Introduction
Cancerous lesions of the renal pelvis and ureter are
malignant tumors that arise from the urothelial (transitional cell
epithelium) mucosa of the renal pelvis or ureter (1). Primary urinary tract urothelial
carcinomas (UTUCs) are rare tumors with an estimated incidence of
1–4 cases/100,000 individuals/year (1). UTUC is much less common than bladder
cancer, and only accounts for ~5% of all urothelial carcinoma cases
(2). A total of 90% of UTUCs are
transitional cell carcinomas (2).
Ureteral tumors are particularly rare (1,3), and their
incidence rate is ~1/4 of that of renal pelvic tumors (1).
The majority of patients with UTUC present with
flank pain or hematuria at the early stages of the disease
(4). Suspected UTUCs are usually
evaluated with intravenous pyelography, retrograde pyelography
(RGP), ultrasonography (UG), computed tomography (CT), upper tract
urinary cytology and cystourethroscopy with biopsy (2,4). Imaging
studies usually reveal a filling defect or an obstructive mass,
which is often associated with hydronephrosis, hydroureter or renal
stones (2). Therefore, despite being
non-invasive methods, imaging studies should not be used as the
sole diagnostic tool for UTUC, due to their low sensitivity for the
detection of small tumors, since numerous causes exist to explain a
filling defect other than UTUC (4,5). Cytology
has a low sensitivity for detecting UTUC, and ureteroscopy is an
invasive method with poor sensitivity, particularly in flat tumors
(4,6).
The epithelium that covers the renal pelvis, bladder and ureter is
mainly transitional. Various tumors may appear in the kidney area,
particularly in the renal medulla portion of the kidney, and often
require to be identified as potential transitional cell carcinoma
of the renal pelvis (4). In addition,
renal pelvis cancer and blood clots in the renal pelvis are often
difficult to identify by imaging techniques (5).
Due to the above mentioned shortcomings, the
identification of sensitive molecular markers for the detection of
UTUC is urgently required. To date, only a limited number of
sensitively accepted markers have been applied to detect urinary
tumors, including ImmunnoCyt™/uCyt+™ and fluorecence in situ
hybridization (FISH) technology (7–9). FISH
technology is generally used for the diagnosis of bladder cancer in
voided urine in China, due to its high sensitivity and specificity,
but its use for detecting UTUCs is limited, due to its low
reliability (10). Luo et al
(10) reviewed the utility of FISH in
the diagnosis of UTUC, but this type of prospective study has not
been conducted in China thus far.
Thus, in the present study, the sensitivity and
specificity of FISH technology for detecting suspicious UTUCs in
voided urine specimens was prospectively estimated, since this type
of specimens are easily collected and accepted by patients.
Materials and methods
Patients and samples
Between May 2011 and August 2014, voided urine from
125 patients (82 males and 43 females; mean age, 61.4 years; range,
45–92 years) with suspicion of UTUC were analyzed prospectively.
Patients were recruited from The First, The Second and The Fifth
Affiliated Hospitals of Chongqing Medical University (Chongqing,
China). Patients with clinical symptoms (gross hematuria, flank
pain) and/or radiographic (UG or CT) abnormalities suggestive of
UTUC, localising hematuria, such as blood efflux from the ureteral
orifice, and atypical obstruction were included in the study. The
patients were followed up for a mean ± standard deviation
observation time of 23.8±7.5 months (range, 5–39 months).
First-time voided urine specimens, which were the first morning
urine specimens at the time of admission to hospital, were
collected for FISH and cytology tests in the morning. Urinary cells
from voided urine were sedimented at 600 g for 10 min in a Sorvall
Legend Mach 1.6R benchtop centrifuge (Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and the cell pellets were divided into two
equal volumes, one of which was used for FISH and one for cytology
analyses. The ethics committee of Chongqing Medical University
(Chongqing, China) approved the present study, and all patients
provided their informed consent prior to participation.
