Introduction
Stromal cells are the most important components of
carcinoma and have a significant role in cancer development
(1). Myofibroblasts constitute the
bulk of the cancer stroma; these cells are activated,
non-transformed fibroblasts that express α-smooth muscle actin
(α-SMA) (2) and are observable in
various human carcinomas (3).
Myofibroblasts are able to promote cancer initiation, angiogenesis,
invasion and metastasis (4) by
secretion of elevated levels of growth factors, chemokines and
matrix metalloproteinases (MMPS) (5,6). The
origin of myofibroblasts in tumors remains to be fully elucidated.
Fibrocytes (7), pericytes (8) and smooth muscle cells (9) are thought to be the precursors of
myofibroblasts. However, certain studies have demonstrated that
bone mesenchymal stem cells (MSCs) may be the source of
myofibroblasts (10–12).
MSCs are defined by their self-renewal, plastic
adherence and multiple differentiation potential (13). MSCs possess the capacity to
differentiate into osteoblasts, adipocytes, chondrocytes, myocytes
and cardiomyocytes depending on the defining microenvironment
(14). Previous studies have reported
that engrafted MSCs are able to differentiate into myofibroblasts
(15) and promote tumor growth in a
rabbit bladder cancer model (16).
MSCs additionally exhibit tropism, which means that they are
attracted to sites of tissue injury, as well as tumor
microenvironments. The tropism of MSCs may be controlled by
inflammatory mediators produced during tissue damage or by the
tumor microenvironment (17,18).
The VX2 tumor is derived from Shope papilloma virus,
which induces malignant papilloma formation of a malignant
epithelial tumor that is a type of squamous cell carcinoma
(19). In a previous study by the
present authors, it was observed that MSCs were able to
differentiate into myofibroblasts in a rabbit VX2 bladder cancer
model (15). In the present study, a
primary culture of MSCs and VX2 cells was utilized to demonstrate
the tropism of MSCs, as well as their capacity to differentiate
into myofibroblasts in VX2 conditioned medium. The results of the
present study provide evidence that MSCs may be the precursor of
myofibroblasts.
Materials and methods
Animals
A total of 6 three-month old male New Zealand
rabbits, weighing ~1.5 kg, were purchased from the Shandong Academy
of Agricultural Sciences (Jinan, China). One three-month old male
New Zealand rabbit, weighing 2 kg, with a VX2 tumor was obtained
from Shanghai Jiao Tong University School of Medicine (Shanghai,
China). The rabbits were maintained in specific pathogen-free
environment at a temperature of 23±1°C with a 12 h light/dark cycle
and supplied rabbit chow and water ad libitum. The present
study was approved by the Ethics Committee of the Shandong
Provincial Hospital Affiliated to Shandong University (Jinan,
China; approval no., 2014-003).
MSC isolation and culture
Rabbit MSCs were aspirated from the bone marrow of
the proximal tibia of one male rabbit. MSCs were isolated as
described previously (10) through
the gradient centrifugation method as follows: The aspirates were
mixed with an equal volume of phosphate-buffered saline (PBS) and
centrifuged (Heraeus™ Pico™ microcentrifuge; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at room temperature (1,200 × g
for 5 min); the pellets were suspended in 5 ml PBS and were added
to 4 ml lymphocyte separating medium (Tianjin Haoyang Biological
Products Technology Co., Ltd., Tianjin, China) and centrifuged
again (2,000 × g for 20 min); the stratum intermedium was suspended
in 6 ml PBS and centrifuged (1,200 × g for 5 min) and the pellets
were suspended in 6 ml PBS and centrifuged at 1,000 × g for 5 min.
Subsequently, the MSC pellets were suspended in low-glucose
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% calf-serum (Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml penicillin-streptomycin
mixture (Shanghai Solarbio Science & Technology Co., Ltd.,
Shanghai, China), followed by plating at an initial seeding density
of 4.0×105/cm2. Nonadherent cells were
removed following 72 h of incubation and the culture medium was
replaced every 3 days. When cells grew to 80% confluence, they were
trypsinized (0.25% trypsin; Sigma-Aldrich, St. Louis, MO, USA) and
subcultured in a 1:2 split. Flow cytometry (FC500 Flow Cytometer;
Beckman Coulter, Inc., Brea, CA, USA) was performed in order to
identify passage 2 MSCs using mouse anti-rabbit cluster of
differentiation (CD) 34 (cat. no. MCA547B; dilution, 1:15),
anti-CD44 (cat. no. MCA806GA; dilution, 1:15), anti-CD105 (cat. no.
MCA1557; dilution, 1:15) and anti-CD109 (cat. no. MCA907F;
dilution, 1:15) primary antibodies (AbD Serotec, Kidlington, UK)
(4°C incubation for 30 min) and sheep anti-mouse fluorescein
isothiocyanate labeled secondary antibody (cat. no. ZDR-5307;
dilution, 1:15; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) (room temperature incubation for 1 h). The
experiment was repeated three times.
VX2 cell isolation and culture
As previously described (10), an aseptically excised tumor (>3 cm
volume), which had been grown in a male New Zealand rabbit for 4
weeks, was cut with scissors into sections (<1 mm in diameter)
following euthanization with 100 mg/kg sodium pentobarbital (New
Asia Pharmaceutical Co., Ltd., Shanghai, China). The sections were
trypsinized in 0.25% trypsin and 0.1% collagenase I (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C for 20 min. The mixture was
filtered using a 200 µm nylon mesh filter and the cells were
suspended in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 15% calf-serum and 100 U/ml
penicillin-streptomycin mixture, followed by plating at an initial
seeding density of 4.0×105/cm2. Nonadherent
cells were removed following 72 h of incubation and the culture
medium was replaced every 2 days. When cells grew to 80%
confluence, they were trypsinized (0.25% trypsin). The cells were
then fixed in 4% paraformaldehyde (Macklin Biochemical Co., Ltd.,
Shanghai, China) at the room temperature for 30 min., stained with
hematoxylin and eosin (Chengdu Rich Science Industry Co., Ltd.,
Chengdu, China) and the morphology of cells was analyzed using a
microscope (CX23; Olympus Corporation, Tokyo, Japan).
