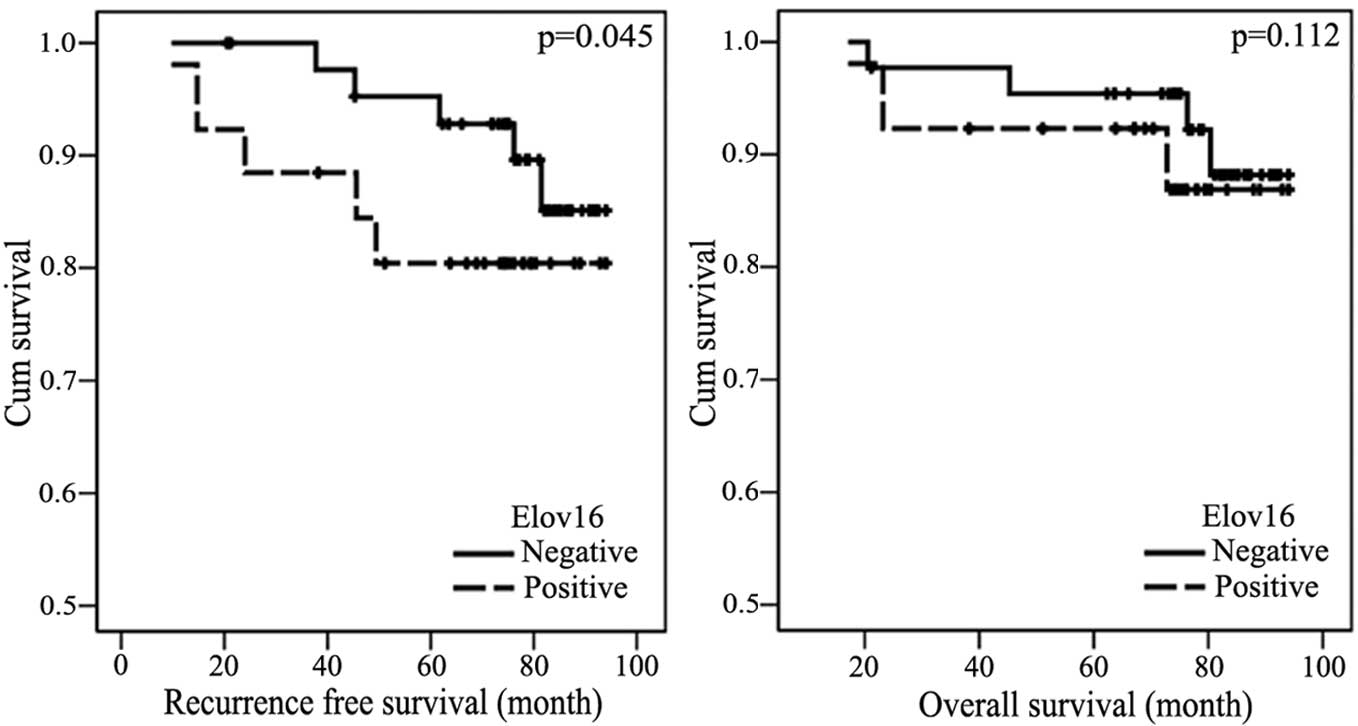
II). The present study performed Kaplan-Meier survival analysis
to estimate the recurrence-free survival time of patients with
Elov16(+) breast cancer. The results demonstrated that patients
with positive Elovl6 expression had a poorer recurrence-free
survival time compared with patients with negative Elov16
expression (P<0.05) (Fig. 3).
 | Table I.Association between Elovl6 expression
and clinicopathological characteristics. |
Table I.
