Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease
in Southeast Asia and Southeast China. Radiotherapy is the primary
treatment for patients with non-metastatic NPC, and concurrent
chemoradiotherapy is considered the standard treatment for
locally-advanced NPC (1,2). Due to radioresistance, certain patients
with NPC present with local recurrences and distant metastases 1–2
years after treatment (3). The
primary reason for the failure to respond to radiotherapy is
radioresistance. Numerous studies have aimed to identify
differentially-expressed proteins (DEPs) associated with cancer
radioresistance between paired parental cell lines and
radioresistant subclones using high-throughput proteomics methods.
These studies focused either on paired parental and radioresistant
cancer cells or immunochemical analyses of tissues from patients
with locoregional failure. These methods have limitations in the
screening for novel biomarkers that can be used for diagnosis and
treatment. Nevertheless, there was no overlap in the proteins
involved in radioresistance in the various studies. This may be due
to distinct tissue specificity or the different methods used.
Although several radioresistant subclones of NPC have been
established (3,4), no animal experiments have been reported
thus far. As described previously (3), we have also established a subclone
(CNE-2R) by exposing CNE-2 cells to a cumulative radiation dose of
64 Gy. CNE-2R and CNE-2 cells have a different survival fraction at
2 Gy value, as well as varying α, β and α/β values, which were
verified by colony formation assays and complementary (c)DNA
microarray analyses (3). A study by
Sekhar et al found that the inhibition of DNA repair due to
the inhibition of nucleophosmin (NPM) shuttling increases the
efficacy of DNA-damaging therapeutic strategies (5), thereby increasing radiosensitivity. In
another study, annexin A3 was selected as a protein of interest in
paired prostate cancer cell lines, and five regulated proteins
(nucleoside diphosphate kinase A, heat shock 70-kDa protein 8,
DNA-(apurinic or apyrimidinic site) lyase, plasminogen activator
inhibitor 1 RNA-binding protein and Ras-related protein Rab-11A)
were validated using western blot analyses. However, annexin A3 was
not validated (6). Nm23-H1 was first
identified as a biological predictor of radioresistance based on
cDNA array and proteomics analyses (7). However, it remains unclear as to whether
it acts as a biomarker.
In the present study, changes in protein expression
associated with radioresistance were investigated. First, possible
DEPs for NPC radioresistance were identified by comparing CNE-2R
cells and parental CNE-2 cells in vitro. Next, three
particularly significant DEPs, NPM1, annexin A3 and nm23-H1, were
validated using western blot assays (8). Comparable radioresistant and
radiosensitive tumor models of NPC were established by
subcutaneously injecting CNE-2R cells and CNE-2 cells into athymic
nude mice. The xenografts were irradiated with fractioned X-ray
irradiation and the growth characterization was studied. Finally,
in order to validate the expression of these proteins in
vivo, NPM1, annexin A3 and nm23-H1 protein expression was
immunohistochemically examined in the tumor tissues. The findings
indicated that NPM1, annexin A3 and nm23-H1 are potential
biomarkers for predicting the response of NPC to radiotherapy.
Materials and methods
Cell lines and cell culture
Poorly-differentiated human NPC CNE-2 cells were
purchased from the Cancer Hospital of Fudan University (Shangha,
China). CNE-2R cells were induced by treating the parental CNE-2
cells with fractioned cobalt-60 γ-ray irradiation (total dose,
6,400 cGy; Theratron 780; Theratronics International Ltd., Kanata,
Canada) (3). The CNE-2R and CNE-2
cells were cultured separately in RPMI 1640 medium (Hyclone; GE
Healthcare Life Sciences, Logan UT, USA) supplemented with 10%
fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), penicillin G (100 kU/l;
(North China Pharmaceutical Co., Ltd., Shijiazhuang, China) and
streptomycin (100 mg/l; Qilu Pharmaceutical Co., Ltd., Jinan,
China). The cells were maintained at 37°C in a 5% CO2
incubator until use.