In vitro migration assay
The tropism of MSCs for VX2 cells was determined
using an in vitro migration assay. MSCs in serum-free
low-glucose DMEM were placed into the upper well of 24 mm tissue
culture Transwell plates (12 µm; EMD Millipore, Billerica, MA, USA)
coated with Matrigel [90 µl endothelial cell medium (Matrigel;
Sigma-Aldrich) diluted in 270 µl low-glucose DMEM]. VX2 cells were
incubated at 37°C in RPMI-1640 medium supplemented with 15%
calf-serum for 48 h. The resulting conditioned medium was aspirated
and prepared for the subsequent experiments. The cells were divided
into three groups as follows, based on the medium placed into the
lower well of the Transwell plates: Control group (low-glucose DMEM
supplemented with 10% calf-serum), Test 1 group (low-glucose DMEM
supplemented with 10% calf-serum and 30% VX2 conditioned medium)
and Test 2 group (low-glucose DMEM supplemented with 10% calf-serum
and 50% VX2 conditioned medium). MSCs were incubated for 12 h at
37°C. The migrated cells were stained using crystal violet (A. B.
Enterprises, Mumbai, India) and observed under a microscope (CX23;
Olympus Corporation). The migration ratio was determined by using a
colorimetric assay (WSL-2 colorimeter; Shanghai Laipade Science
Instruments Co., Ltd., Shanghai, China). All experiments were
performed in triplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR)
MSCs were incubated in conditioned medium
(low-glucose DMEM supplemented with 10% calf-serum and 30% VX2
conditioned medium) for 7 or 14 days at 37°C. Total RNA was
extracted from the MSCs and purified with the RNeasy Mini kit
(Qiagen China Co., Ltd., Shanghai, China), according to the
manufacturer's protocol. RT of the total RNA was performed using
the PrimeScript™ 1st Strand cDNA synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was utilized as the loading control gene,
whereby each RT sample was normalized to the GAPDH level. The PCR
was performed with rabbit primers (Table
I; Invitrogen; Thermo Fisher Scientific, Inc.). The PCR was
performed in a thermal cycler (Gene Cycler™, Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The cycling conditions were as follows:
22 cycles at 94°C for 1 min, 58°C for 60 sec, 72°C for 60 sec and
72°C for 7 min. PCR products were electrophoresed on a 1% agarose
gel containing ethidium bromide (Beijing NuoqiYa Biotechnology Co.,
Ltd., Beijing, China), and were visualized and images were captured
using an ultraviolet transilluminator (UVsolo TS; Biometra GmbH,
Göttingen, Germany). The experiment was performed in triplicate and
diethyl pyrocarbonate water was used as the negative control.
 | Table I.Rabbit-specific primer sequences. |
Table I.
Rabbit-specific primer sequences.
| Primer | Sequence | Size, bp |
|---|
| α-SMA |
GTGTGAGGAAGAGGACAGCA | 391 |
|
|
TACGTCCAGAGGCATAGAGG |
|
| Vimentin |
CTTCTCAGCATCACGACC | 146 |
|
|
ATCTATCTTGCGCTCCTG |
|
| GAPDH |
GAGCTGAACGGGAAACTCAC | 476 |
|
|
GGTCTGGGATGGAAACTGTG |
|
Western blotting
As mentioned previously, MSCs were incubated in
conditioned medium (low-glucose DMEM supplemented with 10%
calf-serum and 30% VX2 conditioned medium) for 7 or 14 days at
37°C. To identify the protein expression of α-SMA and vimentin,
western blotting was performed. Protein extracts were separated
using 14% sodium dodecyl sulfate polyacrylamide gel electrophoresis
and transferred to polyvinylidene difluoride membranes. Following
blocking with 5% non-fat dry milk for 1 h at room temperature, the
membrane was incubated overnight at 4°C with the appropriate
primary antibody (monoclonal mouse anti-rabbit α-SMA; cat. no.
04-1100; dilution, 1:1,000, EMD Millipore; or polyclonal mouse
anti-rabbit vimentin; cat. no. ab45939; dilution, 1:200; Abcam,
Cambridge, UK). Subsequently, the membrane was washed three times
with Tris-Buffered Saline and Tween 20 for 30 min, followed by
incubation with secondary antibody (horseradish
peroxidase-conjugated polyclonal sheep anti-mouse antibody; cat.
no. ZDR-5307; dilution, 1:5,000; Beijing Zhongshan Jinqiao
Biological Technology Co., Ltd., Beijing, China) for 1 h at room
temperature. The labeled proteins were visualized using ECL Western
Blotting Detection System (BestBio Company, Shanghai, China) and
exposed to film. Polyclonal goat anti-rabbit β-actin (cat. no.
TA-09; dilution, 1:500; Beijing Zhongshan Jinqiao Biological
Technology Co., Ltd.) was used as a protein loading control.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between two groups were compared using the
Student's t-test and differences between multiple groups were
analyzed by one-way analysis of variance, using SPSS version 17.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicated a statistically significant difference.
