Introduction
The structural framework of the human body is
provided by the internal skeletal system. At birth, humans have a
total of 270 bones. However, during the various developmental
processes, certain bones fuse with one another and their number is
reduced to 206 in adults (1).
Osteosarcoma is a malignant tumor of the bones that occurs due to
the abnormal proliferation of osteoblast cells. Although it occurs
in all age groups (2,3), it is more common in young adults and
growing children. The survival rate of osteosarcoma patients is
<20%, as they are often diagnosed at advanced stages due to a
lack of definitive diagnostic techniques (4,5).
Currently, the metastatic form of the disease is treated with
chemotherapy, however patient responses to chemotherapy are not
positive enough to ensure long overall survival times (6).
The molecular mechanisms behind osteosarcoma growth
and metastasis are not fully understand (7). Previous studies have reported that the
frequent alterations of tumor suppressor genes, such as tumor
protein 53 and retinoblastoma 1, play a role in osteosarcoma
(8–10). In recent years, the role of Wnt
signaling has been revealed in osteosarcoma development (11). The expression of the B7-H3 protein is
reported in numerous studies of malignant tissues of the human
lungs, stomach, prostate and other organs (12–15). B7-H3
protein is a recently identified B7 family member whose roles
remain controversial (16). A recent
study revealed that B7-H3 protein expression is elevated in
osteosarcoma and assists in tumor progression (16). Further studies on B7-H3 protein are
necessary to identify its role in the primary and metastatic forms
of osteosarcoma in order to assist in understanding the invasive
nature of the tumor. The aim of the present study was to
successfully develop a murine osteosarcoma model by injecting mice
with K7M2 cells, which have the potential to cause osteosarcoma.
The B7-H3 protein expression profile was then analyzed in the
primary and metastatic stages of osteosarcoma.
Subjects and methods
Experimental animals
Female, 9-month-old, BALB/c mice (mean weight, 30 g)
(Jackson Laboratory, Ben Harbor, ME, USA) were maintained under
pathogen-free conditions in the Experimental Therapy Unit,
Department of Orthopedics, The First Affiliated Hospital of Soochow
University (Suzhou, China). All the animals (n=18) were kept under
regular supervision as per the institutional guidelines. The
protocol of the present study was approved by the Institutional
Animal Care and Ethical Committee (The First Affiliated Hospital of
Soochow University), which was formed for the specific purpose of
this project. The animals were regularly provided with food and
water, and handled according to the local ethical regulations.
Murine osteosarcoma model
The mouse model of osteosarcoma was developed by
injecting K7M2 cells (dilution ratio, 104 cells/20 µl)
into the BALB/c strain. The KM72 cells were obtained from American
Type Culture Collection (Manassas, VA, USA), and stored at −130°C
and 25% relative humidify. Cells were grown in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis,
MO, USA) at 37°C and 95% relative humidity with 5–6%
CO2. The exact site of injection was within the bone
marrow space of the femoral bone. The BALB/c strain of mice was
chosen, as these animals have a good ability to develop
osteosarcoma (17). The BALB/c strain
of mice developed spontaneous tumors and the metastatic form of
osteosarcoma following the injection. The mice took 12 days to form
the tumors, as in humans. Mice were sacrificed on the 12th and 20th
days following injection with K7M2 cells, coinciding with the
development of primary and metastatic forms of osteosarcoma. Of the
six mice induced to form the primary tumor, all of the mice
developed a primary tumor on 12th day and three mice developed
tumors at two sites; therefore, a total of nine primary tumor
samples were obtained. Of the six mice induced to form the
metastatic tumor, five developed a metastatic tumor on 20th day and
two developed metastatic tumors at two sites; therefore, a total of
seven metastatic tumor samples were obtained. Control tissue
samples were also obtained from the healthy thigh bones of 6
mice.
Western blot analysis
Samples from normal tissues, and from the primary
and the metastatic forms of osteosarcoma tissue were dissected and
cell lysate was prepared. The protein samples from the cell lysate
were prepared (80 µg/well) and resolved in a 12% gel for sodium
dodecyl sulfate polyacrylamide gel electrophoresis, following the
previously described protocol (18).