Xenograft tumorigenicity assays
BALB/c athymic nude mice (males and females, aged
4–6 weeks; n=36) were obtained from the Animal Laboratory Center of
Guangxi Medical University [Guangxi, China; license number, SCXK
(Gui) 2009-002]. The mice were housed five per cage, and maintained
under specific pathogen-free conditions. The mice were randomly
divided into three groups of 12 animals each: Group A, treated with
CNE-2R cells; group B, treated with CNE-2 cells; and group C,
treated with saline. In group A, CNE-2R cells (1×107 in
a total volume of 0.2 ml) were subcutaneously injected into the
right hind legs of 6 mice, and both the right and left hind legs of
6 mice. In group B, CNE-2 cells (1×107 in a total volume
of 0.2 ml) were subcutaneously injected into the right hind legs of
6 mice, and both the right and left hind legs of 6 mice. The
subcutaneous xenograft tumors were palpated and the diameters
measured every other day using calipers. The volumes of the tumors
were calculated using the following formula: Volume = 0.5 × length
(cm) × width2 (cm). Next, a growth curve of the two
tumors group was generated. Tumor doubling times (DTs) were
calculated using the following formula: DT = ln(2Dt) /
ln(V2 / V1), where V1 and
V2 are the volume estimates in each scan obtained Dt
days apart (9). Tumor-bearing mice
received X-ray irradiation when the tumors reached ~1 cm in the
longest diameter (13 days after cancer cell implantation). The mice
received 16 Gy in 4 fractions delivered over 8 days. Local external
beam radiation was applied (6-MV X-rays at a dose rate of 400
MU/min) using a clinical X-ray therapy unit (Precise Elekta;
Eleckta, Stockholm, Sweden). Right-sided tumors were locally
irradiated, with the rest of the body protected from irradiation
with lead shielding. Single posteroanterior external beam radiation
fields were used. Irradiated (IR) and non-IR (NIR) groups were
created. All animal experiments were performed in accordance with
Guangxi Medical University Ethics Committee guidelines.
Hematoxylin and eosin (H&E)
staining
The mice were sacrificed 2 weeks after irradiation,
and autopsies were performed on all injected mice. Xenograft tumors
were excised, immediately placed in 10% neutral-buffered formalin
and fixed for 24–48 h. Subsequent to fixation, samples were
dehydrated and embedded in paraffin. A series of 4-µm sections were
prepared from each specimen, mounted on poly-lysine-coated glass
slides and dried for 4–5 h at 37°C to promote adhesion. H&E
staining was performed on one section from each specimen.
Tumor tissue immunohistochemistry
Immunohistochemical staining of the paraffin
sections was performed after dewaxing and rehydrating the sections.
Briefly, 4-µm tissue sections were blocked with 3% hydrogen
peroxide for 10 min to inactivate endogenous peroxidase activity.
Once the tissue sections had been autoclaved at 120°C for 10 min in
an antigen retrieval solution [10 mmol/l sodium citrate buffer (pH
6.0)], they were incubated overnight at 4°C with a polyclonal
rabbit NPM1 antibody (cat. no. 10306-1-AP; 1:100 dilution;
ProteinTech Group, Inc., Chicago, IL, USA), a polyclonal rabbit
anti-annexin A3 (cat. no. ab33068; 1:100 dilution; Abcam,
Cambridge, UK) or a monoclonal rabbit nm23-H1 (cat. no. 7948-1;
1:100 dilution; Epitomics, Inc., Burlingame, CA, USA) antibodies.
The sections were then incubated with Biotin-conjugated Affinipure
goat anti-rabbit immunoglobulin G(H+L) (cat. no. SA00004-2; 1:200
dilution; ProteinTech Group, Inc.) for 1 h at room temperature,
followed by incubation with the streptavidin-biotin complex (cat.
no. SA00001-0; 1:200 dilution; ProteinTech Group, Inc.). The color
was developed after incubation for 3–5 min with
3,3′-diaminobenzidine solution (0.05%). The sections were then
counterstained with hematoxylin and mounted. For the negative
controls, the primary antibodies were replaced with
phosphate-buffered saline. The sections were observed using light
microscopy. The degree of NPM1 protein expression was assessed
based on the percentage of positive cells, whereas annexin A3 and
nm23-H1 expression were quantified using a computer-based
quantitative color image analysis software (Image-Pro Plus 6.0;
Media Cybernetics, Bethesda, MD, USA). The mean optical density
(MOD) of 10 views was obtained. The relative amounts of annexin
A3-positive and nm23-H1-positive cells are expressed as a ratio of
the MODs of the CNE-2R and CNE-2 tumors.
