Introduction
Prostate cancer is the most commonly diagnosed
malignancy in elderly men, and the second leading cause of
cancer-associated mortality in the Western world (1,2).
Standard treatments for prostate cancer include
surgery, radiation therapy and androgen deprivation therapy (ADT),
which are usually successful in controlling organ-confined disease.
However, in 30% of patients the disease recurs and becomes androgen
deprivation-resistant (3,4).
Since the 1990's, gonadotropin-releasing hormone
(GnRH) analogues have been the most successful drugs for achieving
ADT (5). Androgen is required for the
function and maintenance of prostate cancer cells, and androgen
deprivation results in an inhibition of growth and decrease in
volume of prostate cancer tumors (6–8). However,
at advanced stages of the disease, almost all tumors develop into
castration-resistant prostate cancer (CRPC), the management of
which is a great challenge (6,8,9). Consequently, there is a clear
requirement for improved treatments to prevent the development of
CRPC, and for novel therapies to treat patients with prostate
cancer.
GnRH-based vaccines represent a promising
anti-hormonal treatment alternative for the treatment of prostate
cancer, since they reduce serum testosterone levels and avoid the
flare response observed when using GnRH analogues (6,10–12). In addition, following the completion
of the immunization procedure, the patient does not require the
administration of any other medication for a prolonged period,
which results in low costs (6,12).
Therefore, these types of vaccines may be highly competitive in the
market (10,11,13).
However, the development of therapeutic vaccines for cancer therapy
presents disadvantages, due to the low immunogenicity of antigens,
tolerance induction and heterogeneity of results among individuals
(6,14,15).
The use of adjuvant combinations such as aluminum
salts (16) and squalene oil
(17), alone or in combination with
saponins, has been recently employed in the development of
preventive vaccines, including the vaccine for human papilloma
virus (18–20). However, adjuvants have not been used
for the development of therapeutic vaccines for advanced cancer
thus far.
The combination of Montanide ISA 51, a type 1
adjuvant, and very small size proteoliposomes (VSSP), a type 2
adjuvant that is derived from the fermentation of Neisseria
meningitides, has been recently used by the authors of the
present study for the generation of anti-GnRH antibodies in healthy
animals with excellent results (12).
The present study demonstrates the humoral immune
response against the autologous GnRH hormone, and its effect on the
inhibition of testosterone levels and tumor development in DD/S
male mice that were injected with hormone-sensitive Shionogi
carcinoma (SC) 115 cells. The SC 115 mice received 6 immunizations
of the GnRH mimetic peptide (GnRHm1-TT)/Montanide ISA 51/VSSP
vaccine candidate. The SC 115 mouse model is a transplantable
androgen-dependent neoplasia similar to human tumors, which may
develop into CRPC in >90% of cases, despite the use of the
maximal androgen blockade (21,22). The
present study also measured the reactivity of specific immune
response to a 40 kDa antigen, which had been previously reported to
be induced by castration, using western blot and interferon (IFN)-γ
enzyme-linked immunospot (ELISPOT) assays (9,22).
Materials and methods
Generation of the synthetic
GnRHm1-TT
GnRHm1-TT was synthesized using conventional solid
phase synthesis (6), at the Center
for Genetic Engineering and Biotechnology of Havana (Havana, Cuba).
L-glycine at position 6 of the natural GnRH (sequence, QHWSYGLRPG)
was substituted for L-proline (sequence, QHWSYPLRPG), and the
epitope QYIKANSKFIGITEL of tetanus toxoid was added to the carboxyl
terminus (6).
For the vaccine emulsion preparation, 750 µg
lyophilized GnRHm1-TT was resuspended in distilled water and mixed
with 120 µg VSSP (obtained from the Center for Molecular
Immunology, Havana, Cuba) to a final volume of 100 µl. The same
quantity of Montanide ISA 51 (Seppic, Paris La Défense, France) was
added in a 1:1 ratio. The vaccine formulation was centrifuged (SCT
15B Himac centrifuge; Hitachi Medical Corporation, Tokyo, Japan) at
3,500 × g for 30 min. The final emulsion was administered to the
mice by subcutaneous injection at four points of the dorsal region.
For the placebo preparation, an identical procedure was performed
with the exception of the peptide, which was not included in the
mixture.
Animal models and experimental
groups
In total, 15 male DD/S mice were maintained at the
Animal Care Unit of the University of Victoria (Victoria, Canada).