Results
MSCs and VX2 cell isolation and
culture
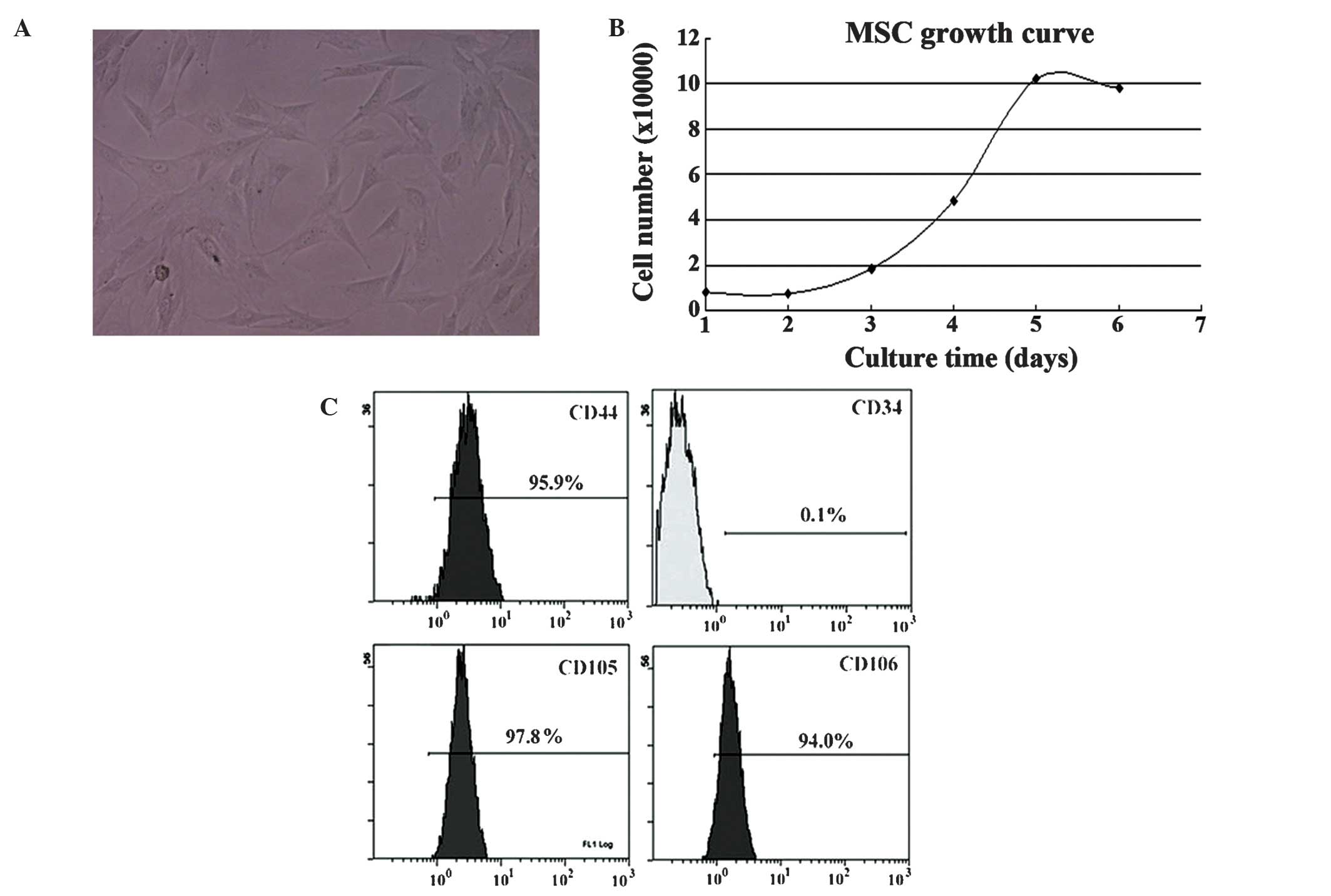
MSCs possessed a spindle shape (Fig. 1A) and their doubling time at passage 2
was ~30 h (Fig. 1B). MSCs were
positive for CD44, CD105 and CD106, but negative for CD34
expression (Fig. 1C). VX2 cells
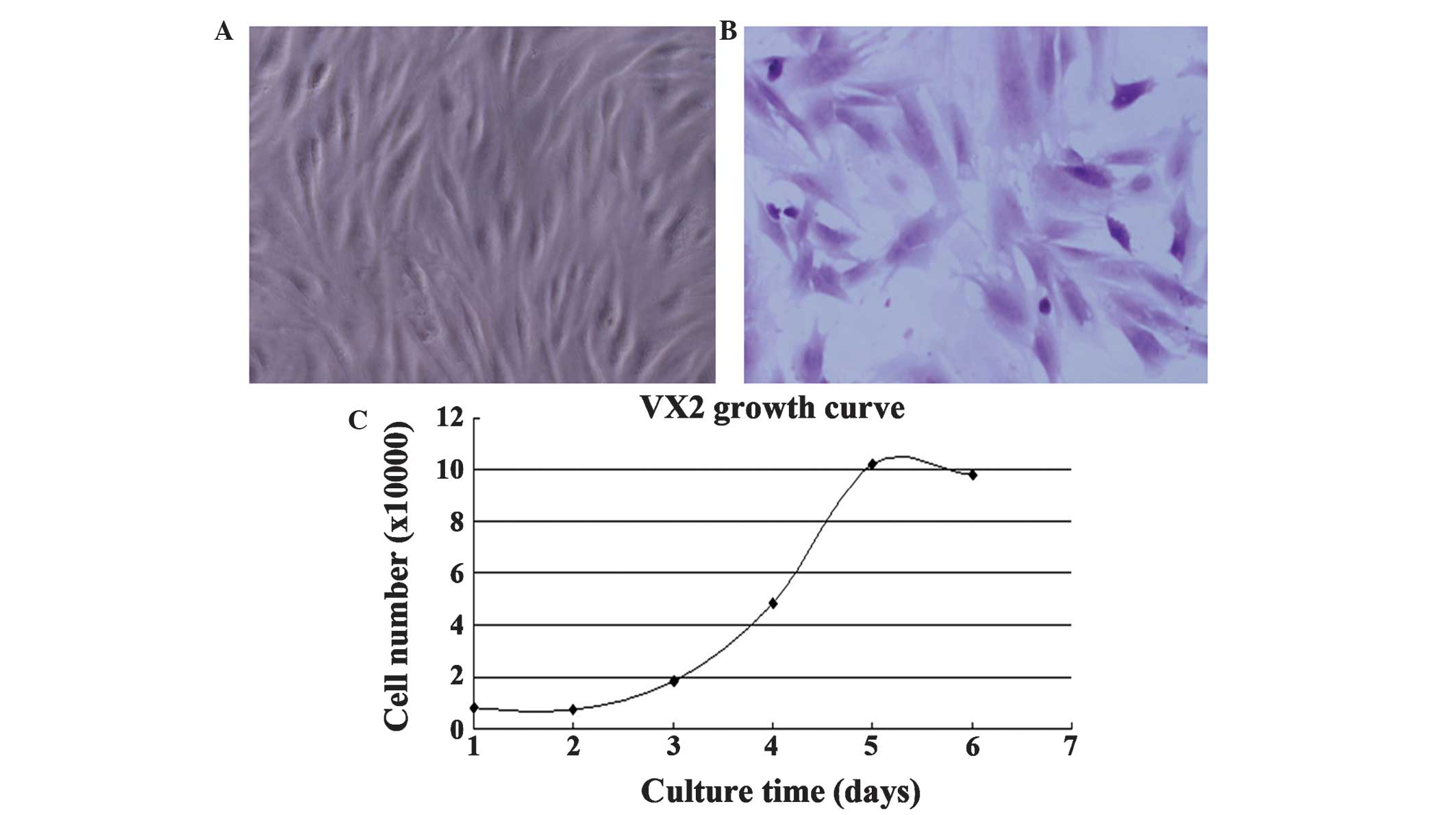
exhibited a spindle or polygon shape (Fig. 2A and B). Their doubling time at
passage 2 was ~22 h (Fig. 2C).