Association between Elovl6 expression
and clinicopathological characteristics.
| Characteristic | Na | Elovl6
(+)b | Elovl6
(−)c | P-value |
|---|
| Tumor size |
|
|
| 0.239 |
| <2
cm | 30 | 14 | 16 |
|
| ≥2
cm | 40 | 12 | 28 |
|
| Node involvement |
|
|
| 0.018 |
|
Positive | 29 | 16 | 13 |
|
|
Negative | 41 | 10 | 31 |
|
| Stage |
|
|
| 0.298 |
| I | 23 | 11 | 12 |
|
| II | 35 | 13 | 22 |
|
|
III | 12 | 2 | 10 |
|
| Grade |
|
|
| 0.295 |
| I | 18 | 7 | 11 |
|
| II | 37 | 14 | 23 |
|
|
III | 15 | 5 | 10 |
|
| ER |
|
|
| 0.412 |
|
Positive | 40 | 17 | 23 |
|
|
Negative | 30 | 9 | 21 |
|
| PR |
|
|
| 0.186 |
|
Positive | 40 | 18 | 22 |
|
|
Negative | 30 | 8 | 22 |
|
| HER2 |
|
|
| 0.960 |
|
Positive | 28 | 10 | 18 |
|
|
Negative | 42 | 16 | 26 |
|
| Diabetes |
|
|
| 0.915 |
|
Positive | 17 | 6 | 11 |
|
|
Negative | 53 | 20 | 33 |
|
| Overweight |
|
|
| 0.062 |
|
Positive | 24 | 13 | 11 |
|
|
Negative | 46 | 13 | 33 |
|
 | Table II.Breast cancer recurrence rate at 5
years post-resection. |
Table II.
Breast cancer recurrence rate at 5
years post-resection.
| Characteristic | N | 5-year disease
recurrence rate, % (n) |
|---|
| All tumors | 70 | 11.4 (8) |
|
Elovl6(+) | 26 | 15.4 (4) |
|
Elovl6(−) | 44 | 9.1 (4) |
| ER(+) tumors | 40 | 7.5 (3) |
|
Elovl6(+) | 17 | 11.8 (2) |
|
Elovl6(−) | 23 | 4.3 (1) |
| PR(+) tumors | 40 | 10 (4) |
|
Elovl6(+) | 18 | 11.1 (2) |
|
Elovl6(−) | 22 | 9.1 (2) |
Discussion
It has been reported that obesity increases the
incidence, progression and mortality associated with breast cancer
primarily in postmenopausal women (27,28). A
rodent model study demonstrated that obese animals deposited excess
nutrients into tumors, and increased expression of PR was
positively correlated with glycolytic and lipogenic enzymes from
tumors (15). In a retrospective
study in Asia, it was observed that obese women experience more
advanced disease with higher axillary lymph node ratio and higher
stage at diagnosis (16). The present
study identified that Elovl6, a key enzyme involved in the
lipogenesis pathway, functioned as a novel prognostic factor in
breast cancer and was correlated with nodal metastasis. These
findings support a previous hypothesis suggesting that the
activation of fatty acid synthesis is required for tumor survival
and carcinogenesis. Enzymes implicated in fatty acid synthesis may
serve as a rational therapeutic target for cancer treatment
(29). Due to the limited number of
study samples, the present study is unable to clarify the
prognostic role of Elovl6 in hormone positive breast cancer.
Therefore, further studies with larger sample sizes are
warranted.
Lipogenesis is considered as a potential target for
the treatment of cancer and several enzymes involved in this
process have been considered as therapeutic targets. The regulation
of FAS in cancer was initially investigated in the 1980s in human
breast cancer cells expressing functioning ERs and PRs (30). High expression of FAS has been
associated with a greater risk of breast cancer-associated
mortality, which has resulted in the investigation of FAS as a
chemotherapy target (31). Orlistat,
an inhibitor of FAS, was originally regarded as an antiobesity
drug, but has now been identified to exert anticancer activity in
oral squamous cell carcinoma (32).