All protocols followed the guidelines of the Canadian Council for
Animal Care (Ottawa, Canada; approval no., NOVO7BHN) and were
approved by the Animal Care Committee at the University of
Victoria. The animals were housed two per cage, under humidity and
temperature controlled conditions and a light/dark cycle of 12 h
intervals. The DD/S mice, which were 8–10 weeks old, were
randomized into 3 groups of 5 mice each, as follows: Group 1
received 5 subcutaneous injections of the vaccine candidate
GnRHm1-TT/Montanide ISA 51/VSSP (12); group 2 received the placebo, which was
administered using the same immunization schedule as group 1; and
group 3 was surgically castrated once the tumor reached 8–10 mm in
diameter.
DD/S mice were injected with SC 115 cells, which
were cultured according to the protocol described by Nesslinger
et al (22), on day 40
subsequent to the beginning of the experiment, when the immunized
and placebo groups had received 3 immunizations. For the tumor
implantation, 5×106 cells in phosphate-buffered saline
(PBS; EMD Millipore, Billerica, MA, USA) were injected
subcutaneously into the flank of the mice, according to the
protocol by Nesslinger et al (22). The tumor was measured daily using a
digital calliper. The tumor volume was calculated using the
following formula: Tumor volume = 4/3π × tumor radius3.
When the tumor reached a volume ≥1.5 cm3, the mice were
sacrificed using anaesthesia. Tumors that were palpable were
considered to be recurring tumors.
Blood extraction and serum
collection
Blood was extracted from the mice (~100 µl) by
puncturing the radial venous with a 26G needle (Terumo Medical
Corporation, Tokyo, Japan), and using heparinized micro-hematocrit
tubes (Drummond Scientific Company, Broomall, PA, USA). The blood
was centrifuged at 3,200 × g for 15 min to obtain the serum, which
was stored at −20°C until further use. Splenocytes from the mice
were isolated according to the protocol described by Nesslinger
et al (22).
Anti-GnRH antibody determination
An enzyme-linked immunosorbent assay (ELISA) was
performed using high-binding Nunc™ 96-well polystyrene plates
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), which were
coated overnight with 10 µg/ml natural GnRH peptide (Center for
Genetic Engineering and Biotechnology of Havana) at 4°C in
carbonate/bicarbonate buffer (0.1 M; pH 9.6; Merck, Kenilworth, NJ,
USA). The serum samples were diluted to 1:50 in PBS (0.1 M; pH 7.4)
for seroconversion, and between 1:1,000 and 1:20,000 for anti-GnRH
titration in PBS (0.1 M; pH 7.4) containing 2% bovine serum albumin
(BSA; Sigma-Aldrich, St Louis, MO, USA). The plates were incubated
for 3 h at 37°C. Subsequently, polyclonal rabbit anti-mouse
immunoglobulin (Ig)G antibody conjugated to peroxidase (cat. no.
A9044; Sigma-Aldrich), which was diluted to 1:10,000 in PBS (0.1 M;
pH 7.4) and 2% BSA, was added to the plates. The antigen-antibody
reaction was detected by addition of o-phenylenediamine (EMD
Millipore) and a H2O2 substrate (EMD
Millipore), which was dissolved in disodium phosphate (0.02 M, pH
5.0; EMD Millipore). The colorimetric reaction was read at 492 nm
using a plate reader (Multiskan™ GO Microplate Spectrophotometer;
Thermo Fisher Scientific, Inc.). Samples were considered to be
positive when the absorbance values were >0.197, which was the
cut-off.
Testosterone determination
Testosterone levels were determined using the
Testosterone radioimmunoassay kit (TESTO-CT2 assay; Cisbio
Bioassays, Codolet, France). The sensitivity of the method, defined
as the detectable concentration equivalent to twice the standard
deviation of the zero-binding value, was ~0.1 nmol/l. The
specificity of the kit for testosterone was >99%. To carry out
the determination, 25 µl serum from each sample was plated directly
in the pre-coated tubes provided in the kit. Duplicates from all
the samples were incubated for 1 h at 37°C. The tubes were washed 3
times with distilled water at room temperature, and read in a gamma
counter (HITACHI-ALOKA AccuFLEX LSC-8000 Scintillation Counter;
Hitachi Medical Corporation). The results were recorded as
nmol/l.