The proteins were then transferred to PVDF membranes (Abcam,
Cambridge, MA, USA), and the membrane was blocked with 5% milk in
Tris-buffered saline with Tween 20. The primary antibody
(monoclonal rat anti-mouse anti-B7-H3 antibody; catalog no. 135605;
BioLegend, San Diego, CA, USA) was used at a 1:500 dilution and
further developed with a secondary antibody [horseradish
peroxidase-conjugated goat anti-rat immunoglobulin (Ig) G; catalog
no., sc-2032; Santa Cruz Biotechnology, Dallas, TX, USA] at a
1:3,000 dilution. Diaminobenzidine (Abcam) was applied as the
chromogen to visualize the proteins. Monoclonal mouse anti-rabbit
glyceraldehyde 3-phosphate dehydrogenase antibody (catalog no.,
ab8245; 1:500; Abcam) was used as the loading control. Protein
signals were visualized using a microscope (Ti-S; Nikon
Corporation, Tokyo, Japan) and signal intensity was analyzed using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Immunohistochemistry
For immunohistochemistry, the tissues were initially
fixed with 10% formalin and paraffin-embedded. Next, slides with
5-µm consecutive sections of tissue were deparaffinized with xylene
and then hydrated. Endogenous peroxidase activity was inhibited by
immersing the slides in freshly prepared 10%
H2O2 and 10% methanol in 1X
phosphate-buffered saline (PBS) for 30 min. In order to assess the
target protein, the tissue sections were incubated with 0.1%
trypsin in 0.1% CaCl2 at 37°C for 10 min. The processed
sections were incubated with mouse monoclonal rabbit anti-human
anti-B7-H3 antibody (1:500; catalog no., SP206; Sigma-Aldrich)
overnight in a humid chamber at 4°C. Subsequently, the sections
were washed thoroughly with 1X PBS and successively incubated with
a suitable secondary antibody (polyclonal horseradish
peroxidase-conjugated goat anti-rabbit IgG; 1:4,000; catalog no.,
6721; Abcam) for 1 h. After being washed, the sections were stained
with diaminobenzidine, which was used as a chromogen. The sections
were counterstained with Ehrlich hematoxylin (Sigma-Aldrich) to
assist in locating the individual cells.
Results
Development of primary and metastatic
osteosarcoma model
For formation of a murine osteosarcoma model, the
standard protocol is for 4-month-old mice to be injected with
aggressive K7M2 cells (106 cells/20 µl). The injected
mice then develop a spontaneous metastatic form of cancer on the
8th day (16). Using this protocol,
it is difficult to differentiate and isolate the primary and
metastatic forms of the disease due to the aggressive nature of
injected K7M2 cells at a dosage of 106 cells/20 µl. In
the present study, the protocol was slightly modified by decreasing
the dosage of K7M2 cells (104 cell/20 µl) and by
increasing the age of the mice (9 month old) so that the aggressive
development of osteosarcoma was limited. Using this modified
protocol, optimized and well controlled isolation of primary and
metastatic forms of osteosarcoma was managed. The mice developed
the primary and metastatic forms of osteosarcoma on the 12th and
20th days, respectively.
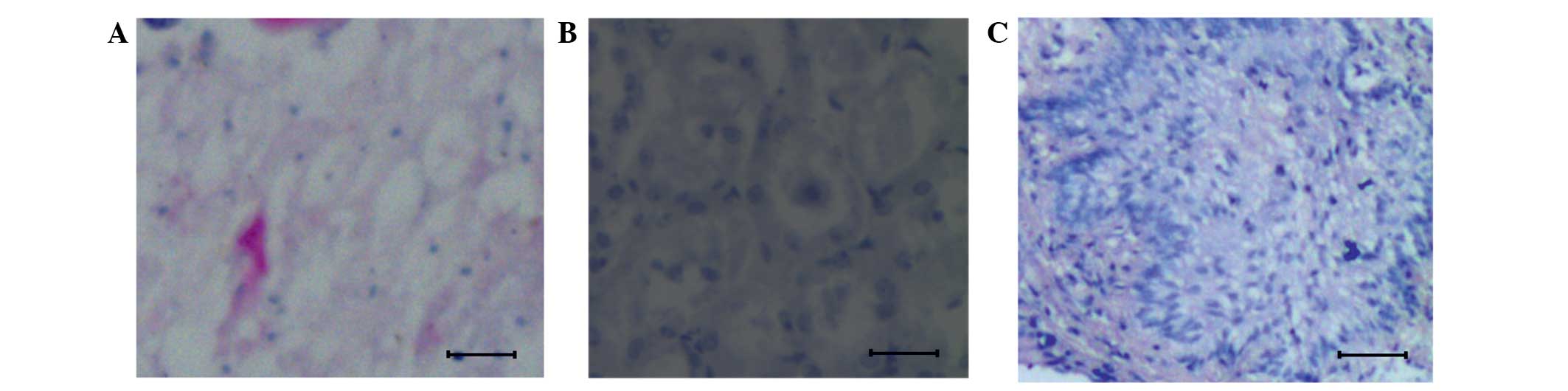
Histological observation
In order to understand the cellular level changes
that were taking place at the time of osteosarcoma formation in the
mouse model following injection with K7M2 cells (104
cell/20 µl), tissue samples from the 12th and 20th days
post-injection were subjected to histological analysis. Results
were compared against data obtained from the control mouse tissues.