Cytology
Urinary cytology was performed according to the
Papanicolau method (11), using an
eosin alcohol 50 solution (Hubei Taikang Medical Equipment Co.,
Ltd., Hubei, China). Cytology was scored as positive, negative or
suspicious by a senior cytologist. Suspicious cytology was defined
as those samples that contained cells with morphologies that could
not be clearly classified as tumor or normal cells.
Histopathological classification was performed according to the
Union for International Cancer Control criteria (8,9).
FISH
Cell pellets were resuspended in 10 ml preheated
hypotonic solution [0.075 mol/l potassium chloride (Hubei Taikang
Medical Equipment Co., Ltd.)], and incubated at 37°C for 25 min,
pipetting three times during this period of time to ensure a
sufficient hypotonic environment for the cells. Next, cells were
sedimented at 600 g for 10 min, and the cell pellets were fixed in
3:1 (v/v) methanol: glacial acetic acid (Hubei Taikang Medical
Equipment Co., Ltd.). The final cell pellet was resuspended in the
appropriate volume of fixing liquid, according to the number of
urinary cells. One or two drops of the resuspended cells (104–106
cells/ml) were seeded onto two glass slides (Hubei Taikang Medical
Equipment Co., Ltd.) and incubated at 60°C for 2 h.
The glass slides were then incubated at 37°C with
RNase A solution [40 ml 2X saline-sodium citrate (SSC) solution
with 60 µl 100 g/ml RNase A; Beijing GP Medical Technologies, Ltd.
(Beijing, China)] for 30 min, followed by incubation with pepsin
solution [40 ml 0.01 M HCl (Hubei Taikang Medical Equipment Co.,
Ltd.) with 160 µl 20 mg/ml pepsin (Beijing GP Medical Technologies,
Ltd.)] at 37°C for 10 min. The slides were then washed twice in 2X
SSC solution for 5 min each, and sequentially dehydrated in 70, 85
and 100% ethanol (3 min each) (Hubei Taikang Medical Equipment Co.,
Ltd.). The slides were then denatured in 70% formamide (Hubei
Taikang Medical Equipment Co., Ltd.)/2X SSC denaturing solution at
73°C for 5 min, and gradient-dehydrated in 70, 85 and 100%
ethanol.
The probe mix used for FISH analysis consisted of
centromere enumeration probes (CEPs) of chromosomes 3, 7 and 17,
and locus-specific identifier probes to the locus 9p21 of the tumor
suppressor gene p16 (Beijing GP Medical Technologies, Ltd.,
Beijing, China). Under dry and dark conditions, the probe mixture
[8 µl hybridization buffer solution (Hubei Taikang Medical
Equipment Co., Ltd.) and 2 µl probe] was prepared, and next
denatured at 73°C for 5 min in an electric-heated thermostatic
water bath (HH.S11-Ni2; Hubei Taikang Medical Equipment Co., Ltd.).
The probe mixture was then added to the slides and incubated
overnight (>17 h) at 42°C for hybridization. In order to prevent
volatilization of the probe mixture volatilize, cover slips were
used to cover the hybridized area, and sealing film was mounted in
the surrounding gap.
The following day, the slides were dehydrated in 70%
ethanol, and naturally dried prior to be stained with
4′,6-diamidino-2-phenylindole (DAPI). Cells with three-color
fluorescent hybridization signals (DAPI/fluorescein
isothiocyanate/tetramethylrhodamine; fluorescence in situ
hybridization detection kit; GP Medical Technologies, Ltd.) were
observed under a microscope (BX51; Olympus Corporation, Tokyo,
Japan). Image analysis was performed with VideoTesT-FISH 2.0
software (Digital Imaging Systems Ltd., Bourne End, UK).
All cases were screened by a cytotechnologist with
specific training in FISH interpretation. Specimens were considered
to be abnormal when presenting >12 cells with heterozygosity
loss or homozygosis loss of solely p16, or >4 aneusomic cells of
>2 other probes. Positive cases were confirmed by a second
cytotechnologist. In addition, specimens were also considered to be
abnormal if contained 25 cells with tetrasomy, which corresponded
to 4 signals in each of the 4 probes analyzed.