Tropism of MSCs to the tumor
microenvironment
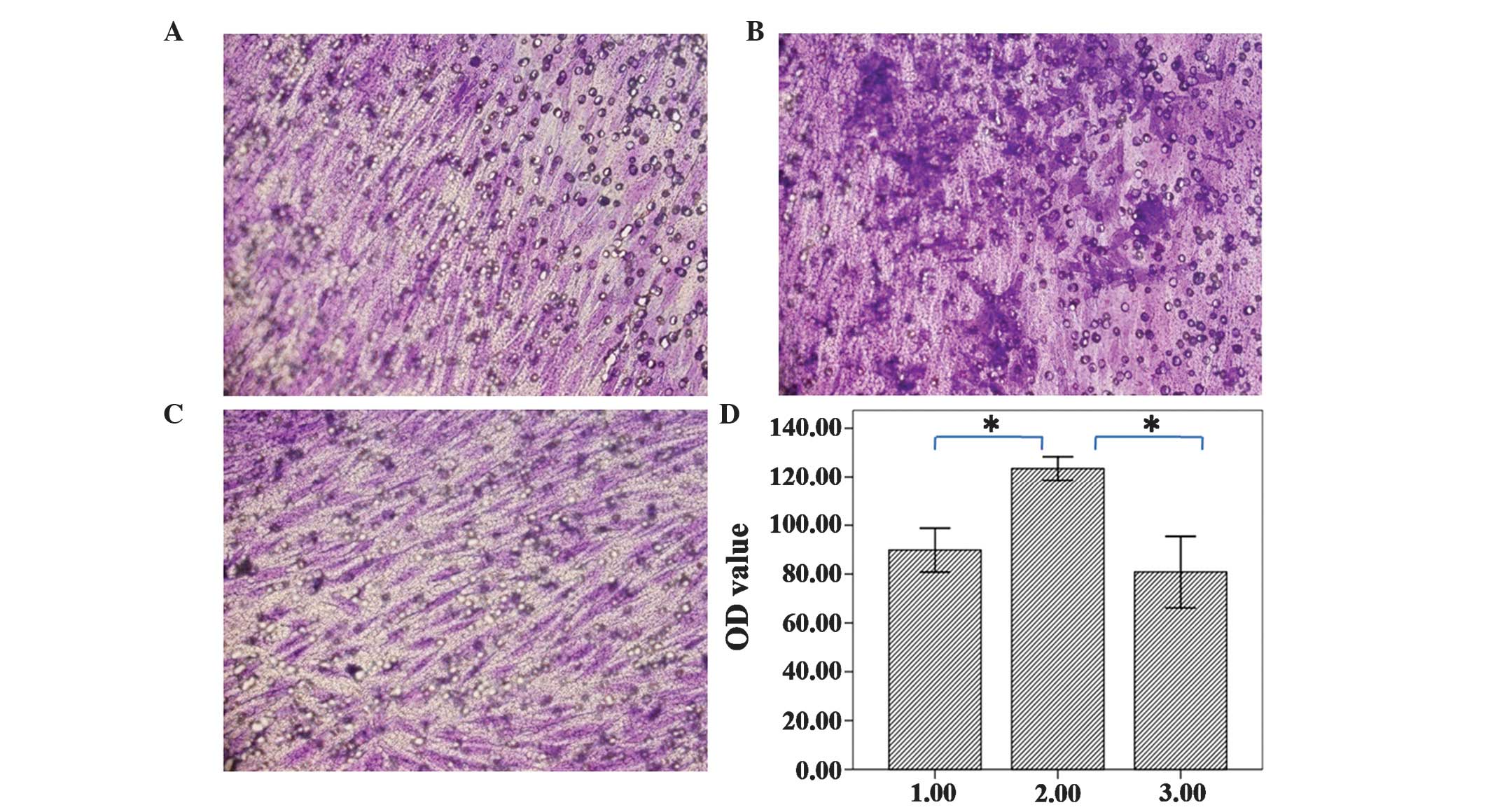
The majority of the MSCs migrated through the
Matrigel in all three groups, and the migrated cells demonstrated
an uneven distribution. The cells of Test 1 group had increased
migration compared with the other groups under microscope
(magnification, ×200; CX53; Olympus Corpoation), which was
additionally confirmed by the results of the colorimetric assay.