Statistical analysis
Data analyses were performed using SPSS version 13.0
(SPSS Inc., Chicago, IL, USA). Differences in protein expression
were compared using a one-way analysis of variance. Significance
levels were further evaluated using Bonferroni's multiple
comparisons tests. All data are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Tumor growth characterization
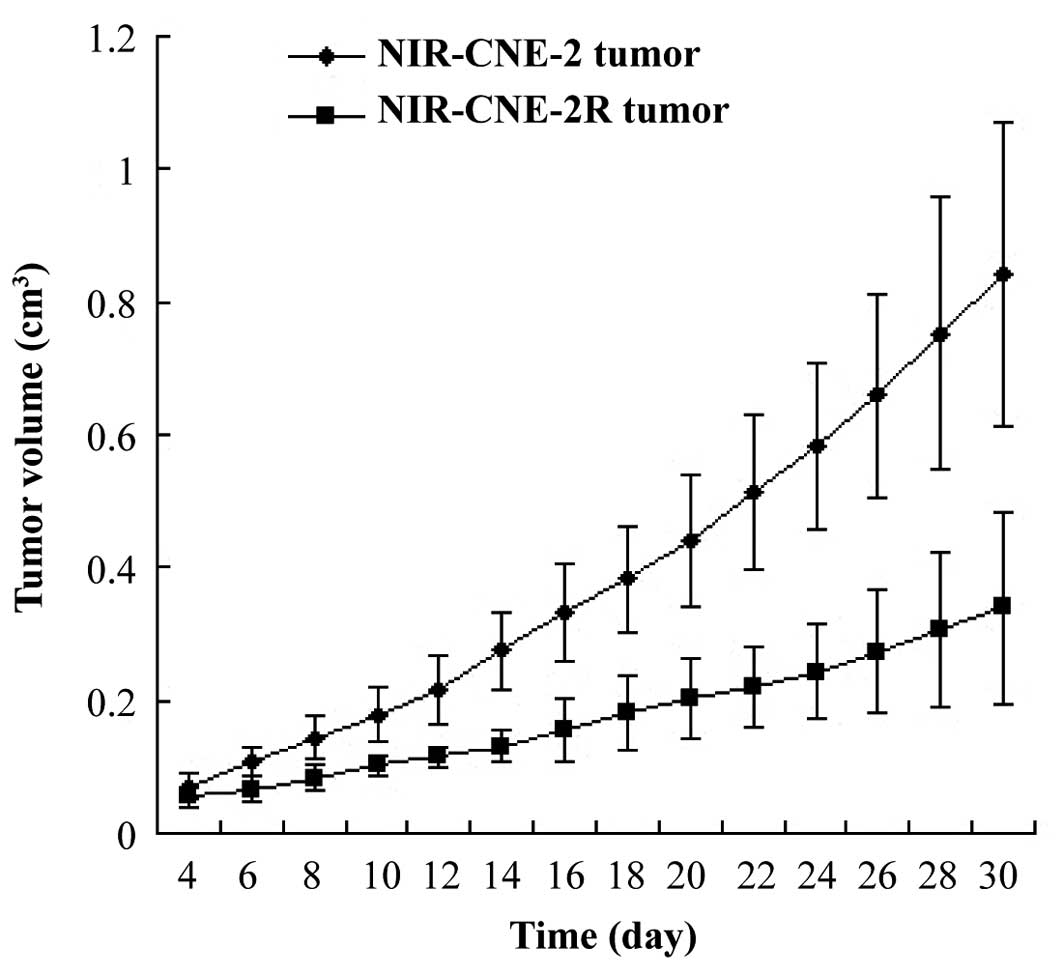
The NIR-CNE-2R tumors grew significantly slower than
the NIR-CNE-2 tumors (P=0.025). The doubling times of the
NIR-CNE-2R and NIR-CNE-2 tumors were 4.8 and 3.9 days,
respectively. CNE-2R tumor volume progression was not inhibited by
irradiation, whereas CNE-2 tumor volume was inhibited (Fig. 1). The tumor volume doubling times for
the IR-CNE-2R and IR-CNE-2 tumors were 6.2 days and 17.1 days,
respectively. The volume increase rate of the IR-CNE-2R tumors was
higher than that of the IR-CNE-2 tumors.
Histological findings
A large amount of tumor necrosis was observed in the
NIR-CNE-2R and NIR-CNE-2 tumors. However, there were only a few
scattered necrotic areas observed in the IR-CNE-2R tumors.
Following irradiation, extensive degeneration and pyknotic cells
were observed in the tumors. Cancer cells were nested, with large
nuclei and abundant cytoplasm, and round or oval hyperchromatic
nuclei. Keratinization was minimal or absent, and the mitotic rates
were variable. The tumor cells possessed morphological
characteristics similar to those of human NPC cells.
