Introduction
O-linked N-acetylglucosamine (O-GlcNAc)
glycosylation (O-GlcNAcylation) is the modification of serine and
threonine groups on nuclear and cytoplasmic proteins by a single
residue of O-GlcNAc. Two enzymes are responsible for cyclic
O-GlcNAcylation: O-GlcNAc transferase (OGT), which catalyzes the
addition of the GlcNAc moiety to target proteins; and O-GlcNAc
hydrolase (OGA), which removes the sugar moiety from proteins.
Analogous to phosphorylation, O-GlcNAcylation changes rapidly and
dynamically in response to changes in the cellular environment
triggered by extracellular stimuli, such as stress (1), nutrients and cell cycle progression.
Furthermore, O-GlcNAcylation is emerging as a key regulator of
cellular biological processes, such as transcription, signal
transduction, cell motility, morphogens, cell metabolism and
development (2–4).
Accumulating studies indicate that perturbations in
cellular O-GlcNAcylation levels are involved in a variety of human
diseases, such as diabetes and neurological disorders (5–8). In recent
years, it has been also determined that O-GlcNAcylation has key
roles in the progression and development of certain types of
cancer. Caldwell et al observed that O-GlcNAc levels and OGT
protein expression increased in the highly metastatic breast cancer
cell lines, which suggested that increased O-GlcNAcylation may be
closely associated with breast tumor progression (9). Gu et al (10) and Mi et al (11) demonstrated that the global O-GlcNAc
level was markedly elevated in breast, lung and colon tumor tissues
compared with matched adjacent tissues; similarly, OGT protein
expression correspondingly changed. In addition, clearly enhanced
O-GlcNAcylation was observed in the metastatic lymph nodes of
breast cancer tissue compared with the corresponding primary tumor
tissues (10,11). A growing number of O-GlcNAc-modified
proteins with important roles in cell growth, proliferation,
motility, differentiation and apoptosis have been identified.
Furthermore, numerous oncogene and tumor suppressor gene products,
such as c-Fos, c-Jun, c-Myc, retinoblastoma protein and p53, have
been revealed to be modified by O-GlcNAc (12–14). These
results implicate O-GlcNAcylation as a crucial regulator that may
have an effect on tumorigenesis, migration and metastasis.
At present, thyroid cancer represents ~2.5% of new
cancer diagnoses in the United States and the incidence of
malignant thyroid tumor is increasing. Thyroid malignancies are
categorized into papillary carcinoma, follicular carcinoma,
medullary thyroid carcinoma, anaplastic thyroid carcinoma (ATC),
primary thyroid lymphoma (rare) and primary thyroid sarcoma (rare).
ATC has the highest mortality rate of all the thyroid tumors,
exhibiting a high mitotic rate, and lymphovascular and vascular
invasion (15). It rapidly invades
surrounding tissues, such as the trachea, or even transfers to the
lymph nodes, lungs, liver, chest and femur. Treatment of ATC is
typically palliative in its intent, as the disease is rarely
curable and almost always fatal, with worse prognoses associated
with large tumors, distant metastases, acute obstructive symptoms
and leukocytosis. Therefore, ATC management demands rapid, complex
and integrated multidisciplinary decision-making, and the
underlying malignancy-associated mechanism require further
investigation.
Although it has been proposed that aberrant O-GlcNAc
metabolism is associated with the malignant properties of a variety
of thyroid cancer cell types, and previous studies have indicated
that O-GlcNAcylation participates in the functional regulation of
ATC cells by modulating Akt1 activity (16,17), the
exact roles of O-GlcNAc in thyroid cancer pathogenesis and
progression remain to be established.
In the present study, the global O-GlcNAcylation
level was altered through OGT overexpression, OGT silencing or OGA
inhibition in ATC cells, and the effects of O-GlcNAcylation on the
malignant properties of thyroid tumors were determined.
Materials and methods
Cell cultures and treatments
8305C ATC cells (European Collection of
Authenticated Cell Cultures, Salisbury, UK) were grown in Advanced
Minimum Essential medium (MEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) medium supplemented with 2 mM glutamine
(Gibco; Thermo Fisher Scientific, Inc.) and 5% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere containing 5% CO2 at 37°C. THJ-11T human ATC
cells (Cell Resource Center, Institute of Basic Medical Sciences,
China Academy of Medical Sciences, Beijing, China) were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. Both cell lines were treated with 5 µM
Thiamet-G (Sigma-Aldrich, St. Louis, MO, USA) for 48 h or the
indicated time period.
A vector expressing Flag-tagged human
nuclear/cytoplasmic OGT (pCMV-Flag-OGT) was provided by Dr. Jin Won
Cho (Department of Biology, Yonsei University, Seoul, South Korea)
(18). Transfections were conducted
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions.
The shRNA-expressing lentiviral vector pLKO.1-puro,
a pCMV Δ8.2 packaging plasmid construct (encoding gag, pol, rev)
and pCMV-VSV-G envelope plasmid were provided by Dr. William C.