Statistical analysis
Statistical analysis for evaluating the differences
between cytology and FISH was performed using two-sided Fisher's
exact test. When not applicable, χ2 test was performed
instead (7). P<0.05 was considered
to indicate a statistically significant difference. All analyses
were performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
A total of 125 voided urine specimens from 125
patients were collected in the present study. The average follow-up
time following cytology and FISH assay was 23.8 months (range, 5–39
months). Of the 125 patients, 8 could not be contacted until the
last follow-up, and were therefore excluded from the study. The
voided urine specimens from the remaining 117 patients were
analyzed in the present study, 79 of whom were men and 38 women.
Cytology could not be performed in 6 of the 117 samples due to
insufficient number of cells in these urine samples, while the
fluorescence signals of FISH test in 7 specimens could not be
interpreted. As an insufficient number of cells in urine is
typically voided during FISH, all these patients, which were
regarded as non-diagnostic, were included in the subsequent
statistical analysis in order to estimate the overall sensitivity
and specificity of FISH. Of the 117 patients, 19 had histologically
confirmed UTUC, of whom, 6 exhibited stage pTa disease, 5 stage pT1
disease, 5 stage pT2 disease and 3 stage pTis disease (7 G1, 8 G2
and 4 G3).
Tables I and II indicate the characteristics of the study
population. Table I contains the
cytology data of 117 patients, while their FISH data is presented
in Table II. Table III reveals the sensitivity,
specificity and predictive value of FISH and cytology tests.
 | Table I.Cytology data compared with histology
data (n=117). |
Table I.
Cytology data compared with histology
data (n=117).
|
| Histology |
|---|
|
|
|
|---|
| Cytology | Urothelial
lesions | Negative | Total |
|---|
| Positive | 8 | 0 | 8 |
| Negative | 10 | 93 | 103 |
| Nondiagnostic | 1 | 5 | 6 |
| Total | 19 | 98 | 117 |
 | Table II.FISH data compared with histology data
(n=117). |
Table II.
FISH data compared with histology data
(n=117).
|
| Histology |
|---|
|
|
|
|---|
| FISH | Urothelial
lesions | Negative | Total |
|---|
| Positive | 16 | 4 | 20 |
| Negative | 2 | 88 | 90 |
| Nondiagnostic | 1 | 6 | 7 |
| Total | 19 | 98 | 117 |
 | Table III.Sensitivity, specificity and
predictive value of FISH data compared with cytology data. |
Table III.
Sensitivity, specificity and
predictive value of FISH data compared with cytology data.
| Histology, %
(n=117) | Cytology, % | FISH, % | P-value |
|---|
| Sensitivity | 42.11 (8/19) | 84.21 (16/19) | 0.01a |
| Specificity | 94.90 (93/98) | 89.80 (88/98) | 0.09 |
| False positive | 0.00 (0/8) | 20.00 (4/20) | 0.24 |
| False negative | 9.71 (10/103) | 2.20 (2/90) | 0.02a |
| PPV | 100.00 (8/8) | 80.00 (16/20) | 0.24 |
| NPV | 90.29 (93/103) | 97.78 (88/90) | 0.02a |
The overall sensitivity of FISH to detect UTUC from
voided urine specimens was 84.21% (16/19), whereas that of cytology
was 42.11% (8/19) (P=0.01). The overall specificity of FISH to
detect UTUC from voided urine specimens was 89.80% (88/98),
compared with 94.90% (93/98) of cytology (P=0.09). Due to the
limited number of specimens, the differences in grade and
muscle-invasiveness of tumors were not compared. A total of 6
specimens could not be diagnosed by cytology (1 of which exhibited
positive histology and 5 negative), as the cellular morphology was
obscure or the number of cells in the sample was insufficient. A
total of 7 specimens could not be diagnosed by FISH (1 of which
exhibited positive histology and 6 negative), since their
fluorescence signals were faint or the number of cells in the
sample was insufficient.