The data clearly showed that in the control, the bone tissues are
well organized, as shown in the Fig.
1A. By contrast, in the mice injected with K7M2 cells, a
primary form of osteosarcoma was present on 12th day (Fig. 1B) and a metastatic form of
osteosarcoma on the 20th day (Fig.
1C). The primary form of osteosarcoma showed a less organized
tissue structure, whereas in the metastatic form of osteosarcoma,
the individual cells were heavily replicated.
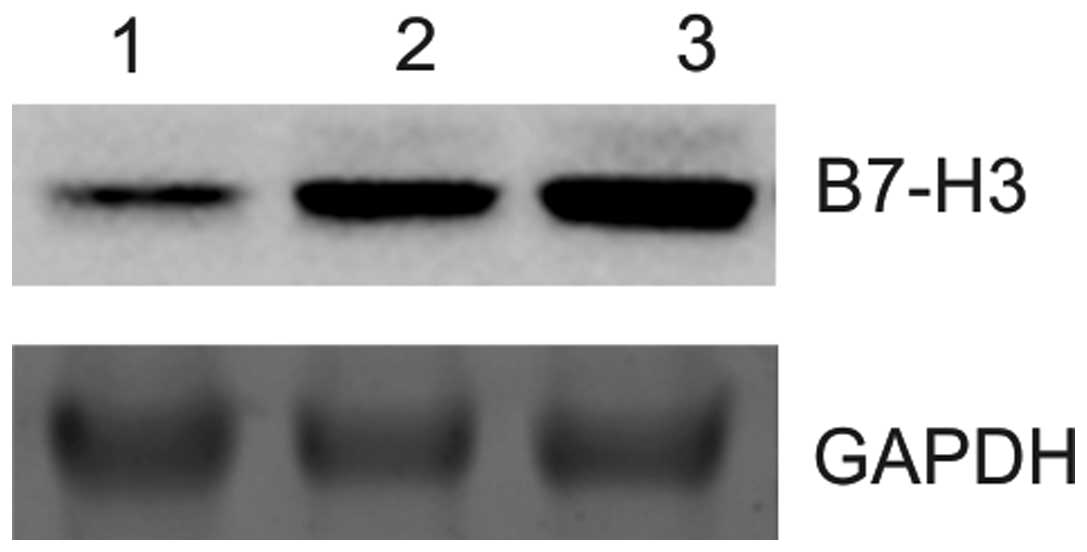
B7-H3 protein expression in control
tissues, and primary and metastatic forms of osteosarcoma
To examine B7-H3 expression, the protein samples
were prepared from the control tissue, and the osteosarcoma tissues
of primary and metastatic form, and then subjected to western
blotting analysis using the antibody against B7-H3 protein. The
expression pattern of B7-H3 protein in the control, primary and
metastatic tissues is shown in Fig.
2A–C. The results were assessed based on the intensity of the
signals obtained. When compared with the control tissue, the
primary form of osteosarcoma exhibited 3-fold increased expression
of B7-H3 protein. The metastatic form of osteosarcoma showed a
5-fold higher level of B7-H3 protein expression compared with the
control and primary osteosarcoma tissues.