Aside from extensive studies examining insulin resistance and
lipogenesis, a limited amount of research has focused on the role
of Elovl6 in cancer biology. In a study investigating pediatric
primary central nervous system germ cell tumors, the expression of
Elovl6 mRNA was observed to be abundant in germinoma (33). To analyze the oncogenic role of
Elovl6, a phosphatase and tensin homolog-null mouse model
demonstrated that Elovl6 expression was significantly higher in HCC
tissue compared with adjacent NASH liver tissue (24). Among the enzymes involved in de
novo lipogenesis, inhibition of ATP citrate lyase (ACLY)
results in apoptosis and growth suppression in human cancer cells
(34). In addition, ACLY depletion
induces phosphorylation of AMP-activated protein kinase and
coincides with Elov16 downregulation (35). Notably, it was reported that
diethylnitrosamine-induced carcinogenesis, which mimics
inflammatory events in early hepatocarcinogenesis, increased
C18/C16 ratio and hepatic lipids. To clarify the emerging role of
Elovl6 in cancer biology, a recent study analyzed human liver
samples from patients with NASH or NASH-related HCC (36). The results demonstrated that the NASH
and NASH-related HCC tissues exhibited an elevated expression of
Elovl6 (36). When combined, these
results suggest that Elovl6 may be regarded as an oncogenic protein
in the process of lipogenesis.
Elovl6 deficiency reduces SREBP-1 and peroxisome
proliferator-activated receptor α, in addition to altering hepatic
fatty acid composition. Elovl6 knockout mice are unique as their
insulin resistance increases without amelioration of
hepatosteatosis and obesity (37).
This emphasizes the importance of tissue fatty acid composition in
insulin sensitivity, which may be controlled by Elovl6 activity
(37). A novel, selective and potent
active inhibitor for mammalian Elovl6 has been identified. In a
previous study, chronic treatment with an oral inhibitor of Elovl6
in animals with diet-induced obesity resulted in a reduction in
hepatic fatty composition (38).
However, the same inhibitor failed to improve insulin sensitivity
via Elovl6 inhibition in several other animal models (38). Based on the implication of Elovl6
expression in breast cancer, further studies are required to
evaluate the therapeutic potential of Elovl6 inhibition in breast
cancer treatment.
In conclusion, the present study demonstrated that
Elovl6, a microsomal enzyme that regulates fatty acid metabolism
and insulin sensitivity, was associated with a poor outcome in
patients with operable breast cancer. In addition, the
overexpression of Elovl6 was associated with malignant involvement
of regional lymph nodes and shorter recurrence free survival. These
results suggest that Elovl6 may function as a prognostic predictor
in human breast cancer and may hold promise as a potential strategy
for cancer chemoprevention and treatment.
Acknowledgements
The project was funded in part by Chi-Mei Medical
Center, Tainan, Taiwan (grant nos. CMNCKU10004 and CMHFR10204).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
SREBP1
|
sterol regulatory element-binding
protein 1
|
|
FAS
|
fatty acid synthase
|
|
NASH
|
non-alcoholic steatohepatitis
|
|
ACLY
|
ATP citrate lyase
|
References
|
1
|
Andersen DK: Diabetes and cancer: Placing
the association in perspective. Curr Opin Endocrinol Diabetes Obes.
20:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundaram S, Johnson AR and Makowski L:
Obesity, metabolism and the microenvironment: Links to cancer. J
Carcinog. 12:192013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogden CL, Carroll MD, Curtin LR, McDowell
MA, Tabak CJ and Flegal KM: Prevalence of overweight and obesity in
the United States, 1999–2004. JAMA. 295:1549–1555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu NF: Prevalence of obesity in Taiwan.
Obes Rev. 6:271–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergström A, Pisani P, Tenet V, Wolk A and
Adami HO: Overweight as an avoidable cause of cancer in Europe. Int
J Cancer. 91:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng YH, Velazquez-Torres G, Gully C, Chen
J, Lee MH and Yeung SC: The impact of type 2 diabetes and
antidiabetic drugs on cancer cell growth. J Cell Mol Med.