Hahn (Harvard Medical School and Dana-Farber Cancer Institute,
Boston, MA, USA). Small interfering RNA duplexes targeting the
coding region of human OGT mRNA sequences were used to suppress OGT
enzyme levels in the 8305C and THJ-11T cells. The human
shOGT-targeting sequence was GGATGCTTATATCAATTTAGG. A control shRNA
oligonucleotide sequence that did not match with any known human
coding cDNA was used as the control: sense,
CCGGTACGTGACACGTTCGGAGAATTCTCGAGAATTCTCCGAACGTGTCACGTTTTTTG and
antisense,
AATTCAAAAAACGTGACACGTTCGGAGAATTCTCGAGAATTCTCCGAACGTGTCACGTA. shRNA
oligos were cloned into the PLKO.1 vector by the AgeI and EcoRI
sites. The recombinant plasmid PLKO.1-shRNA was cotransfected with
Δ8.2 and VSV-G plasmids into HEK293 cells (Cell Resource Center,
Institute of Basic Medical Sciences, China Academy of Medical
Sciences), which were used as the virus packaging cells based on
their high replication rate and transfection efficiency for
efficient virus production. Following 48 h of transfection, the
cell medium was collected and centrifuged at 6,000 × g for 3 min at
4 °C. The virus supernatant was obtained and used to infect ATC
cells. The stable infected cells were selected with 8 µg/ml
puromycin (Sigma-Aldrich) for 2 weeks.
Western blotting
Cells were lysed in lysis buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% NP40, 1 mM EDTA, 1 mM
Na3VO4, 10 mM NaF; Sigma-Aldrich] containing
a protease inhibitor cocktail (Roche Molecular Diagnostics,
Branchburg, NJ, USA) and 5 µM PUGNAc (an OGA inhibitor; Toronto
Research Chemicals Inc., Toronto, Canada). Proteins were isolated
from the total cell lysate by centrifugation at 12,000 × g for 10
min at 4°C, followed by collection of the supernatant. Protein
samples (50 µg) were separated by 12% SDS-PAGE (Sigma-Aldrich),
transferred to Immobilon-P membranes (EMD Millipore, Billerica, MA,
USA), and incubated with antibodies against O-GlcNAc (RL2; mouse
monoclonal; dilution, 1:1,000; catalog no., MA1-072; Thermo Fisher
Scientific, Inc.), OGT (F-12; mouse monoclonal; dilution, 1:500;
catalog no., sc-74546; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and GAPDH (D-6; mouse monoclonal; dilution, 1:5,000; catalog
no., sc-166545; Santa Cruz Biotechnology, Inc.). Following
incubation with primary antibodies overnight at 4°C, the membranes
were washed 3 times with phosphate-buffered saline and incubated
with horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibody (dilution, 1:2,000; catalog
no., sc-2005; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Protein expression was detected using Amersham ECL Prime Western
Blotting Detection Reagent (GE Healthcare Life Sciences, Chalfont,
UK).
Cell proliferation assay
Cell viability and proliferation were assessed by
MTT dye conversion. Briefly, cells were seeded 10,000 cells per
well in a 96-well flat bottom plate. Cells were allowed to grow for
48 h, then 20 µl MTT (5 mg/ml in phosphate-buffered saline) was
then added to each well. After 4 h incubation at 37°C, cells were
lysed by the addition of 200 µl dimethylsulfoxide. Absorbance was
measured at 570 nm using a Tecan Spectra Rainbow Microplate Reader
(Tecan Austria GmbH, Grödig, Austria).
Colony formation ability assay
A soft agar colony formation assay was performed as
described (2). Briefly, cells
(5×103) were suspended in 1 ml top agar medium (cell
culture medium supplied with 0.4% agar). The cell suspension was
then overlaid onto 1.5 ml bottom agar medium (cell culture medium
supplied with 0.8% agar) in six-well tissue culture plates in
triplicate. Fresh medium was added to plates every 3 days as a
feeder layer. On days 12–18, the number of colonies was counted in
six random fields at ×40 magnifications (BX50; Olympus Corp.,
Tokyo, Japan).
Cell migration assay
A cell migration assay was performed as previously
described (19). Cell migration was
assayed using Transwell chambers (6.5 mm; Corning Incorporated,
Corning, NY, USA) with 8 µm pore membranes. The lower chamber was
filled with 600 µl NIH-3T3 conditioned medium (Cell Resource
Center, Institute of Basic Medical Sciences, China Academy of
Medical Sciences) with or without OGA inhibitors (5 µM Thiamet-G).
Cells (5×104) were suspended in 100 µl medium (MEM or
RPMI-1640 with 1% fetal calf serum) and plated into the upper
chamber with or without OGA inhibitors. After 20 h, the number of
cells appearing by crystal violet staining (for 20 min; Shanghai
Haoran Biological Technology Co., Ltd., Shanghai, China) on the
undersurface of the polycarbonate membranes was scored visually in
five random fields at ×100 magnification using a light microscope
(BX50; Olympus Corp.).
Cell invasion assay
For cell invasion assays, the upper face of the
membrane was covered with 70 µl Matrigel (1 mg/ml; BD Biosciences,
Franklin Lakes, NJ, USA). The invasion assay was performed using
the same procedure as the migration assay, except that the
incubation time of the experiment was prolonged to 24 h.