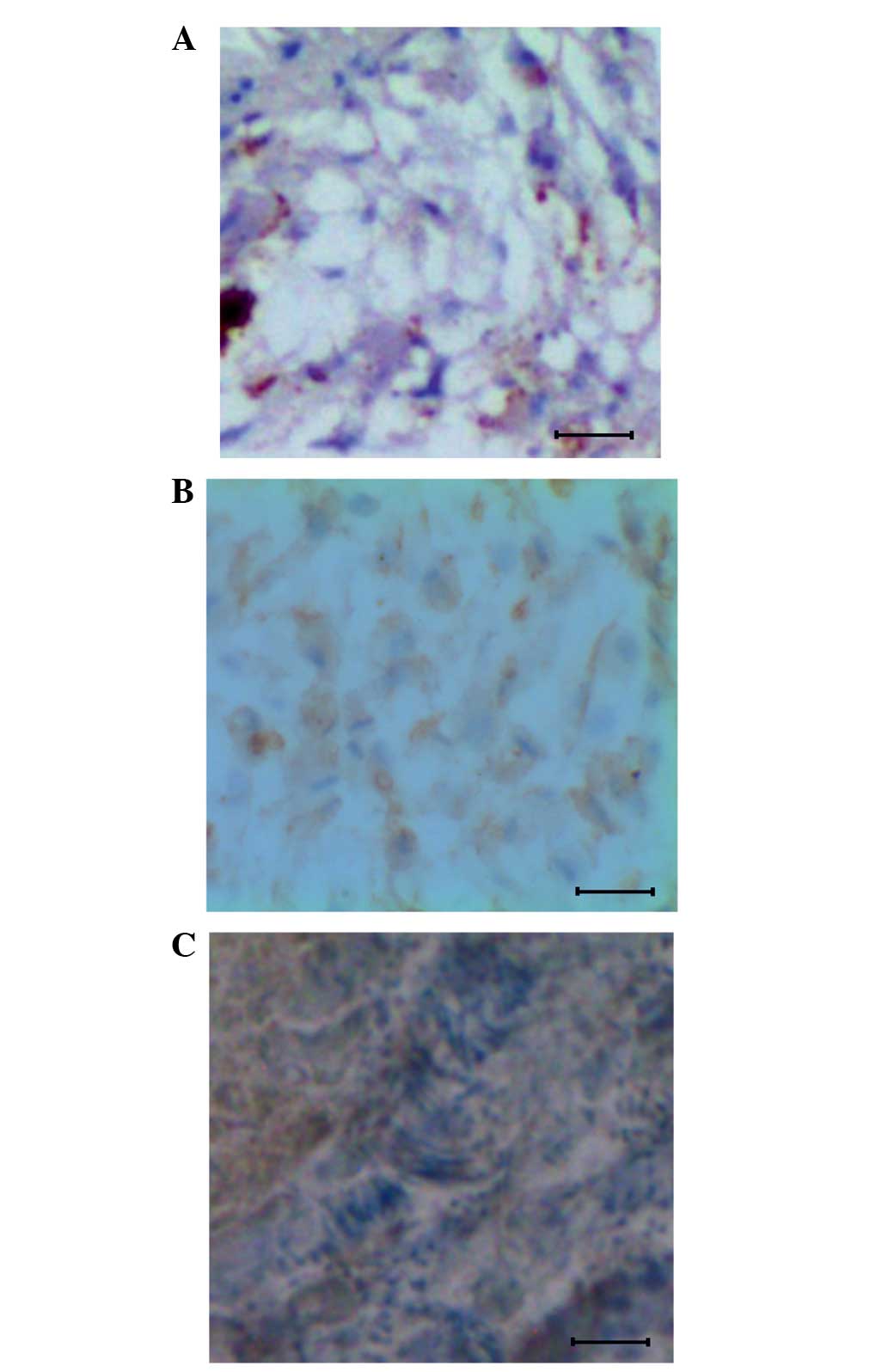
Overexpression of B7-H3 protein in the
proliferative cells of osteosarcoma tissues
The contribution of B7-H3 protein in the formation
of the primary and metastatic forms of osteosarcoma was studied by
immunohistochemistry using the anti-B7-H3 antibody. For this study,
control tissues and mouse osteosarcoma samples from the 12th and
20th day post-injection were subjected to immunohistochemistry. The
results showed that the B7-H3 expression was restricted in the
control tissue (Fig. 3A), whereas the
expression was uniformly distributed among the cells at the primary
osteosarcoma stage. It was also noted that the protein was highly
expressed in the plasma membrane and cytoplasm of these cells
(Fig. 3B). In the metastatic form of
osteosarcoma, a higher expression level of B7-H3 protein was
observed (Fig. 3C).