In total, 10 false-negative samples were diagnosed
by cytology. These samples were also analyzed by FISH, and 8 of
them rendered a positive result. FISH analysis produced 2
false-negatives; these samples were also analyzed by cytology, and
were negative. A total of 4 false-positive samples analyzed by FISH
were also analyzed by cytology, and all rendered a negative result.
The positive predictive value (PPV) and negative predictive value
(NPV) of FISH were 80.00% (16/20) (P=0.24 vs. cytology) and 97.78%
(88/90) (P=0.02 vs. cytology), respectively, whereas the PPV and
NPV of cytology were 100.00% (8/8) and 90.29% (93/103),
respectively.
Table IV presents the
histopathological classification and other findings of 19 UTUCs in
the last follow-up screening of 19 cases. Of these 19 patients (15
males and 4 females; mean age, 64 years; range, 48–82 years), 3
exhibited gross hematuria and 5 microhematuria. The average
follow-up time was 21.42 months (range, 6–36 months). One patient
experienced recurrence of bladder cancer in the left ureter after
33 months of bladder cancer excision.
 | Table IV.Histopathological classification and
other findings of 19 upper tract urothelial carcinomas. |
Table IV.
Histopathological classification and
other findings of 19 upper tract urothelial carcinomas.
| No. | Gender | Age (years) | H | Bladder cancer
recurrence | Location of
lesion | Follow-up time
(months) | TCC
stage/grade | Cytology | FISH |
|---|
| 1 | Male | 54 | MH | Yes | LU | 33 | pT2/G2 | Positive | Positive |
| 2 | Male | 68 | None | No | RRP | 28 | pTa/G1 | Negative | Positive |
| 3 | Male | 72 | None | No | LU | 36 | pT2/G3 | Positive | Positive |
| 4 | Female | 71 | None | No | LRP | 35 | pT1/G2 | Negative | Positive |
| 5 | Male | 65 | MH | No | LRP | 29 | pTis/G1 | Positive | Negative |
| 6 | Male | 82 | None | No | RRP | 26 | pT1/G2 | Negative | Positive |
| 7 | Male | 48 | GH | No | RRP | 31 | pTis/G1 | Positive | Positive |
| 8 | Female | 63 | None | No | LRP | 22 | pTa/G2 | Negative | Positive |
| 9 | Male | 77 | GH | No | LRP | 24 | pT2/G3 | Positive | Positive |
| 10 | Male | 65 | None | No | RU | 19 | pT1/G1 | Positive | Negative |
| 11 | Male | 51 | MH | No | RU | 24 | pTa/G1 | Negative | Positive |
| 12 | Female | 53 | None | No | LRP | 12 | pT1/G2 | Negative | Positive |
| 13 | Male | 73 | None | No | RU | 9 | pTa/G2 | Negative | Positive |
| 14 | Female | 61 | None | No | LRP | 8 | pTis/G1 | Negative | Positive |
| 15 | Male | 56 | MH | No | RU | 16 | pTa/G2 | Nondiagnostic | Nondiagnostic |
| 16 | Male | 76 | None | No | LRP | 23 | pT2/G3 | Negative | Positive |
| 17 | Male | 49 | GH | No | RRP | 17 | pTa/G1 | Negative | Positive |
| 18 | Male | 68 | None | No | LRP | 9 | pT1/G3 | Negative | Positive |
| 19 | Male | 64 | MH | No | RRP | 6 | pT2/G2 | Positive | Positive |
Fig. 1 reveals
abnormal and normal FISH signals of CEP3, CEP7, CEP17 and gene
locus-specific p16. Fig. 1A and B
represent tumor cells, while Fig. 1C and
D represent normal cells.