Statistical analysis
All experiments were repeated at least 3 times. Data
were analyzed by Student's t-test using SPSS software (version
16.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. Data are presented
as the mean ± standard error of the mean.
Results
Regulation of O-GlcNAcylation
expression in the ATC cell lines
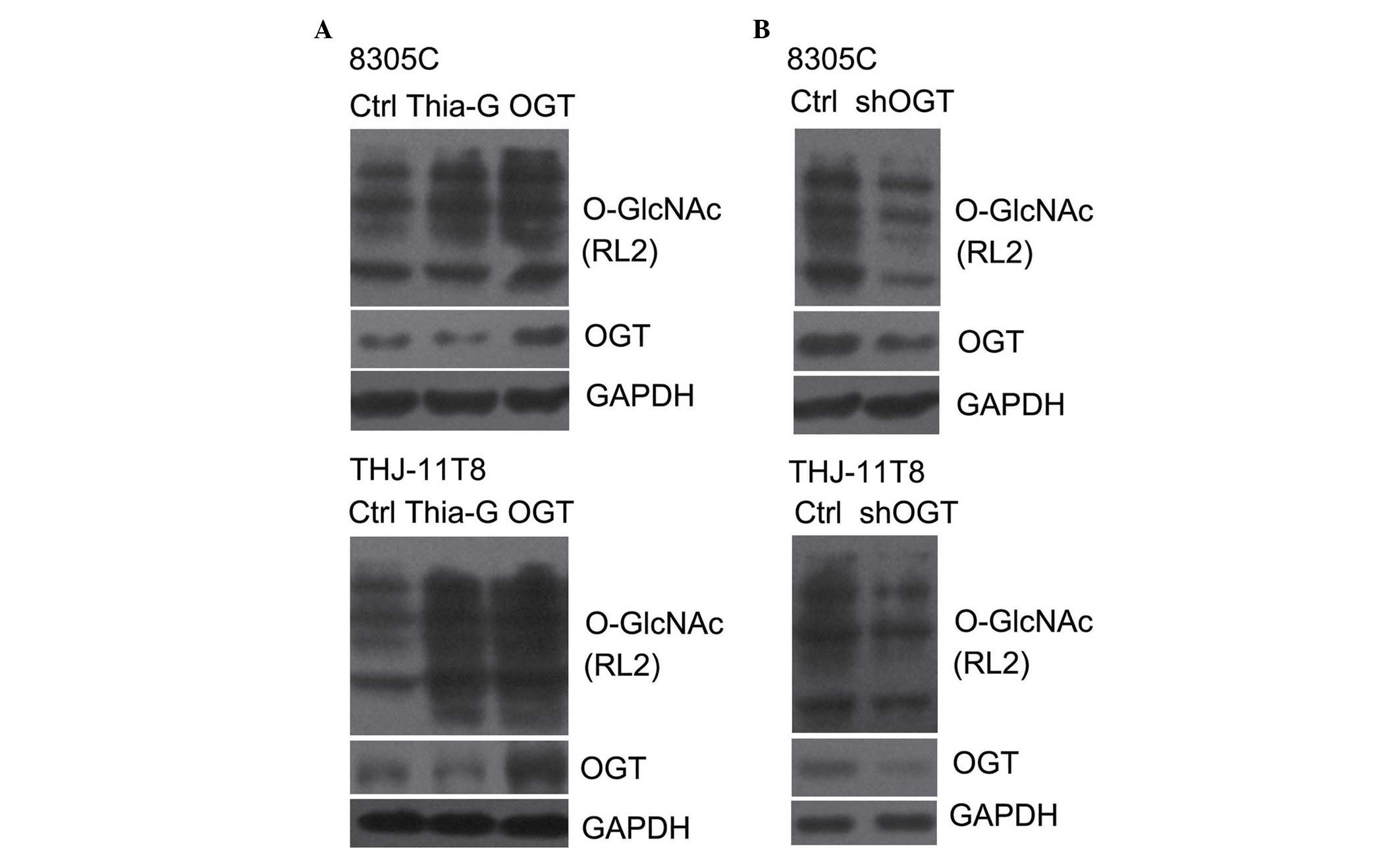
To investigate whether O-GlcNAcylation is important
in thyroid cancer malignancy, the level of O-GlcNAcylation was
elevated by the overexpression of OGT or the inhibition of OGA, and
decreased by the silencing of OGT in the 8305C and THJ-11T ATC cell
lines. OGT overexpression (pCMV-Flag-OGT) and Thiamet-G, a highly
potent and selective OGA inhibitor, effectively increased the
O-GlcNAcylation in 8305C and THJ-11T cells (Fig. 1A) (20).
By contrast, shOGT markedly reduced OGT protein expression levels,
as well as O-GlcNAcylation levels, in 8305C and THJ-11T cells
(Fig. 1B).