Discussion
The functional role of B7-H3 protein is
controversial; two different theories exist, as it has been
identified as with immunoinhibitory and immunoenhancing roles
(19). Recent studies have linked the
overexpression of the protein with a range of cancers, including
lung (20), prostate (21), breast (22) and gastric (23) cancer, and osteosarcoma (16), while the knockdown of the protein is
linked with other cancer types, for example, human oral squamous
cell cancer (24). Therefore,
research is required in order to provide further elucidation of the
functions of the B7-H3 protein in different cancerous tissues, as
well as to study its role in different model systems.
Osteosarcoma is a malignant tumor of the bone that
mostly affects younger individuals. A good model system that mimics
the disease in humans is necessary for performing research that
assists in refining the knowledge in this field. Controversy exists
with regard to the B7-H3 protein, with one debatable point being
the different responses in the in vitro and in vivo
study of various cancer types (25).
Mice have a natural ability to form osteosarcoma spontaneously
(26) and exhibit useful responses
after triggering the disease condition artificially. The present
study was performed using the BALB/c strain of mice, which has the
potential to form the primary and metastatic forms of osteosarcoma
in the 12th and 20th days post-injection, respectively. This method
of osteosarcoma formation assists in evaluating the failure of the
immune system that supports osteosarcoma formation and
progression.
In the present study, the histological data
suggested that the well-organized structure of the tissues becomes
disturbed with osteosarcoma progression (Fig. 1A–C). This particularly occurs in the
metastatic form of osteosarcoma, where clumps of proliferative
cancerous cells are observed on the 20th day (Fig. 1C), thus showing the aggressive nature
of osteosarcoma development. The gradual increase in the expression
of B7-H3 protein as the osteosarcoma progressed indicated that the
disease progression is associated with the level of B7-H3 protein
expression (Figs. 2 and 3). Also, this may suggest that it is
directly or indirectly involved in the development of osteosarcoma.
Wang et al (16) also proposed
a possible association between osteosarcoma and B7-H3, stating that
B7-H3 inhibits CD8+ T cell infiltration into the tumor
tissue and thereby suppresses the development of tumor
immunogenicity. The highest disease severity is achieved in the
metastatic form of osteosarcoma and at that stage, significant
overexpression of B7-H3 protein may support tissue dissemination. A
previous study has suggested that the overexpression of B7-H3
protein may reduce the survival time of an affected patient to 5
years, and that this may be due to the worsening of immune system
function as the disease progresses (16). Further research is required in this
area to identify the functional aspect of this misbehavior.
In summary, the present study indicates that the
primary and metastatic forms of osteosarcoma show varied
histological characteristics. The data also supports that the
costimulatory molecule B7-H3 elicits higher levels of expression as
the disease progresses. Overall, this data may aid in developing a
therapeutic intervention to access this type of cancer.
References
|
1
|
Marshall Cavendish Corporation: Mammal
anatomy: An illustrated guide. Cavendish Square Publishing. NY:
2010.
|
|
2
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Errani C, Longhi A, Rossi G, Rimondi E,
Biazzo A, Toscano A, Alì N, Ruggieri P, Alberghini M, Picci P, et
al: Palliative therapy for osteosarcoma. Expert Rev Anticancer
Ther. 11:217–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meyers PA, Heller G, Healey J, Huvos A,
Lane J, Marcove R, Applewhite A, Vlamis V and Rosen G: Chemotherapy
for nonmetastatic osteogenic sarcoma: The Memorial Sloan-Kettering
experience. J Clin Oncol. 10:5–15. 1992.PubMed/NCBI
|
|
5
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
PosthumaDeBoer J, Witlox M, Kaspers G and
Van Royen B: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brun J, Dieudonné FX, Marty C, Müller J,
Schüle R, Patiño-García A, Lecanda F, Fromigué O and Marie PJ: FHL2
silencing reduces Wnt signaling and osteosarcoma tumorigenesis in
vitro and in vivo. PloS One. 8:e550342013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patiño-García A, Piñeiro ES, Díez MZ,
Iturriagagoitia LG, Klüssmann FA and Ariznabarreta LS: Genetic and
epigenetic alterations of the cell cycle regulators and tumor
suppressor genes in pediatric osteosarcomas. J Pediatr Hematol
Oncol. 25:362–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuchiya T, Sekine K, Hinohara S, Namiki
T, Nobori T and Kaneko Y: Analysis of the p16INK4, p14ARF, p15,
TP53 and MDM2 genes and their prognostic implications in
osteosarcoma and Ewing sarcoma. Cancer Genet Cytogenet. 120:91–98.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wadayama B, Toguchida J, Shimizu T,
Ishizaki K, Sasaki MS, Kotoura Y and Yamamuro T: Mutation spectrum
of the retinoblastoma gene in osteosarcomas. Cancer Res.