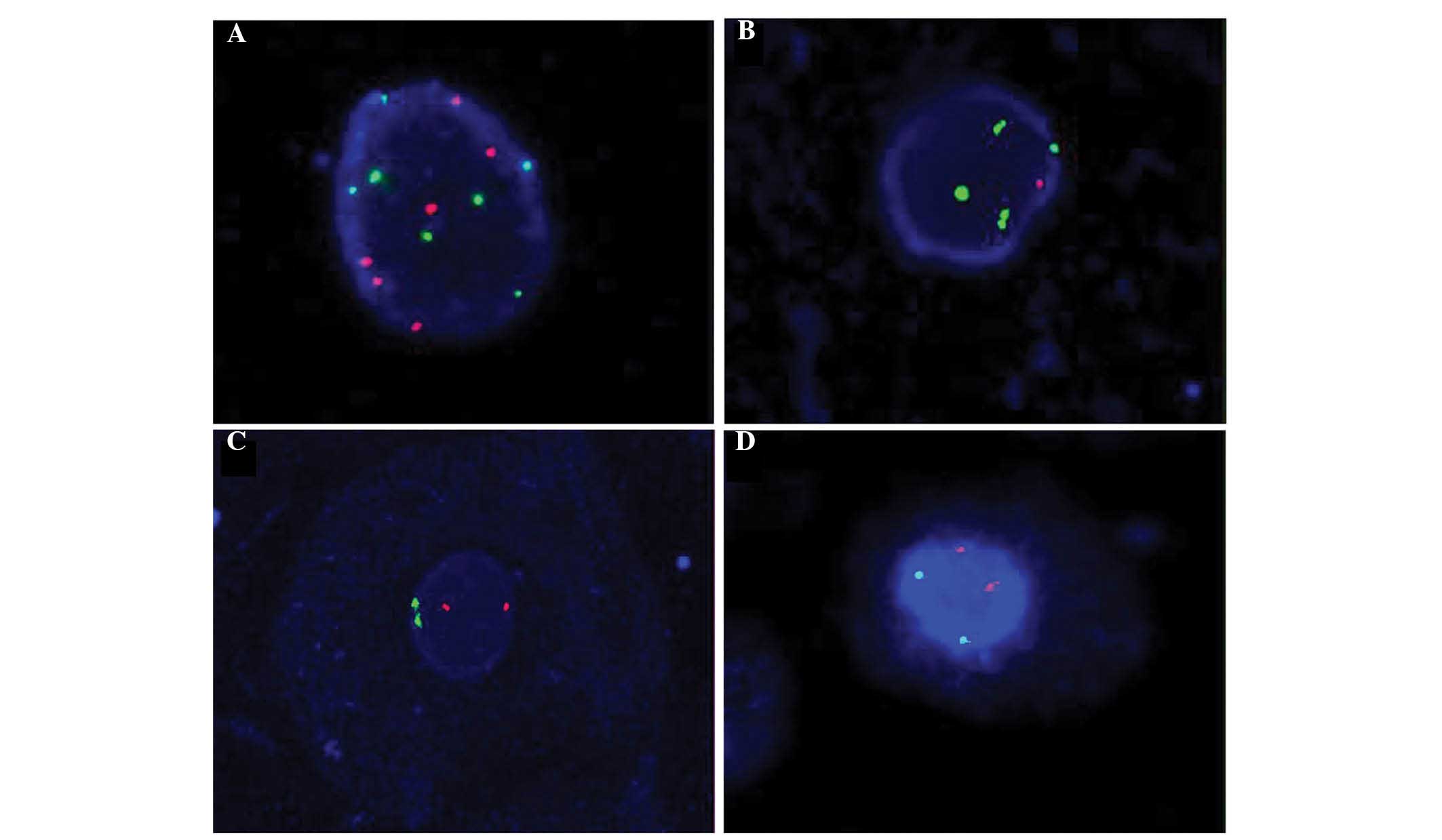 | Figure 1.Representative images of abnormal and
normal FISH signals of CEP3, CEP7, CEP17 and GLP p16. (A) Abnormal
FISH signals of CEP3 (red, 6 dots) and CEP7 (green, 7 dots). (B)
Abnormal FISH signals of GLP p16 (red, 1 dot) and CEP17 (green, 4
dots). (C) Normal FISH signals of CEP3 (red, 2 dots) and CEP7
(green, 2 dots). (D) Normal FISH signals of GLP p16 (red, 2 dots)
and CEP17 (green, 2 dots). Magnification, ×1,000. FISH,
fluorescence in situ hybridization; CEP, centromere
enumeration probe; GLP, gene locus-specific. |
Discussion
UTUC is not a frequent type of urological cancer,
accounting for only 5% of all transitional cell carcinomas of the
urinary system (12). UTUCs tend to
present high grade and stage, and are usually associated with poor
prognosis (12). Therefore, early
diagnosis and effective treatment for UTUC is imperative (4,13).