15:825–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng YH, Lin CY, Huang WT, Wu CL, Fang JL,
et al: Diabetes mellitus impairs the response to intra-arterial
chemotherapy in hepatocellular carcinoma. Med Oncol. 28:1080–1088.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coates RJ, Clark WS, Eley JW, Greenberg
RS, Huguley CM and Brown RL: Race, nutritional status, and survival
from breast cancer. J Natl Cancer Inst. 82:1684–1692. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caan BJ, Kwan ML, Hartzell G, Castillo A,
Slattery ML, Sternfeld B and Weltzien E: Pre-diagnosis body mass
index, post-diagnosis weight change, and prognosis among women with
early stage breast cancer. Cancer Causes Control. 19:1319–1328.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kroenke CH, Chen WY, Rosner B and Holmes
MD: Weight, weight gain, and survival after breast cancer
diagnosis. J Clin Oncol. 23:1370–1378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okwan-Duodu D, Umpierrez GE, Brawley OW
and Diaz R: Obesity-driven inflammation and cancer risk: Role of
myeloid derived suppressor cells and alternately activated
macrophages. Am J Cancer Res. 3:21–33. 2013.PubMed/NCBI
|
|
15
|
Giles ED, Wellberg EA, Astling DP,
Anderson SM, Thor AD, Jindal S, Tan AC, Schedin PS and Maclean PS:
Obesity and overfeeding affecting both tumor and systemic
metabolism activates the progesterone receptor to contribute to
postmenopausal breast cancer. Cancer Res. 72:6490–6501. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaviani A, Neishaboury M, Mohammadzadeh N,
Ansari-Damavandi, et al: Effects of obesity on presentation of
breast cancer, lymph node metastasis and patient survival: A
retrospective review. Asian Pac J Cancer Prev. 14:2225–2229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pandey PR, Xing F, Sharma S, Watabe M, Pai
SK, Iiizumi-Gairani M, Fukuda K, Hirota S, Mo YY and Watabe K:
Elevated lipogenesis in epithelial stem-like cell confers survival
advantage in ductal carcinoma in situ of breast cancer. Oncogene.
32:5111–5122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhajda FP, Jenner K, Wood FD, Hennigar
RA, Jacobs LB, Dick JD and Pasternack GR: Fatty acid synthesis: A
potential selective target for antineoplastic therapy. Proc Natl
Acad Sci USA. 91:6379–6383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furuta E, Pai SK, Zhan R, Bandyopadhyay S,
Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, et al:
Fatty acid synthase gene is up-regulated by hypoxia via activation
of Akt and sterol regulatory element binding protein-1. Cancer Res.
68:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alo' PL, Visca P, Marci A, Mangoni A,
Botti C and Di Tondo U: Expression of fatty acid synthase (FAS) as
a predictor of recurrence in stage I breast carcinoma patients.
Cancer. 77:474–482. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang D, Tai LK, Wong LL, Chiu LL, Sethi
SK and Koay ES: Proteomic study reveals that proteins involved in
metabolic and detoxification pathways are highly expressed in
HER-2/neu-positive breast cancer. Mol Cell Proteomics. 4:1686–1696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vazquez-Martin A, Colomer R, Brunet J and
Menendez JA: Pharmacological blockade of fatty acid synthase (FASN)
reverses acquired autoresistance to trastuzumab (Herceptin by
transcriptionally inhibiting ‘HER2 super-expression’ occurring in
high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells.
Int J Oncol. 31:769–776. 2007.PubMed/NCBI
|
|
23
|
Matsuzaka T, Shimano H, Yahagi N, Kato T,
Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, et
al: Crucial role of a long-chain fatty acid elongase, Elovl6, in
obesity-induced insulin resistance. Nat Med. 13:1193–1202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Muir K, Hazim A, He Y, Peyressatre M, Kim
DY, Song X and Beretta L: Proteomic and lipidomic signatures of
lipid metabolism in NASH-associated hepatocellular carcinoma.