54:3042–3048. 1994.PubMed/NCBI
|
|
11
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: Implications for therapy
in osteosarcoma. 2011.
|
|
12
|
Boland JM, Kwon ED, Harrington SM,
Wampfler JA, Tang H, Yang P and Aubry MC: Tumor B7-H1 and B7-H3
expression in squamous cell carcinoma of the lung. Clinical Lung
Cancer. 14:157–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arigami T, Uenosono Y, Hirata M, Yanagita
S, Ishigami S and Natsugoe S: B7-H3 expression in gastric cancer: A
novel molecular blood marker for detecting circulating tumor cells.
Cancer Sci. 102:1019–1024. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arigami T, Narita N, Mizuno R, Nguyen L,
Ye X, Chung A, Giuliano AE and Hoon DS: B7-H3 ligand expression by
primary breast cancer and associated with regional nodal
metastasis. Ann Surg. 252:1044–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang X, Thompson RH, Al-Ahmadie HA, Serio
AM, Reuter VE, Eastham JA, Scardino PT, Sharma P and Allison JP:
B7-H3 and B7x are highly expressed in human prostate cancer and
associated with disease spread and poor outcome. Proc Natl Acad
Sci. 104:19458–19463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Zhang Q, Chen W, Shan B, Ding Y,
Zhang G, Cao N, Liu L and Zhang Y: B7-H3 is overexpressed in
patients suffering osteosarcoma and associated with tumor
aggressiveness and metastasis. PloS One. 8:e706892013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khanna C, Prehn J, Yeung C, Caylor J,
Tsokos M and Helman L: An orthotopic model of murine osteosarcoma
with clonally related variants differing in pulmonary metastatic
potential. Clin Exp Metastasis. 18:261–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamidouche Z, Haÿ E, Vaudin P, Charbord P,
Schüle R, Marie PJ and Fromigué O: FHL2 mediates
dexamethasone-induced mesenchymal cell differentiation into
osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2
expression. FASEB J. 22:3813–3822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang HY, Chu M, Zheng LW, Zwahlen RA, Luo
J, Zou DH and Sun ST: Transgenic B7-H3 therapy induces
tumor-specific immune response in human oral squamous cell cancer:
An in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 106:721–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu YH, Zhang GB, Wang JM and Hu HC: B7-H3
and CD133 expression in non-small cell lung cancer and correlation
with clinicopathologic factors and prognosis. Saudi Med J.
31:980–986. 2010.PubMed/NCBI
|
|
21
|
Yuan H, Wei X, Zhang G, Li C, Zhang X and
Hou J: B7-H3 over expression in prostate cancer promotes tumor cell
progression. J Urol. 186:1093–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed BA: Awareness and practice of breast
cancer and breast-self examination among university students in
Yemen. Asian Pac J Cancer Prev. 11:101–105. 2009.
|
|
23
|
Biglarian A, Hajizadeh E, Kazemnejad A and
Zayeri F: Determining of prognostic factors in gastric cancer
patients using artificial neural networks. Asian Pac J Cancer Prev.
11:533–536. 2010.PubMed/NCBI
|
|
24
|
Nygren M, Tekle C, Ingebrigtsen V and
Fodstad O: B7-H3 and its relevance in cancer; immunological and
non-immunological perspectives. Front Biosci (Elite Ed). 3:989–993.
2010.
|
|
25
|
Lu P, Liu R, Ma EM, Yang TJ and Liu JL:
Functional analysis of B7-H3 in colonic carcinoma cells. Asian Pac
J Cancer Prev. 13:3899–3903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tinkey PT, Lembo TM, Evans GR, Cundiff JH,
Gray KN and Price RE: Postirradiation sarcomas in Sprague-Dawley
rats. Radiat Res. 149:401–404. 1998. View
Article : Google Scholar : PubMed/NCBI
|