O-GlcNAcylation increases ATC cell
viability
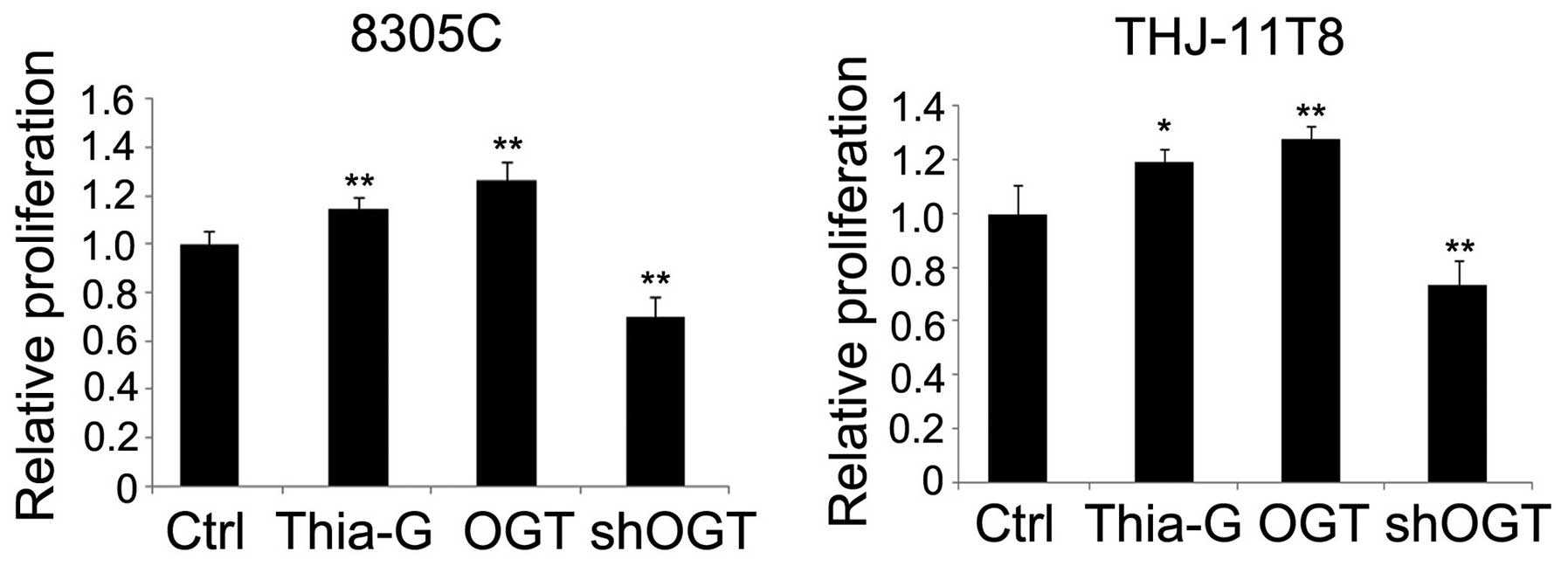
To determine whether O-GlcNAcylation affects cell
viability, 8305C and THJ-11T ATC cells were treated to produce OGT
overexpression (pCMV-Flag-OGT), OGA inhibition (5 µM Thiamet-G) or
OGT silencing (shOGT) cell groups. The cells were grown in medium
with serum for 48 h and an MTT assay for cell proliferation was
performed. Proliferation of 8305C and THJ-11T cells increased by
~27 and ~15% (P<0.01) following OGT overexpression, and ~28
(P<0.01) and ~19% (P<0.05) following OGA inhibition,
respectively, relative to the control. Conversely, the
proliferation of OGT-silenced 8305C and THJ-11T cells was decreased
by ~30 and ~27%, respectively, compared with the control
(P<0.01; Fig. 2).