The results for the control group, Test 1 group and Test 2 groups
were 0.0898±0.0110, 0.1235±0.0059 and 0.0808±0.0179, respectively,
which indicated that Test 1 group cells had migrated significantly
more compared with the other groups (Fig.
3). These results suggested that 30% VX2 conditioned medium may
induce increased myofibroblast generation compared with 50% VX2
conditioned medium, which may mean that 30% VX2 conditioned medium
is the most suitable concentration for tropism of myofibroblast
cells.
MSCs differentiate into
myofibroblasts
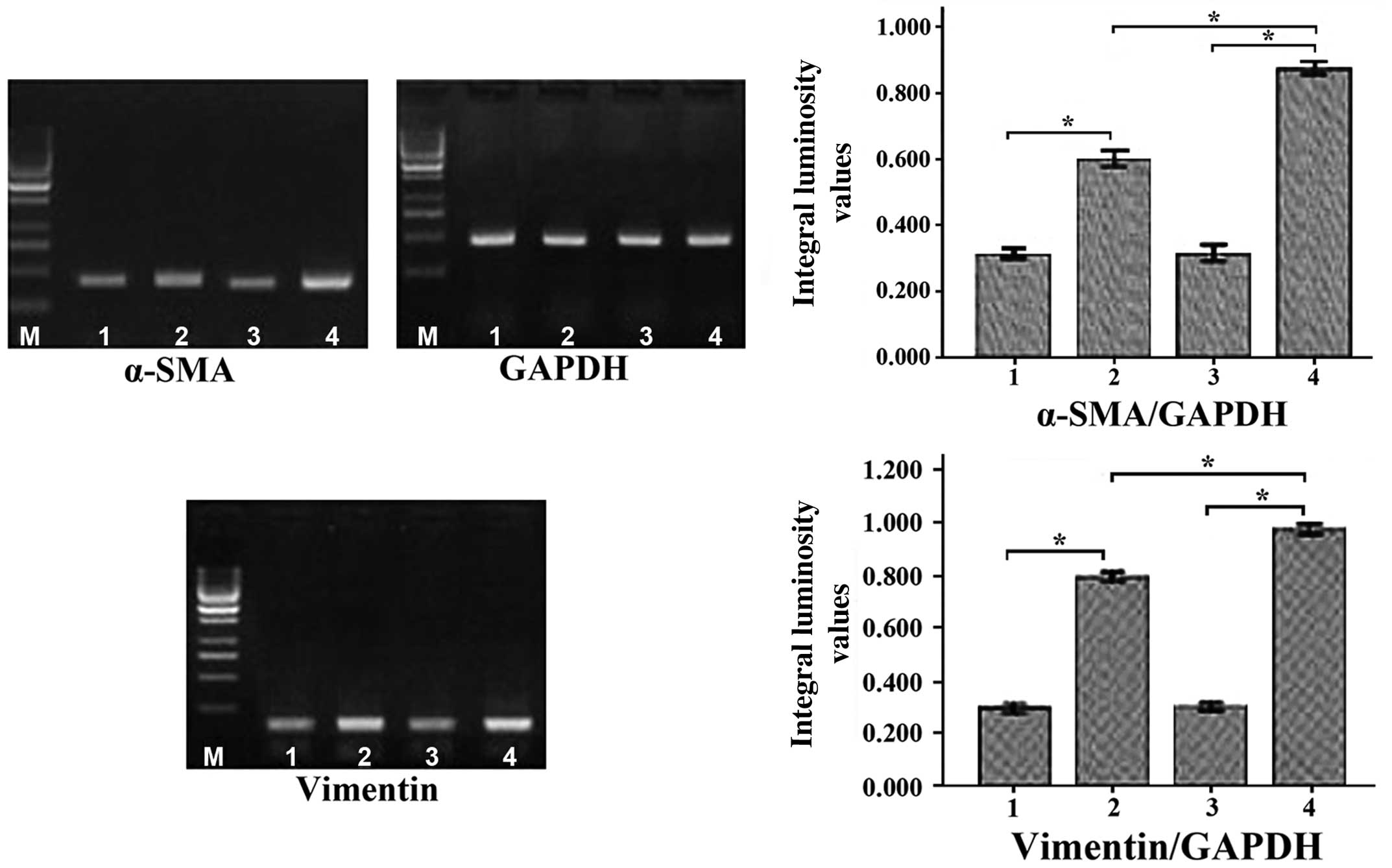
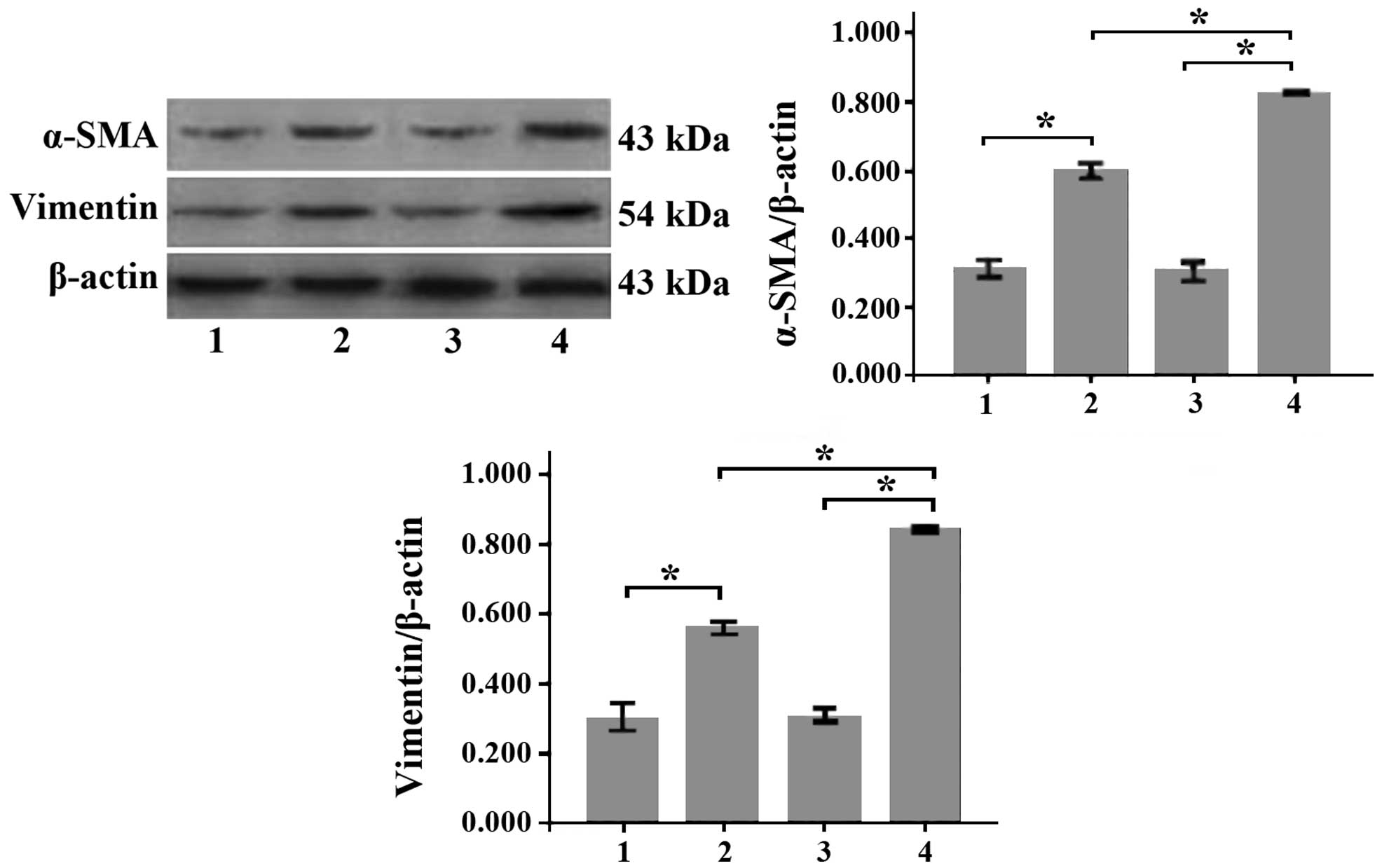
Following incubation of MSCs with conditioned medium
for 7 and 14 days, it was subsequently observed that the mRNA
levels of α-SMA and vimentin (myofibroblast markers) (11) had significantly increased in a
time-dependent manner (P<0.01; Fig.
4). The protein levels of α-SMA and vimentin were additionally
observed to have increased significantly in a time-dependent manner
(P<0.01; Fig. 5).
Discussion
Myofibroblasts were first identified in granulation
tissue by Gabbiani and Majno (20) in
1972, and have been subsequently observed in a wide range of normal
and abnormal tissues (21,22). Prior to the study by Gabbiani and
Majno, there had not been an exact definition of myofibroblasts, as
their appearance and function was not invariable within different
tissues. Currently, it is generally accepted that myofibroblasts
possess similar features to fibroblasts and smooth muscle cells, as
they express the fibroblast marker vimentin and the smooth muscle
marker α-SMA (23). Myofibroblasts
have roles in contraction, secretion and synthesis, and possess a
significant role in injury healing, organogenesis and tissue
molding (24,25).