NPM1, annexin A3 and nm23-H1
immunohistochemical analysis
Immunohistochemical analyses were used to compare
NPM1, annexin A3 and nm23-H1 protein expression in the CNE-2 and
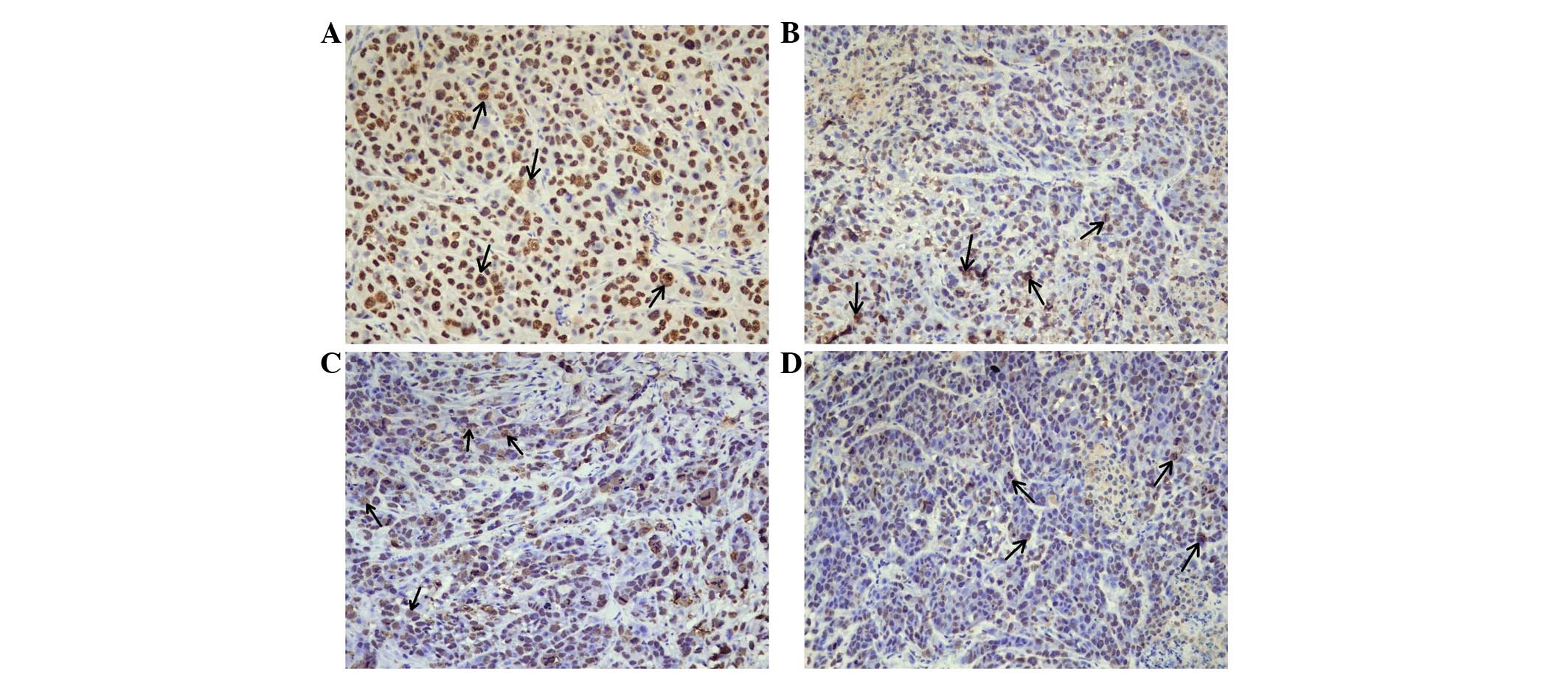
CNE-2R tumor tissues. For NPM1 immunohistochemistry, positive cells
were distinguished by brown staining in the nuclei of the cancer