O-GlcNAcylation enhances ATC cell
colony formation ability
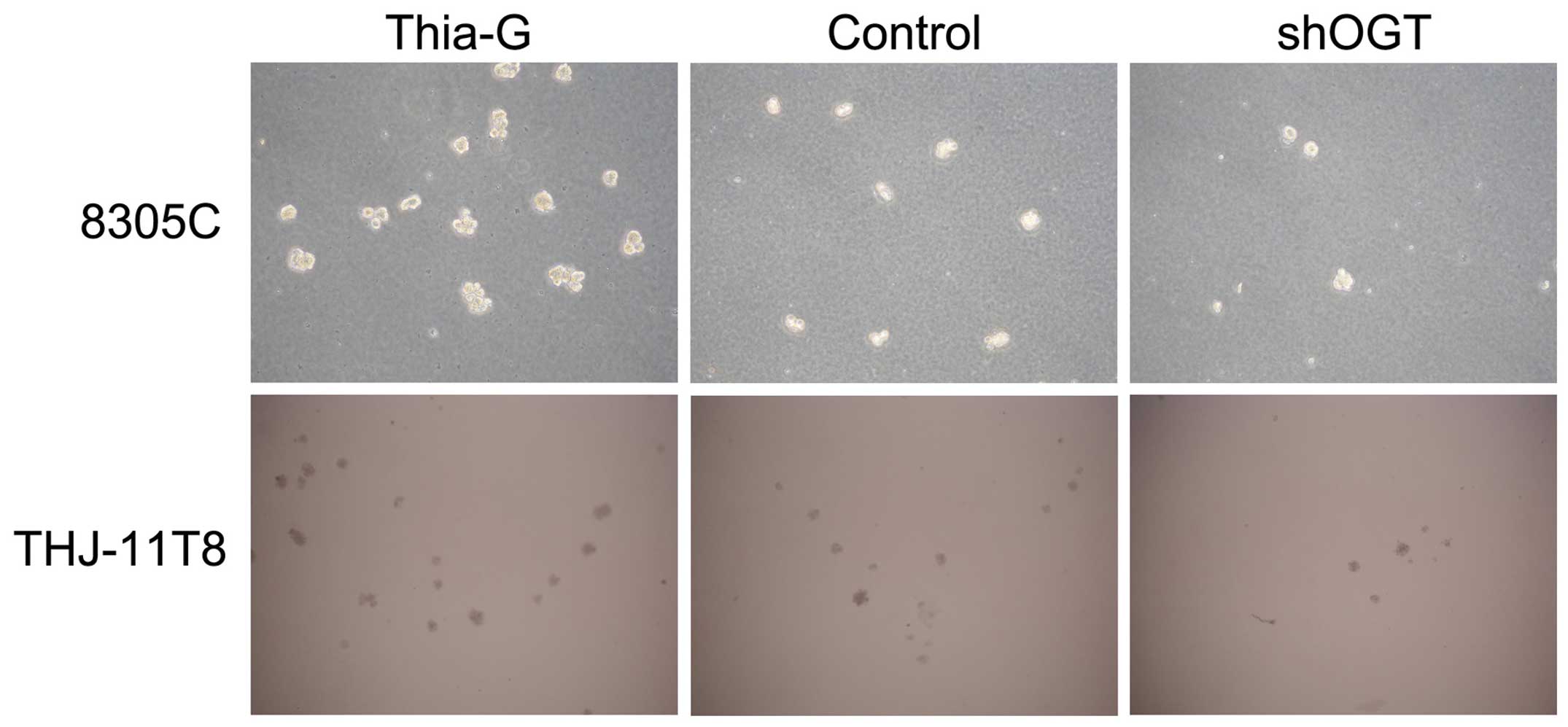
To investigate whether O-GlcNAcylation affects the
colony formation ability (anchorage-independent growth) of ATC
cells, soft agar colony formations assays were conducted. The
assays revealed that OGA inhibition (Thiamet-G) markedly increased
the colony formation ability of 8305C and THJ-11T cells compared
with the control. By contrast, OGT silencing (shOGT) markedly
reduced the colony formation ability compared with the control
(Fig. 3). These results suggest that
O-GlcNAcylation is important for maintaining the
anchorage-independent growth of ATC cells.
O-GlcNAcylation promotes ATC cells
motility
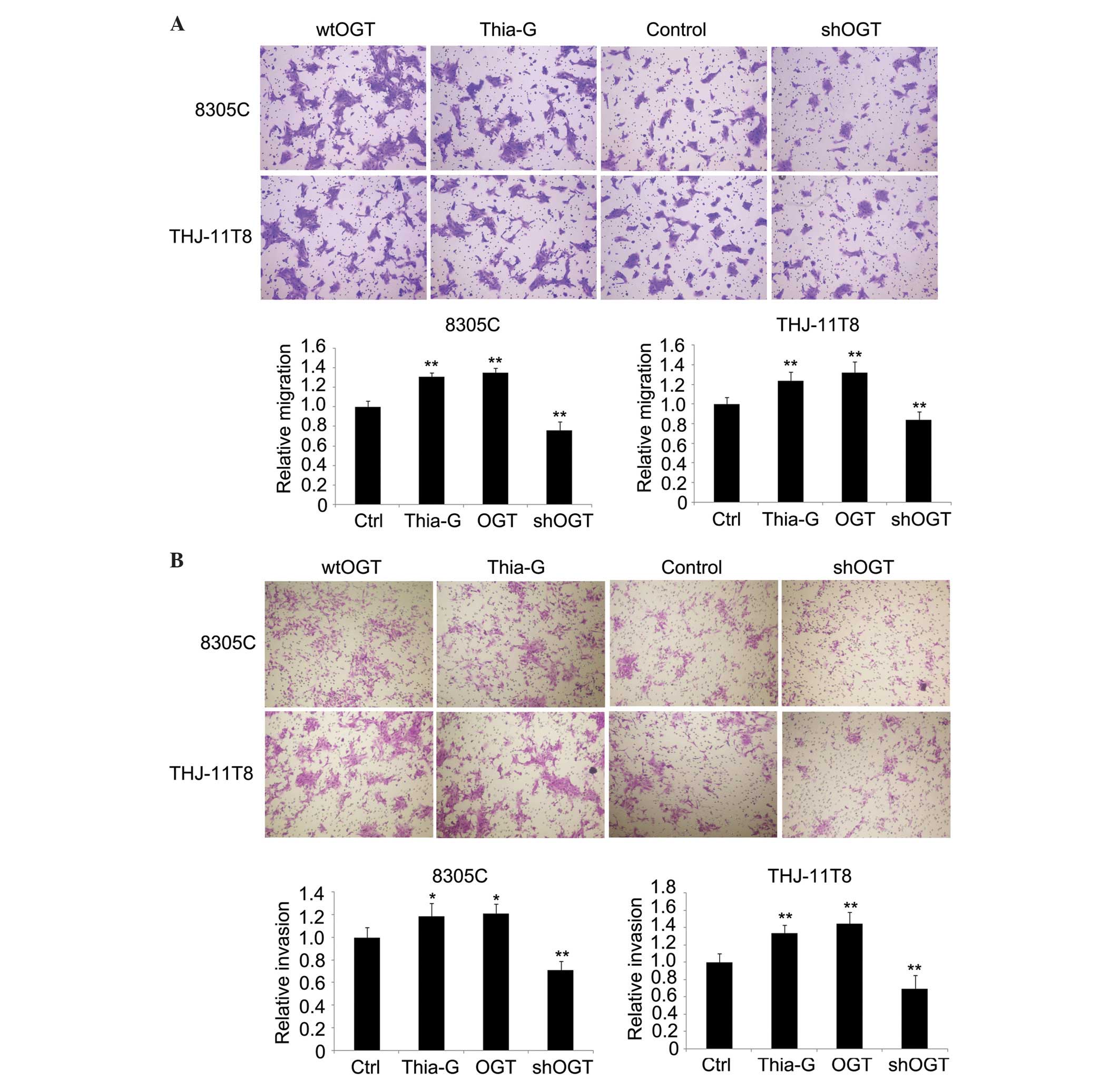
The role of O-GlcNAcylation in thyroid cancer cell
metastasis was detected by migration and invasion assays in
vitro. The results indicated that OGT overexpression and OGA
inhibition significantly enhanced cell migration and invasion in
8305C and THJ-11T cells, whereas OGT silencing significantly
inhibited cell migration and invasion compared with the control
(P<0.01; Fig. 4A and B).
Discussion
The present study analyzed the effect of
O-GlcNAcylation in ATC progression and development. The results
demonstrated that O-GlcNAcylation enhanced the malignancy of ATC by
promoting cellular viability, anchorage-independent growth,
migration and invasion, all of which are considered to be among the
fundamental properties of malignant cells. These results suggest
that the increase of O-GlcNAcylation may initiate and promote ATC
formation and metastasis to other lesions.
To change the O-GlcNAc level, three methodological
approaches were used. On cells group was transfected with
pCMV-Flag-OGT, which efficiently increased OGT protein expression
levels, as well as O-GlcNAcylation. The next cells group were
treated with Thiamet-G, a potent and selective OGA inhibitor.
Prolonged cellular treatment with Thiamet-G is known to elevate
O-GlcNAc modification of numerous proteins and has been a useful
tool for the investigation of cellular responses affected by
O-GlcNAc modification. However, Thiamet-G treatment increased
O-GlcNAcylation and reduced OGT protein expression, possibly due to
the feedback inhibition induced by elevated O-GlcNAcylation. Taking
into account the effect of O-GlcNAcylation, the RNA interference