Myofibroblasts are an important component of
tumors/carcinomas; these cells are known as
tumor/carcinoma-associated myofibroblasts and differ from the
myofibroblasts observed within normal tissues (26). Tumor/carcinoma-associated
myofibroblasts are perpetually activated, and do not undergo
apoptosis or elimination, which results in tumor/carcinoma growth
and development (27). It has been
reported that myofibroblasts promote tumor/carcinoma growth and
progression by secretion of growth factors, chemokines and MMPs
(28). In prostate cancer,
myofibroblasts promote cancer cell growth and invasion by
increasing the expression of chemokine (C-X-C motif) ligand
(CXCL)12, CXCL14, MMP2 and MMP3 (29). It has been reported that
myofibroblasts may promote cancer cell growth, angiogenesis and
invasion by increasing the expression of CXCL12, MMP9 and MMP14 in
breast cancer (30). Due to the
accumulating evidence of their cancer-promoting effects,
myofibroblasts may be promising novel therapeutic targets for the
treatment of cancer (31).
The source of tumor/carcinoma-associated
myofibroblasts remains to be elucidated. Certain previous studies
reported that tumor/carcinoma-associated myofibroblasts may be
derived from myofibroblasts within normal tissue, which are
activated by transforming growth factor (TGF)β1 and basic
fibroblast growth factor (32,33).
Another study reported that bone marrow was the primary source of
tumor/carcinoma-associated myofibroblasts (34), and an alternative study proposed that
epithelial to mesenchymal transition may be responsible for the
origin of myofibroblasts (35). The
present study demonstrated that myofibroblasts may be derived from
bone marrow MSCs. The following characteristics of MSCs suggest
that they may be the precursors of myofibroblasts: Multiple
differentiation potential and tropism (36). The results of the present study
revealed that MSCs were able to differentiate into myofibroblasts
in the presence of conditioned medium, which suggested that MSCs
may be the primary source of myofibroblasts. The mechanism
underlying this process remains to be elucidated. It was inferred
that chemokines/cytokines secreted by tumor/cancer cells had a
significant role. Tumor/cancer cells produce epidermal growth
factor, platelet-derived growth factor, vascular endothelial growth
factor and TGFβ1, and TGFβ1 in particular promotes the
transdifferention process (37). A
previous study observed that tumor/cancer-derived lysophosphatidic
acid was involved in the differentiation of MSCs to myofibroblasts
(38).
In the present study, the cells of Test 1 group had
increased migration compared with the control group, which
demonstrated the tropism of MSCs to the tumor microenvironment,
which was consistent with previous studies (39,40).
Furthermore, it is notable that migrated cells were significantly
increased in the 30% VX2 conditioned medium group compared with the
50% VX2 conditioned medium group, however, these results contradict
the results of previous studies (39,40). There
may be various reasons for this result. Culturing MSCs is
challenging and minor alterations in the composition of media may
cause cell death. The composition of VX2 conditioned medium was
complex and included a wide range of chemokines/cytokines and
metabolic products. The results of the migration assay suggested
that 30% VX2 conditioned medium may be more appropriate, rather
than 50% VX2 conditioned medium. There were a number of limitations
of the present study. A component analysis of the VX2 conditioned
medium was not performed to additionally investigate the mechanism
of MSC differentiation into myofibroblasts. These underlying
mechanisms are of great interest for future studies.
In conclusion, MSC differentiation into
myofibroblasts observed in the tumor/cancer stroma may be mediated
by a range of chemokines/cytokines produced by the tumor/cancer,
and this may be the primary source of tumor/cancer-associated
myofibroblasts.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant nos. 30672104 and 30900549) and
the Scientists Fund of Shandong Province (grant no.
2007BS03060).
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Wang XC, Katso R, Butler R, Hanby AM,
Poulsom R, Jones T, Sheer D and Ganesan TS: H-RYK, an unusual
receptor kinase: Isolation and analysis of expression in ovarian
cancer. Mol Med. 2:189–203. 1996.PubMed/NCBI
|
|
2
|
Polanska UM and Orimo A:
Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting
mesenchymal cells. J Cell Physiol. 228:1651–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fonseca FP, Coletta RD, Azevedo MB, Prado
RAC, Pires SAM, Miyahara GI, Carlos R, Farthing P, Hunter KD,
Speight PM, et al: Stromal myofibroblasts in squamous cell
carcinoma of the tongue in young patients - a multicenter
collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol.
118:483–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jezierska-Drutel A, Rosenzweig SA and
Neumann CA: Role of oxidative stress and the microenvironment in
breast cancer development and progression. Adv Cancer Res.
119:107–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bianchetti L, Barczyk M, Cardoso J,
Schmidt M, Bellini A and Mattoli S: Extracellular matrix
remodelling properties of human fibrocytes. J Cell Mol Med.
16:483–495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Göritz C, Dias DO, Tomilin N, Barbacid M,
Shupliakov O and Frisén J: A pericyte origin of spinal cord scar
tissue. Science. 333:238–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coen M, Gabbiani G and Bochaton-Piallat
ML: Myofibroblast-mediated adventitial remodeling: An
underestimated player in arterial pathology. Arterioscler Thromb
Vasc Biol. 31:2391–2396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Direkze NC, Hodivala-Dilke K, Jeffery R,
Hunt T, Poulsom R, Oukrif D, Alison MR and Wright NA: Bone marrow
contribution to tumor-associated myofibroblasts and fibroblasts.
Cancer Res. 64:8492–8495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mishra PJ, Mishra PJ, Glod JW and Banerjee
D: Mesenchymal stem cells: Flip side of the coin. Cancer Res.