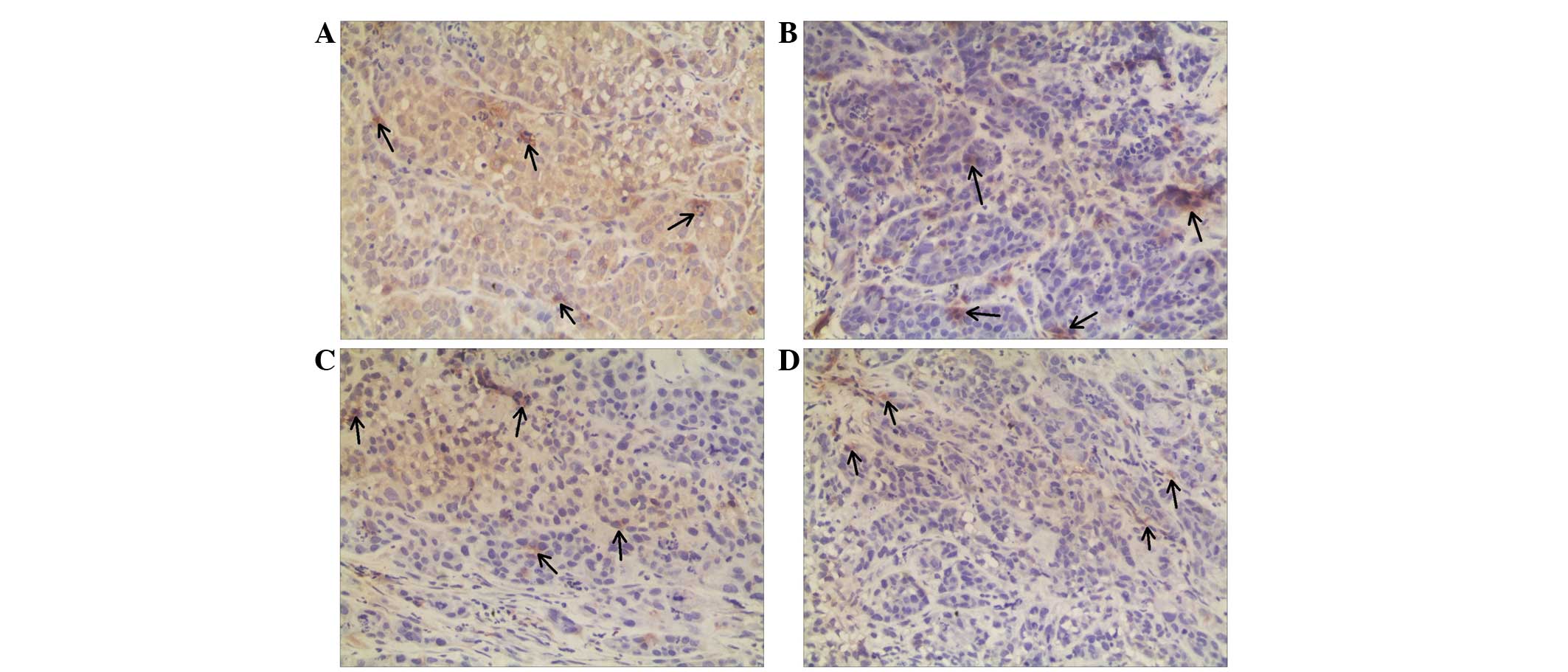
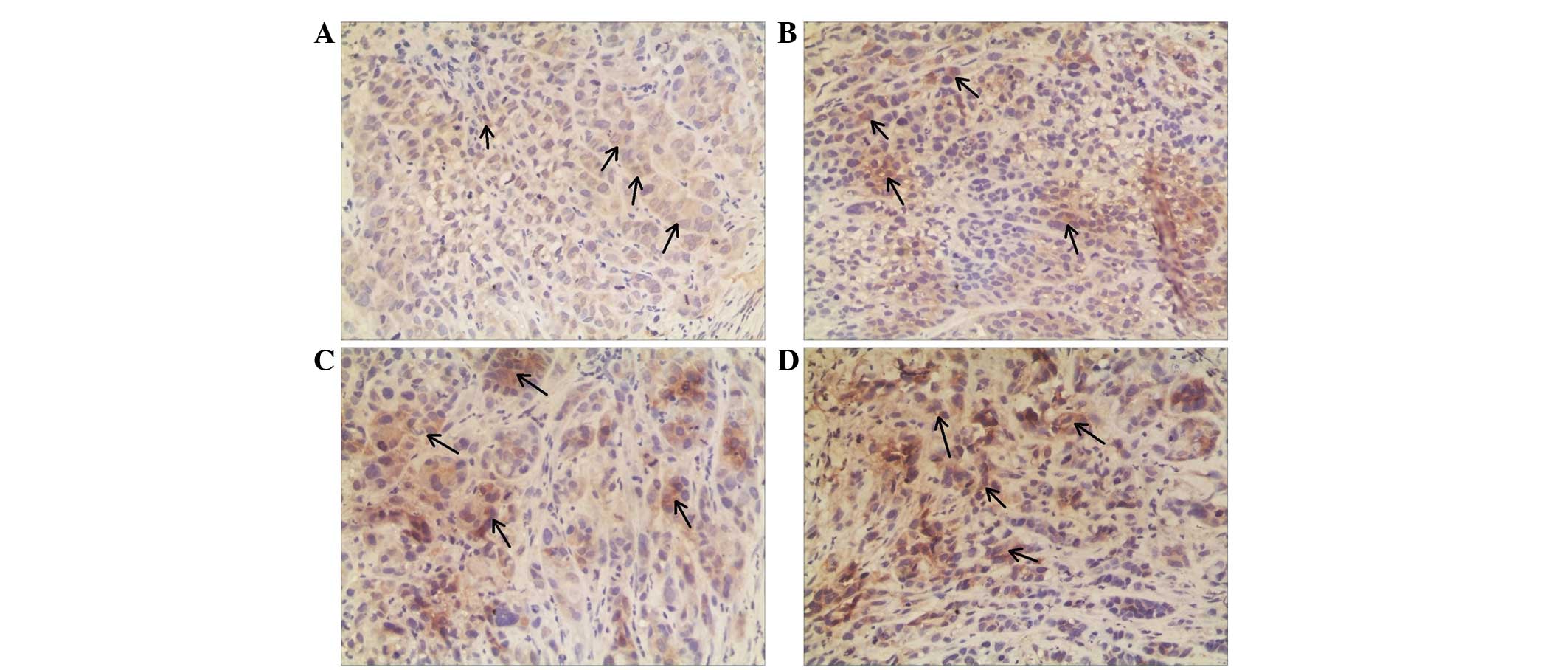
cells (Fig. 2). Annexin A3 and
nm23-H1 were predominately expressed in the cytoplasm; therefore,
brown granules in the cytoplasm of the carcinoma cells and
interstitial cells were considered positive (Figs. 3 and 4).