method was used to reduce expression of OGT in a third group of
cells. All of the methods were similarly effective in altering the
O-GlcNAc level in cellular proteins.
At present, the function of O-GlcNAcylation is
undergoing thorough investigation. O-GlcNAcylation has important
roles in certain human diseases, such as diabetes, neurologic
disorders and cardiovascular disease (2). However, there is no unified view as to
the role of O-GlcNAc in tumor pathogenesis and progression. Studies
by Caldwell et al (9), Gu
et al (10) and Mi et
al (11) indicated that more
O-GlcNAcylation may be closely associated with breast, lung and
colon tumor progression. O-GlcNAc levels and OGT protein expression
increased in highly metastatic breast cancer cell lines, and lung
and colon tumor tissues in comparison with matched adjacent
tissues. The lysosomal β-N-acetylglucosaminidase is a type of
O-GlcNAcase. The enhancement of its degradative activity has been
observed to be associated with O-GlcNAcylation level in a variety
of human cancer types (21–30). Additionally, a previous study proposed
OGA activity to be significantly higher and O-GlcNAcylation to be
lower in tumor tissue compared with corresponding normal tissue
(31). Similarly, a different study
demonstrated increased activity of OGA activity and decreased
O-GlcNAc levels in thyroid cancer compared with benign lesions
(16). By contrast, the present study
demonstrated that an increase in O-GlcNAcylation was advantageous
for the malignancy of ATC cells; however, the two different
findings were not mutually contradictory. The former study claimed
that O-GlcNAcylation was decreased in tumor tissues, particularly
in proteins in the molecular mass range of 45–65 kDa. However, this
study also stated that the variety of modified proteins was greater
in different molecular mass ranges in the majority of tumor
tissues. Therefore, the roles and mechanisms of O-GlcNAcylation in
carcinoma development are complex, and may be context-dependent and
influenced by cell type or oncogenic events acquired during the
course of tumor evolution. O-GlcNAcylation of specific proteins may
be more important for the behavior of cancer cells than the global
O-GlcNAc level. Thus, further investigations of specific O-GlcNAc
modified proteins in tumor cells should be conducted.