69:1255–1258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tschumperlin DJ, Liu F and Tager AM:
Biomechanical regulation of mesenchymal cell function. Curr Opin
Rheumatol. 25:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao HF, Chen J, Xu ZS and Zhang KQ:
Distribution and differentiation of mesenchymal stem cells in tumor
tissue. Chin Med J (Engl). 122:712–715. 2009.PubMed/NCBI
|
|
16
|
Zhang K, Shi B, Chen J, Zhang D, Zhu Y,
Zhou C, Zhao H, Jiang X and Xu Z: Bone marrow mesenchymal stem
cells induce angiogenesis and promote bladder cancer growth in a
rabbit model. Urol Int. 84:94–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zoja C, Garcia PB, Rota C, Conti S,
Gagliardini E, Corna D, Zanchi C, Bigini P, Benigni A, Remuzzi G
and Morigi M: Mesenchymal stem cell therapy promotes renal repair
by limiting glomerular podocyte and progenitor cell dysfunction in
adriamycin-induced nephropathy. Am J Physiol Renal Physiol.
303:F1370–F1381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brennen WN, Denmeade SR and Isaacs JT:
Mesenchymal stem cells as a vector for the inflammatory prostate
microenvironment. Endocr Relat Cancer. 20:R269–R290. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rous P, Kidd JG and Smith WE: Experiments
on the cause of the rabbit carcinomas derived from virus-induced
papillomas. II. Loss by the Vx2 carcinoma of the power to immunize
hosts against the papilloma virus. J Exp Med. 96:159–174. 1952.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabbiani G and Majno G: Dupuytren's
contracture: Fibroblast contraction? An ultrastructural study. Am J
Pathol. 66:131–146. 1972.PubMed/NCBI
|
|
21
|
Angadi PV, Kale AD and Hallikerimath S:
Evaluation of myofibroblasts in oral submucous fibrosis:
correlation with disease severity. J Oral Pathol Med. 40:208–213.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta K, Metgud R and Gupta J: Evaluation
of stromal myofibroblasts in oral leukoplakia, oral submucous
fibrosis, and oral squamous cell carcinoma - an immunohistochemical
study. J Cancer Res Ther. 11:893–898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmitt-Gräff A, Desmoulière A and
Gabbiani G: Heterogeneity of myofibroblast phenotypic features: An
example of fibroblastic cell plasticity. Virchows Arch. 425:3–24.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saw VP, Schmidt E, Offiah I, Galatowicz G,
Zillikens D, Dart JK, Calder VL and Daniels JT: Profibrotic
phenotype of conjunctival fibroblasts from mucous membrane
pemphigoid. Am J Pathol. 178:187–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayrand D, Laforce-Lavoie A, Larochelle S,
Langlois A, Genest H, Roy M and Moulin VJ: Angiogenic properties of
myofibroblasts isolated from normal human skin wounds.
Angiogenesis. 15:199–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lúcio PS, Cavalcanti AL, Alves PM, Godoy
GP and Nonaka CF: Myofibroblasts and their relationship with oral
squamous cell carcinoma. Braz J Otorhinolaryngol. 79:112–118.
2013.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mertens JC, Fingas CD, Christensen JD,
Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts
LR, Sirica AE and Gores GJ: Therapeutic effects of deleting
cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res.
73:897–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Fan X and Houghton J: Tumor
microenvironment: The role of the tumor stroma in cancer. J Cell
Biochem. 101:805–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Ying G, Wang J, Jung Y, Lu J, Zhu
J, Pienta KJ and Taichman RS: Characterization of phosphoglycerate
kinase-1 expression of stromal cells derived from tumor
microenvironment in prostate cancer progression. Cancer Res.
70:471–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu M, Peluffo G, Chen H, Gelman R, Schnitt
S and Polyak K: Role of COX-2 in epithelial-stromal cell
interactions and progression of ductal carcinoma in situ of
the breast. Proc Natl Acad Sci USA. 106:3372–3377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth - bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC,
Dang TD and Rowley DR: Reactive stroma in human prostate cancer:
Induction of myofibroblast phenotype and extracellular matrix
remodeling. Clin Cancer Res. 8:2912–2923. 2002.PubMed/NCBI
|
|
33
|
Lewis MP, Lygoe KA, Nystrom ML, Anderson
WP, Speight PM, Marshall JF and Thomas GJ: Tumor-derived TGF-β1
modulates myofibroblast differentiation and promotes
HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer.
90:822–832. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe
T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J, et al:
Bone-marrow-derived myofibroblasts contribute to the cancer-induced
stromal reaction. Biochem Biophys Res Commun. 309:232–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mishra PJ, Mishra PJ, Humeniuk R, Medina
DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW and Banerjee D:
Carcinoma-associated fibroblast-like differentiation of human
mesenchymal stem cells. Cancer Res. 68:4331–4339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oktem G, Sercan O, Guven U, Uslu R, Uysal
A, Goksel G, Ayla S and Bilir A: Cancer stem cell differentiation:
TGFβ1 and versican may trigger molecules for the organization of
tumor spheroids. Oncol Rep. 32:641–649. 2014.PubMed/NCBI
|
|
38
|
Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM,
Cho M, Suh DS, Yoon MS, Chang CL, Jung JS and Kim JH:
Cancer-derived lysophosphatidic acid stimulates differentiation of
human mesenchymal stem cells to myofibroblast-like cells. Stem
Cells. 26:789–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ho IA, Yulyana Y, Sia KC, Newman JP, Guo
CM, Hui KM and Lam PY: Matrix metalloproteinase-1-mediated
mesenchymal stem cell tumor tropism is dependent on crosstalk with
stromal derived growth factor 1/C-X-C chemokine receptor 4 axis.
FASEB J. 28:4359–4368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berger L, Shamai Y, Skorecki KL and
Tzukerman M: Tumor specific recruitment and reprogramming of
mesenchymal stem cells in tumorigenesis. Stem Cells. 34:1011–1026.
2016. View Article : Google Scholar : PubMed/NCBI
|