NPM1 and annexin A3 expression was significantly lower in the
NIR-CNE-2R tumors compared with that in the NIR-CNE-2 tumors
(P=0.007 and P=0.005; Tables I and
II), whereas Nm23-H1 expression was
significantly higher (P=0.036; Table
III). These results are in agreement with results from our
previous in vitro study (9).
Annexin A3 expression was significantly downregulated in the
IR-CNE-2R tumors compared with the IR-CNE-2 tumors (P=0.003;
Table II). However, Nm23-H1 protein
expression was significantly upregulated (P=0.004; Table III). NPM1 levels were slightly
upregulated in the IR-CNE-2R tumors compared with the NIR-CNE-2
tumors; however, the difference was not significant (P=0.731;
Table I).
 | Table I.NPM1 protein expression in xenograft
tumor tissues. |
Table I.
NPM1 protein expression in xenograft
tumor tissues.
| Tumors | NPM1, % |
|---|
| NIR-CNE-2 | 56.3±5.2a |
| NIR-CNE-2R | 36.0±4.2b |
| IR-CNE-2 | 35.3±5.5c |
| IR-CNE-2R | 36.3±4.7 |
 | Table II.MOD of annexin A3 protein in xenograft
tumor tissues. |
Table II.
MOD of annexin A3 protein in xenograft
tumor tissues.
| Tumors | MOD value of annexin
A3 |
|---|
| NIR-CNE-2 |
0.062±0.009a,b |
| NIR-CNE-2R |
0.035±0.012c |
| IR-CNE-2 |
0.051±0.009d |
| IR-CNE-2R | 0.029±0.007 |
 | Table III.MOD of nm23-H1 protein in xenograft
tumor tissues. |
Table III.
MOD of nm23-H1 protein in xenograft
tumor tissues.
| Tumors | MOD value of
nm23-H1 |
|---|
| NIR-CNE-2 |
0.043±0.007a,b |
| NIR-CNE-2R |
0.056±0.007c |
| IR-CNE-2 |
0.046±0.007d |
| IR-CNE-2R | 0.079±0.009 |
Discussion
Previous studies have screened for DEPs associated
with radioresistance in NPC (4,10). One
study identified seven upregulated genes in radioresistant
subclones of CNE2. Among these, gp96 and growth differentiation
factor 15 showed the highest expression (10). Feng et al (4) identified 34 DEPs using proteomics
methods. It was found that the downregulation of 14-3-3σ and
Maspin, and the upregulation of heat shock protein family A (Hsp70)
member 5 and manganese superoxide dismutase correlated with NPC
radioresistance. However, none of these DEPs were validated in
vivo.
To identify more reliable markers for
radioresistance, a sub-line (termed CNE-2R) was established by
exposing CNE-2 cells to a cumulative irradiation dose of 64 Gy
(3). In our previous study, proteomic
analyses were used to search for potential biomarkers of
radioresistance. NPM1, annexin A3 and Nm23-H1 proteins were among
the 16 identified DEPs that were regulated more than 2-fold
(11). Furthermore, a comparable
radioresistant and radiosensitive tumor model of human NPC was
established, and the level of these three proteins was compared
in vivo. The results indicated that NPM1, annexin A3 and
Nm23-H1 protein expression likely correlate with NPC
radioresistance.
NPM1 (also known as nucleolar phosphoprotein B23) is
a molecular chaperone involved in numerous cellular processes,
including centrosome duplication, ribosome biogenesis, cell-cycle
progression (12) and DNA damage
repair (13). Enhanced NPM1
expression causes uncontrolled cell growth. In tumor cells, NPM
overexpression is associated with increased cell growth and
proliferation. It has been demonstrated that NPM1 protein levels
are inversely associated with cell doubling time in human cancer
cells (14). Higher NPM expression is
associated with local recurrence rate and/or better disease-free
survival in certain types of cancers; therefore, it has been
proposed that NPM acts as a marker for oral squamous cell
carcinomas (15). Loss of NPM
expression contributes to tumorigenesis via its interaction with
the protein alternate reading frame, thereby controlling genomic
stability (16). Recently, the
association between NPM expression and DNA repair has been
elucidated. Sekhar et al (5)
demonstrated that the inhibition of DNA repair by NPM shuttling
inhibition increased the efficacy of DNA-damaging therapeutic
strategies such as ionizing radiation. In the present study, NPM1
protein expression was significantly lower in the CNE-2R cells
compared with the CNE-2 cells. In vivo tests also showed
that NPM1 expression was lower in the NIR-CNE-2R tumors compared
with the NIR-CNE-2 tumors. Notably, NPM1 protein expression was
slightly higher in the IR-CNE-2R tumors compared with the IR-CNE-2
tumors, which appears paradoxical with NPM1 protein expression
levels in non-irradiated tumors. We propose that this may be as the
IR-CNE-2R cells have acquired increased DNA damage repair
activity.