Simultaneously, to verify the regulatory effect of O-GlcNAcylation
on the malignancy of ATC, in vivo studies should also be
performed.
O-GlcNAcylation is an inducible and dynamically
cycling post-translational modification that can regulate various
cellular biological processes (2,32,33); for example, cell cycle, protein
stability, apoptosis, growth and the cellular stress response
(9,32–34). As a
stress sensor, O-GlcNAc levels rapidly and dynamically increase in
multiple mammalian cell lines in response to different cellular
stresses (35); thus, cells remodel
their metabolic and signaling pathways to promote survival.
O-GlcNAcylation may protect cells from oxidative stress injury
through enhancing Forkhead box O4 transcriptional activity
(36). Raising O-GlcNAcylation may
also render cells more thermotolerant (37). In addition, lowering O-GlcNAcylation
by reducing OGT levels or blocking hexosamine biosynthetic
pathway-sensitized cells to apoptotic stimuli (1). The aberrant metabolism or growth of
primary tumor cells is challenged by multiple forms of stimuli and
stress, including reactive oxygen species, extracellular matrix
components, basement membranes, nutrient deprivation and hypoxia,
and attack by the immune system. To defend against
microenvironmental death stimuli, tumor cells require metastasis
and exposure to novel microenvironments (38). The present study indicates that
O-GlcNAcylation elevation may benefit cancer cells to resist
apoptotic stimuli, accelerating tumor formation and
progression.
In conclusion, the results of the present study
demonstrated that O-GlcNAcylation increased the malignancy of
anaplastic thyroid carcinoma cells by promoting cellular growth,
colony formation, and migration and invasion. Therefore,
understanding the association between abnormal O-GlcNAcylation and
cancerous behavior may be significant, and O-GlcNAcylation may be a
potential target for the diagnosis and therapy of cancer.
References
|
1
|
Zachara NE, O'Donnell N, Cheung WD, Mercer
JJ, Marth JD and Hart GW: Dynamic O-GlcNAc modification of
nucleocytoplasmic proteins in response to stress. A survival
response of mammalian cells. J Biol Chem. 279:30133–30142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart GW, Housley MP and Slawson C: Cycling
of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins.
Nature. 446:1017–1022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wells L, Vosseller K and Hart GW:
Glycosylation of nucleocytoplasmic proteins: Signal transduction
and O-GlcNAc. Science. 291:2376–2378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanover JA: Glycan-dependent signaling:
O-linked N-acetylglucosamine. FASEB J. 15:1865–1876. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dias WB and Hart GW: O-GlcNAc modification
in diabetes and Alzheimer's disease. Mol Biosyst. 3:766–772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lazarus BD, Love DC and Hanover JA:
O-GlcNAc cycling: Implications for neurodegenerative disorders. Int
J Biochem Cell Biol. 41:2134–2146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuzwa SA and Vocadlo DJ: O-GlcNAc and
neurodegeneration: Biochemical mechanisms and potential roles in
Alzheimer's disease and beyond. Chem Soc Rev. 43:6839–6858. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caldwell SA, Jackson SR, Shahriari KS,
Lynch TP, Sethi G, Walker S, Vosseller K and Reginato MJ: Nutrient
sensor O-GlcNAc transferase regulates breast cancer tumorigenesis
through targeting of the oncogenic transcription factor FoxM1.
Oncogene. 29:2831–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zachara NE and Hart GW: Cell signaling,
the essential role of O-GlcNAc! Biochim Biophys Acta. 1761:599–617.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chou TY, Hart GW and Dang CV: C-Myc is
glycosylated at threonine 58: A known phosphorylation site and a
mutational hot spot in lymphomas. J Biol Chem. 270:18961–18965.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamemura K, Hayes BK, Comer FI and Hart
GW: Dynamic interplay between O-glycosylation and O-phosphorylation
of nucleocytoplasmic proteins. Alternative
glycosylation/phosphorylation of THR-58, a known mutational hot
spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem.
277:19229–19235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu MI, Vassilopoulou-Sellin R, Lustig R
and Lamont JP: Thyroid and parathyroid cancers. Pazdur R, Wagman
LD, Camphausen KA and Hoskins WJ: Cancer Management: A
Multidisciplinary Approach (11th). UMB Medica. (Norwalk, CT). 1–4.
2008.
|
|
16
|
Krzeslak A, Pomorski L and Lipinska A:
Elevation of nucleocytoplasmic beta-N-acetylglucosaminidase
(O-GlcNAcase) activity in thyroid cancers. Int J Mol Med.
25:643–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krześlak A, Jóźwiak P and Lipińska A:
Down-regulation of β-N-acetyl-D-glucosaminidase increases Akt1
activity in thyroid anaplastic cancer cells. Oncol Rep. 26:743–749.