Cancer Res. 73:4722–4731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng L, Nagabhushan M, Pretlow TP, Amini
SB and Pretlow TG: Expression of E-cadherin in primary and
metastatic prostate cancer. Am J Pathol. 148:1375–1380.
1996.PubMed/NCBI
|
|
26
|
Cheng L, Pan CX, Zhang JT, Zhang S, Kinch
MS, Li L, Baldridge LA, Wade C, Hu Z, Koch MO, et al: Loss of
14-3-3sigma in prostate cancer and its precursors. Clin Cancer Res.
10:3064–3068. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Willett WC, Colditz GA, Hunter
DJ, Manson JE, Rosner B, Speizer FE and Hankinson SE: Waist
circumference, waist:hip ratio, and risk of breast cancer in the
Nurses' Health Study. Am J Epidemiol. 150:1316–1324. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reeves GK, Pirie K, Beral V, Green J,
Spencer E and Bull D: Million Women Study Collaboration: Cancer
incidence and mortality in relation to body mass index in the
Million Women Study: Cohort study. BMJ. 335:11342007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zaidi N, Lupien L, Kuemmerle NB, Kinlaw
WB, Swinnen JV and Smans K: Lipogenesis and lipolysis: The pathways
exploited by the cancer cells to acquire fatty acids. Prog Lipid
Res. 52:585–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chalbos D, Joyeux C, Galtier F, Escot C,
Chambon M, Maudelonde T and Rochefort H: Regulation of fatty acid
synthetase by progesterone in normal and tumoral human mammary
glands. Rev Esp Fisiol. 46:43–46. 1990.PubMed/NCBI
|
|
31
|
Wang Y, Kuhajda FP, Li JN, Pizer ES, Han
WF, Sokoll LJ and Chan DW: Fatty acid synthase (FAS) expression in
human breast cancer cell culture supernatants and in breast cancer
patients. Cancer Lett. 167:99–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agostini M, Almeida LY, Bastos DC, Ortega
RM, Moreira FS, Seguin F, Zecchin KG, Raposo HF, Oliveira HC,
Amoêdo ND, et al: The fatty acid synthase inhibitor orlistat
reduces the growth and metastasis of orthotopic tongue oral
squamous cell carcinomas. Mol Cancer Ther. 13:585–595. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wong JM, Chi SN, Marcus KJ, Levine BS,
Ullrich NJ, MacDonald S, Lechpammer M and Goumnerova LC: Germinoma
with malignant transformation to nongerminomatous germ cell tumor.
J Neurosurg Pediatr. 6:295–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Migita T, Okabe S, Ikeda K, Igarashi S,
Sugawara S, et al: Inhibition of ATP citrate lyase induces an
anticancer effect via reactive oxygen species: AMPK as a predictive
biomarker for therapeutic impact. Am J Pathol. 182:1800–1810. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Migita T, Okabe S, Ikeda K, Igarashi S,
Sugawara S, Tomida A, Soga T, Taguchi R and Seimiya H: Inhibition
of ATP citrate lyase induces triglyceride accumulation with altered
fatty acid composition in cancer cells. Int J Cancer. 135:37–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kessler SM, Simon Y, Gemperlein K,
Gianmoena K, Cadenas C, Zimmer V, Pokorny J, Barghash A, Helms V,
van Rooijen N, et al: Fatty acid elongation in non-alcoholic
steatohepatitis and hepatocellular carcinoma. Int J Mol Sci.
15:5762–5773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuzaka T and Shimano H: Elovl6: A new
player in fatty acid metabolism and insulin sensitivity. J Mol Med
(Berl). 87:379–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shimamura K, Nagumo A, Miyamoto Y,
Kitazawa H, Kanesaka M, Yoshimoto R, Aragane K, Morita N, Ohe T,
Takahashi T, et al: Discovery and characterization of a novel
potent, selective and orally active inhibitor for mammalian ELOVL6.
Eur J Pharmacol. 630:34–41. 2010. View Article : Google Scholar : PubMed/NCBI
|