Annexins are a structurally homologous family of
calcium-dependent phospholipid-binding proteins that includes 12
members (17). Annexins have diverse
roles regulating membrane trafficking, cell division,
differentiation and apoptosis (18).
Moreover, they also function in carcinogenesis (19). Annexin A3 is relatively uncommon and
is not as well studied as annexin A1 and A2. Although it has not
been extensively studied in NPC cells, annexin A3 has prognostic
relevance in prostate cancer (20),
lung adenocarcinoma (21) and
papillary thyroid cancer (22).
Skvortsova et al (6)
identified annexin A2 and A3 as novel biomarkers in prostate cancer
cell lines using two-dimensional difference gel electrophoresis and
matrix-assisted laser desorption ionization time-of-flight
(TOF)/TOF-mass spectrometry; however, annexin A3 was not verified.
In the present study, annexin A3 was first screened as a
downregulated protein in the CNE-2R cells, and was also verified
using western blot analyses (11).
In vivo tests showed that annexin A3 expression was
significantly lower in the NIR-CNE-2R tumors than in the NIR-CNE-2
tumors. These results suggested that annexin A3 may function in NPC
radioresistance. However, its mechanism of action requires further
clarification.
The Nm23-H1 protein, encoded by the Nm23-H1
gene, is a ubiquitously distributed nuclear diphosphate kinase that
catalyzes the phosphorylation of nucleoside diphosphates (23). Recently, the 3′-5′ exonuclease
activity of Nm23-H1 was found to be important for DNA repair, as it
maintains genomic stability after ionizing or ultraviolet
irradiation (24,25). Kim et al (7) found that the overexpression of Nm23-H1,
and specifically its nuclear translocation, may be a powerful
predictor of radioresistance in head and neck squamous cell
carcinoma. These studies suggested that the functional mechanism of
Nm23-H1 nuclear translocation may play a role in DNA damage repair,
which may affect radioresistance. The present study found that
Nm23-H1 protein expression was significantly higher in the CNE-2R
cells compared with the CNE-2 cells in vitro, as well as
being higher in the IR-CNE-2R tumors compared with the IR-CNE-2
tumors. We propose that Nm23-H1 may correlate with DNA damage
repair. It is likely that the CNE-2R xenograft tumor cells acquired
more DNA damage after irradiation. We hypothesize that this is the
reason for the CNE-2R cells obtaining radioresistance.
In conclusion, the present study established a
comparable radioresistant and radiosensitive tumor model of human
NPC. Furthermore, it was found that abnormal NPM1, annexin A3 and
nm23-H1 expression may contribute to NPC radioresistance and thus
be potential biomarkers for predicting NPC response to
radiotherapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30860329) and the GuangXi
Natural Science Foundation of China (no. 0832229).
References
|
1
|
Lee AW, Tung SY, Ngan RK, Chappell R, Chua
DT, Lu TX, Siu L, Tan T, Chan LK, Ng WT, et al: Factors
contributing to the efficacy of concurrent-adjuvant chemotherapy
for locoregionally advanced nasopharyngeal carcinoma: Combined
analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer. 47:656–666.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP,
Tang LL, Guo Y, Lin AH, Zeng XF and Ma J: Preliminary results of a
prospective randomized trial comparing concurrent chemoradiotherapy
plus adjuvant chemotherapy with radiotherapy alone in patients with
locoregionally advanced nasopharyngeal carcinoma in endemic regions
of china. Int J Radiat Oncol Biol Phys. 71:1356–1364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Y, Zhu XD, Qu S, Li L, Su F, Li Y,
Huang ST and Li DR: Identification of genes involved in
radioresistance of nasopharyngeal carcinoma by integrating gene
ontology and protein-protein interaction networks. Int J Oncol.
40:85–92. 2012.PubMed/NCBI
|
|
4
|
Feng XP, Yi H, Li MY, Li XH, Yi B, Zhang
PF, Li C, Peng F, Tang CE, Li JL, et al: Identification of
biomarkers for predicting nasopharyngeal carcinoma response to
radiotherapy by proteomics. Cancer Res. 70:3450–3462. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekhar KR, Reddy YT, Reddy PN, Crooks PA,
Venkateswaran A, McDonald WH, Geng L, Sasi S, Van Der Waal RP, Roti
JL, et al: The novel chemical entity YTR107 inhibits recruitment of
nucleophosmin to sites of DNA damage, suppressing repair of DNA
double-strand breaks and enhancing radiosensitization. Clin Cancer
Res. 17:6490–6499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skvortsova I, Skvortsov S, Stasyk T, Raju
U, Popper BA, Schiestl B, von Guggenberg E, Neher A, Bonn GK, Huber
LA and Lukas P: Intracellular signaling pathways regulating
radioresistance of human prostate carcinoma cells. Proteomics.