ImmunoCyt/uCyt+ test, voided or clean catch urine cytology,
radiographic or ultrasound imaging and ureteroscopy are the most
common diagnostic methods for UTUC (7). However, urine cytology has been reported
to be of little value in evaluating unclear or suspicious findings
of the upper urinary tract obtained by imaging studies (14,15), which
have a sensitivity of 30–50% (16,17).
Furthermore, radiographic and ultrasound imaging have low
sensitivity for the detection of carcinoma in situ in small
lesions, and ureteroscopy is an invasive method which patients
prefer to avoid (4).
FISH was previously reported to have a high
sensitivity and specificity for detecting bladder cancer (18). Few retrospective studies on FISH
demonstrated that FISH had high sensitivity and specificity in
detecting UTUC, contrarily to cytology (4,7,10). However, the majority of those studies
were retrospective, and the data available are insufficient
(4,7,10). Thus,
prospective, randomized studies are required. In addition,
specimens were detected mainly in clean catch urine, with a limited
number of specimens being detected in voided urine (7). However, patients usually do not accept
collecting clean catch urine, but would rather collect voided
urine, particularly in China (7,19). In
addition, reference standards of positive FISH are based on the
diagnostic criteria of bladder cancer (9). However, exfoliative cells of the upper
ureter or renal pelvis are present in low quantities in voided
urine and are susceptible to contamination by bladder cells.
Therefore, the diagnostic criteria of FISH in voided urine should
be amended moderately.
In the present study, UTUC was detected in voided
urine by FISH within a prospective randomized trial. If the number
of cells following centrifugation was not sufficient for visual
analysis with the naked eye, all the resuspended cells would be
seeded onto glass slides, and the number of aneusomic cells in the
whole slide would be counted, in order to increase FISH
sensitivity. Combining microscopic observation with analysis of
selected images enabled to exclude non-specific signals, thus
improving FISH specificity. If numerous hemameba were present,
samples would be observed using the DAPI channel with differential
cell nucleus refractivity between epithelial cells and hemameba, in
order to decrease the interference of hemameba.