2011.PubMed/NCBI
|
|
18
|
Yang WH, Park SY, Nam HW, Kim Do H, Kang
JG, Kang ES, Kim YS, Lee HC, Kim KS and Cho JW: NF{kappa}B
activation is associated with its O-GlcNAcylation state under
hyperglycemic conditions. Proc Natl Acad Sci USA. 105:17345–17350.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Zhang J, Mi W, Yang J, Han F, Lu X
and Yu W: Silencing of GM3 synthase suppresses lung metastasis of
murine breast cancer cells. Breast Cancer Res. 10:R12008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuzwa SA, Shan X, Macauley MS, Clark T,
Skorobogatko Y, Vosseller K and Vocadlo DJ: Increasing O-GlcNAc
slows neurodegeneration and stabilizes tau against aggregation. Nat
Chem Biol. 8:393–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okochi T, Seike H, Higashino K, Hada T,
Watanabe S, Yamamura Y, Ito F, Matsuda M, Osafune M, Kotake T and
Sonoda T: Alteration of hexosaminidase isoenzymes in human renal
carcinoma. Cancer Res. 39:1829–1834. 1979.PubMed/NCBI
|
|
22
|
Narita M, Taniguchi N, Makita A, et al:
Elevated activity of beta-hexosaminidase and sulfhydryl
modification in the B-variant of human lung cancer. Cancer Res.
43:5037–5042. 1983.PubMed/NCBI
|
|
23
|
Gil-Martin E, Gil-Seijo S, Nieto-Novoa C
and Fernández-Briera A: Elevation of acid glycosidase activities in
thyroid and gastric tumors. Int J Biochem Cell Biol. 28:651–657.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gil-Martín E, Rodríguez-Berrocal FJ, de la
Páez Cadena M and Fernández-Briera A: N-acetyl-beta-hexosaminidase
activity and isoenzymes in human gastric adenocarcinoma. Oncology.
56:142–154. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luqmani Y, Temmim L, Memon A, Abdulaziz L,
Parkar A, Ali M, Baker H, Motawy M and Fayaz S: Measurment of serum
N-acetyl beta glucosaminidase activity in patients with breast
cancer. Acta Oncol. 38:649–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oktem F, Yazicilar O, Güvenç MG, Toprak M,
Uzun H, Aydin S and Uslu E: Urinary N-acetyl-beta-D-glucosaminidase
levels in patients with laryngeal squamous cell carcinoma. J
Otolaryngol. 36:233–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wielgat P, Walczuk U, Szajda S, Bień M,
Zimnoch L, Mariak Z and Zwierz K: Activity of lysosomal
exoglycosidases in human gliomas. J Neurooncol. 80:243–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borzym-Kluczyk M, Olszewska E,
Radziejewska I, Lewszuk A and Zwierz K: Isoenzymes of
N-acetyl-beta-hexosaminidase in human pleomorphic adenoma and
healthy salivary glands: A preliminary study. Clin Chem Lab Med.
46:131–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szajda SD, Snarsk J, Jankowska A,
Puchalski Z and Zwierz K: Isoenzymes A and B of
N-acetyl-beta-D-hexosaminidase in serum and urine of patients with
pancreatic cancer. Hepatogastroenterology. 55:695–698.
2008.PubMed/NCBI
|
|
30
|
Olszewska E, Borzym-Kluczyk M, Rzewnicki
I, Rutkowska J, Knas M, Rogowski M, Waniewska E and Wielgosz R:
Hexosaminidase as a new potential marker for larynx cancer. Clin
Biochem. 42:1187–1189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slawson C, Pidala J and Potter R:
Increased N-acetyl-beta-glucosaminidase activity in primary breast
carcinomas corresponds to a decrease in N-acetylglucosamine
containing proteins. Biochim Biophys Acta. 1537:147–157. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanover JA, Krause MW and Love DC: The
hexosaminidase signaling pathway: O-GlcNAc cycling in feast or
famine. Biochim Biophys Acta. 1800:80–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Butkinaree C, Park K and Hart GW: O-linked
beta-Nacetylglucosamine (O-GlcNAc): Extensive crosstalk with
phosphorylation to regulate signaling and transcription in response
to nutrients and stress. Biochim Biophys Acta. 1800:96–106. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shafi R, Iyer SP, Ellies LG, O'Donnell N,
Marek KW, Chui D, Hart GW and Marth JD: The O-GlcNAc transferase
gene resides on the X chromosome and is essential for embryonic
stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA.
97:5735–5739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dehennaut V, Lefebvre T, Sellier C, Leroy
Y, Gross B, Walker S, Cacan R, Michalski JC, Vilain JP and Bodart
JF: O-linked N-acetylglucosaminyltransferase inhibition prevents
G2/M transition in Xenopus laevis oocytes. J Biol Chem.
282:12527–12536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ho SR, Wang K, Whisenhunt TR, Huang P, Zhu
X, Kudlow JE and Paterson AJ: O-GlcNAcylation enhances FOXO4
transcriptional regulation in response to stress. FEBS Lett.
584:49–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sohn KC, Lee KY, Park JE and Do SI: OGT
functions as a catalytic chaperone under heat stress response: A
unique defense role of OGT in hyperthermia. Biochem Biophys Res
Commun. 322:1045–1051. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|