8:4521–4533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SH, Lee SY, Park HR, Sung JM, Park AR,
Kang S, Kim BG, Choi YP, Kim YB and Cho NH: Nuclear localization of
Nm23-H1 in head and neck squamous cell carcinoma is associated with
radiation resistance. Cancer. 117:1864–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Huang S, Zhu X, Zhou Z, Liu Y, Qu S
and Guo Y: Identification of radioresistance-associated proteins in
human nasopharyngeal carcinoma cell lines by proteomic analysis.
Cancer biother Radiopharm. 28:380–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geddes DM: The natural history of lung
cancer: A review based on rates of tumour growth. Br J Dis Chest.
73:1–17. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang JT, Chan SH, Lin CY, Lin TY, Wang
HM, Liao CT, Wang TH, Lee LY and Cheng AJ: Differentially expressed
genes in radioresistant nasopharyngeal cancer cells: Gp96 and
GDF15. Mol Cancer Ther. 6:2271–2279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He F, Luo W, Zhang Q, Guo Y, Liu MZ and Ma
J: Retrospective analysis of effectiveness of intensity-modulated
radiotherapy combined with chemotherapy or not for locoregionally
advanced nasopharyngeal carcinoma. Zhonghua Yi Xue Za Zhi.
93:2292–2295. 2013.(In Chinese). PubMed/NCBI
|
|
12
|
Brady SN, Maggi LB Jr, Winkeler CL, Toso
EA, Gwinn AS, Pelletier CL and Weber JD: Nucleophosmin protein
expression level, but not threonine 198 phosphorylation, is
essential in growth and proliferation. Oncogene. 28:3209–3220.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koike A, Nishikawa H, Wu W, Okada Y,
Venkitaraman AR and Ohta T: Recruitment of phosphorylated NPM1 to
sites of DNA damage through RNF8-dependent ubiquitin conjugates.
Cancer Res. 70:6746–6756. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Derenzini M, Sirri V, Trerè D and Ochs RL:
The quantity of nucleolar proteins nucleolin and protein B23 is
related to cell doubling time in human cancer cells. Lab Invest.
73:497–502. 1995.PubMed/NCBI
|
|
15
|
Coutinho-Camillo CM, Lourenço SV,
Nishimoto IN, Kowalski LP and Soares FA: Nucleophosmin, p53 and
Ki-67 expression patterns on an oral squamous cell carcinoma tissue
microarray. Hum Pathol. 41:1079–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grisendi S, Mecucci C, Falini B and
Pandolfi PP: Nucleophosmin and cancer. Nat Rev Cancer. 6:493–505.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev
Mol Cell Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kollermann J, Schlomm T, Bang H, Schwall
GP, von Eichel-Streiber C, Simon R, Schostak M, Huland H, Berg W,
Sauter G, et al: Expression and prognostic relevance of annexin A3
in prostate cancer. Eur Urol. 54:1314–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YF, Xiao ZQ, Li MX, Li MY, Zhang PF,
Li C, Li F, Chen YH, Yi H, Yao HX and Chen ZC: Quantitative
proteome analysis reveals annexin A3 as a novel biomarker in lung
adenocarcinoma. J Pathol. 217:54–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung EJ, Moon HG, Park ST, Cho BI, Lee SM,
Jeong CY, Ju YT, Jeong SH, Lee YJ, Choi SK, et al: Decreased
annexin A3 expression correlates with tumor progression in
papillary thyroid cancer. Proteomics Clin Appl. 4:528–537.
2010.PubMed/NCBI
|
|
23
|
Lascu L: The nucleoside diphosphate
kinases 1973–2000. J Bioenerg Biomembr. 32:213–214. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang M, Jarrett SG, Craven R and Kaetzel
DM: YNK1, the yeast homolog of human metastasis suppressor NM23, is
required for repair of UV radiation- and etoposide-induced DNA
damage. Mutat Res. 660:74–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jarrett SG, Novak M, Dabernat S, Daniel
JY, Mellon I, Zhang Q, Harris N, Ciesielski MJ, Fenstermaker RA,
Kovacic D, et al: Metastasis suppressor NM23-H1 promotes repair of
UV-induced DNA damage and suppresses UV-induced melanomagenesis.
Cancer Res. 72:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|