In the present study, the sensitivity of FISH for
detecting UTUC was higher than that of cytology (P=0.01), and the
rate of false-negatives of FISH was lower than that of cytology
(P=0.02). In addition, the NPV of FISH was superior to that of
cytology (P=0.02). However, in terms of specificity, rate of
false-positives and PPV, FISH was similar to cytology. Contrarily
to a previous study by Huang et al (17), who reported a sensitivity of 100% for
FISH in detecting UTUC, the sensitivity identified in the present
study was low. This difference may be due to the fact that the
majority of patients in the cohort analyzed by Huang et al
(17) exhibited high-grade UTUCs, for
which the FISH assay has higher sensitivity than for low-grade
tumors (17,20). The sensitivity of FISH for the
detection of UTUC identified in the present study was similar to
that reported by Gruschwitz et al (21), but higher than that reported by
Reynolds et al (9). There is a
possible reason for this difference. In the study by Reynolds et
al (9), specimens were considered
to be abnormal by FISH if 10 cells with near-tetrasomy or
tetrasomy, or 5 cells with hypertetrasomy were detected. However,
the cut-off value used in the present study was lower, based on the
characteristics of the Chinese healthy donors who provided voided
urine as a control. In the study by Reynolds et al (9), the samples consisted of ureteral
washings and renal pelvis brushings, while voided urine specimens
were used instead in the present study. Thus, the proportion of
upper ureteral epitheliums as contaminated by bladder transitional
epitheliums is low in the present study, compared with the study by
Reynolds et al (9). Thus, the
authors recommend in their study to apply a high cut-off value of
20%, due to the enrichment in upper tract urothelial cells in their
samples, whereas the cut-off value of 4% used in the present study
still rendered high sensitivity in detecting patients with
UUT-UCCs, due to the reduced number of false positives detected as
a consequence of as a result of contamination by bladder
transitional epitheliums in voided urine specimens. Therefore, the
present authors recommend 4% as the cut-off value, which led to
84.21% sensitivity, 89.80% specificity, 2.20% rate of
false-negatives and 97.78% NPV for the detection of UTUCs in voided
urine by FISH. However, this low cut-off value increased the
false-positivity (20.00%) and decreased the PPV (80.00%) of FISH.
In the present study, 2 false-negative results of FISH that were
positive by cytology corresponded to low-grade tumor specimens, and
7 out of 10 false-negative results of cytology that were positive
by FISH were high-grade tumor specimens. One specimen was
nondiagnostic by FISH and cytology, due to an insufficient number
of cells, but urethroscopy with biopsy during the follow-up
demonstrated that it was a pTa G1 tumor in the right ureter.
In summary, the present data demonstrated that it is
possible to apply FISH as a method to detect UTUC in voided urine
specimens. FISH displayed good sensitivity and specificity when the
cut-off value was 4%, but this increased its rate of
false-positivity and decreased its PPV. In the present study, FISH
was observed to be a clinical reliable method for the detection of
UTUC, with a higher sensitivity than cytology and equal
specificity. In order to improve FISH specificity, non-specific
signals were excluded between microscopic observation and analysis
upon image selection. With regard to positive FISH results, further
examination or follow-up are strongly recommended.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (Beijing, China; grant
no. 81100443), Chongqing Yuzhong District Science and Technology
Plan (project no. 20120214) and Chongqing Municipal Health Bureau
(scientific research project no. 2013-2-151).
References
|
1
|
Oya M and Kikuchi E: Committee for
Establishment of Clinical Practice Guideline for Management of
Upper Tract Urothelial Carcinoma; Japanese Urological Association:
Evidenced-based clinical practice guideline for upper tract
urothelial carcinoma (summary - Japanese Urological Association,
2014 edition). Int J Urol. 22:3–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta R, Paner GP and Amin MB: Neoplasms
of the upper urinary tract: A review with focus on urothelial
carcinoma of the pelvicalyceal system and aspects related to its
diagnosis and reporting. Adv Anat Pathol. 15:127–139. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colin P, Koenig P, Ouzzane A, Berthon N,
Villers A, Biserte J and Rouprêt M: Environmental factors involved
in carcinogenesis of urothelial cell carcinomas of the upper
urinary tract. BJU Int. 104:1436–1440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marín-Aguilera M, Mengual L, Ribal MJ,
Musquera M, Ars E, Villavicencio H, Algaba F and Alcaraz A: Utility
of fluorescence in situ hybridization as a non-invasive
technique in the diagnosis of upper urinary tract urothelial
carcinoma. Eur Urol. 51:409–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mills IW, Laniado ME and Patel A: The role
of endoscopy in the management of patients with upper urinary tract
transitional cell carcinoma. BJU Int. 87:150–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiener HG, Mian C, Haitel A, Pycha A,
Schatzl G and Marberger M: Can urine bound diagnostic tests replace
cystoscopy in the management of bladder cancer? J Urol.
159:1876–1880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mian C, Mazzoleni G, Vikoler S, Martini T,
Knüchel-Clark R, Zaak D, Lazica A, Roth S, Mian M and Pycha A:
Fluorescence in situ hybridisation in the diagnosis of upper
urinary tract tumours. Eur Urol. 58:288–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH and Wittekind CH: Urological
tumours: Bladder. TNM Classification of Malignant Tumours (6th).
John Wiley & Sons. (Hoboken, NJ). 199–202. 2002.
|
|
9
|
Reynolds JP, Voss JS, Kipp BR, Karnes RJ,
Nassar A, Clayton AC, Henry MR, Sebo TJ, Zhang J and Halling KC:
Comparison of urine cytology and fluorescence in situ
hybridization in upper urothelial tract samples. Cancer Cytopathol.
122:459–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo B, Li W, Deng CH, Zheng FF, Sun XZ,
Wang DH and Dai YP: Utility of fluorescence in situ
hybridization in the diagnosis of upper urinary tract urothelial
carcinoma. Cancer Genet Cytogenet. 189:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
May M, Hakenberg OW, Gunia S, Pohling P,
Helke C, Lübbe L, Nowack R, Siegsmund M and Hoschke B: Comparative
diagnostic value of urine cytology, UBC-ELISA, and fluorescence
in situ hybridization for detection of transitional cell
carcinoma of urinary bladder in routine clinical practice. Urology.
70:449–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oosterlinck W, Solona E, van der Meijden
AP, Sylvester R, Böhle A, Rintala E and Lobel B: European
Association of Urology: EAU guidelines on diagnosis and treatment
of upper tract transitional cell carcinnoma. Eur Urol. 46:147–154.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stewart GD, Bariol SV, Grigor KM, Tolley
DA and McNeill SA: A comparison of the pathology of transitional
cell carcinoma of the bladder and upper urinary tract. BJU Int.
95:791–793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akkad T, Brunner A, Pallwein L, Gozzi C,
Bartsch G, Mikuz G, Steiner H and Verdorfer I: Fluorescence in
situ hybridization for detecting upper urinary tract tumors - a
preliminary report. Urology. 70:753–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zincke H, Aguilo JJ, Farrow GM, Utz DC and
Khan AU: Significance of urinary cytology in the early detection of
transitional cell cancer of the upper urinary tract. J Urol.
116:781–783. 1976.PubMed/NCBI
|
|
16
|
Lodde M, Mian C, Wiener H, Haitel A, Pycha
A and Marberger M: Detection of upper urinary tract transitional
cell carcinoma with ImmunoCyt: A preliminary report. Urology.
58:362–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang WT, Li LY, Pang J, Ruan XX, Sun QP,
Yang WJ and Gao X: Fluorescence in situ hybridization assay
detects upper urinary tract transitional cell carcinoma in patients
with asymptomatic hematuria and negative urine cytology. Neoplasma.
59:355–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song M-J, Lee H-M and Kim S-H: Clinical
usefulness of fluorescence in situ hybridization for
diagnosis and surveillance of bladder cancer. Cancer Genet
Cytogenet. 198:144–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shan Z, Wu P, Zheng S, Tan W, Zhou H, Zu
Y, Qi H, Zhang P, Peng H and Wang Y: Evaluation of upper urinary
tract tumors by FISH in Chinese patients. Cancer Genet Cytogenet.
203:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen AA and Grasso M: Is there a role for
FISH in the management and surveillance of patients with upper
tract transitional-cell carcinoma? J Endourol. 22:1371–1374. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gruschwitz T, Gajda M, Enkelmann A, Grimm
MO, Wunderlich H, Horstmann M and Junker K: FISH analysis of
washing urine from the upper urinary tract for the detection of
urothelial cancers. Int Urol Nephrol. 46:1769–1774. 2014.
|