Western blot analysis to detect
anti-Shionogi proteins
Western blot analysis was performed as previously
described (23). Briefly, proteins
(400 µg) from SC 115 tumor cells (obtained from the Nelson Lab,
University of Victoria, Victoria, Canada) were separated using 12%
standard polyacrylamide gel electrophoresis (Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and transferred to nitrocellulose
membranes (Thomas Scientific, Swedesboro, NJ, USA. A molecular
weight marker 20–80 KD (Sigma-Aldrich) was used. The sera of the
mice from the various groups were diluted in dilution buffer
(dilution, 1:500), which contained PBS (50 mM), 5% dry milk powder
(Sigma-Aldrich), 0.1% Tween 20 (EMD Millipore), 50 mmol/l Tris (EMD
Millipore) and 150 mmol/l sodium chloride (EMD Millipore), and
incubated for 1 h at room temperature with the nitrocellulose
membrane, using the Mini Protean II® MultiScreen
Apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
nitrocellulose membrane was washed, incubated for 1 h at room
temperature with polyclonal horseradish peroxidase-conjugated goat
anti-mouse IgG secondary antibody (H+L; cat. no. 115-035-003;
dilution, 1:10,000; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA), and visualized using enhanced
chemiluminescence.
IFN-γ ELISPOT assay
MultiScreen-IP Filter Plates (96 wells; 0.45 µm pore
size; EMD Millipore, Billerica, MA, USA) were pre-wet with 70%
ethanol, followed by three washes with sterile PBS
(Gibco®; Thermo Fisher Scientific, Inc.). The plates
were incubated overnight at 4°C with 50 µl/well monoclonal rat
anti-mouse IFN-γ antibody (10 µg/ml; clone, AN18; catalog no.,
3321-3-250; Mabtech, Inc., Cincinnati, OH, USA). Following three
washes with PBS, the plates were blocked with T-cell medium
(Sigma-Aldrich; RPMI 1640 supplemented with 10% fetal bovine serum,
1 mM sodium pyruvate, 2 mM L-glutamine, 100 µg/ml
penicillin/streptomycin and 25 µM 2-mercaptoethanol) for 2 h at
37°C. In total, 3×105 splenocytes from the mice were
added to each well. Polyadenylate-binding protein nuclear 1
(PABPN1; the Nelson Lab, University of Victoria) and concanavalin A
(Sigma-Aldrich) were added to the wells at a final concentration of
10 and 2 µg/ml, respectively. T-cell medium was used as a negative
control. The samples were run in triplicate. The plates were
incubated for 24 h at 37°C. Subsequent to washing six times with 50
mM PBS containing 0.05% Tween 20, 100 µl biotinylated anti-mouse
IFN-γ was added to each well. The plates were incubated for 2 h at
37°C, and then washed 12 times with PBS at room temperature
containing 0.05% Tween 20. Avidin-biotin peroxidase complex
(VECTASTAIN® ABC kit; Vector Laboratories, Inc.,
Burlingame, CA, USA) was added to the plates for 5–10 min. The
development was stopped by rinsing the plates with water. Air-dried
plates were read for enumeration of spots using an ELISPOT reader
with KS ELISPOT 4.4 software (Carl Zeiss AG, Oberkochen,
Germany).
Statistical analysis
For the statistical analysis to enable a parametric
evaluation of the data, analysis of variance and Student's t
tests were used. The treatment groups were compared using the
Student's t-test. For animal survival analysis Kaplan-Meier
analysis was used in addition to the log rank test for statistical
associations. A one-way analysis of variance was used for antibody
and testosterone analysis. Prism GraphPad version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) was used to conduct statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Anti-GnRH antibodies, generated by the
vaccine candidate GnRHm1-TT/Montanide ISA 51/VSSP, induce
testosterone ablation in DD/S mice implanted with SC 115
tumors
The development of the vaccine formulation
GnRHm1-TT/Montanide ISA 51/VSSP is a promising candidate to
generate a productive response against GnRH, which presents the
advantage of combining a type 1 and a type 2 adjuvants (12,24). To
investigate how this vaccine candidate affects a tumoral model, the
present study performed an experiment in DD/S mice implanted with
the hormone-sensitive SC 115 tumor cell line, which has been
extensively used to study the conversion from androgen-dependent to
androgen-independent neoplasia (7,21).
Shionogi tumors are initially androgen-dependent, and are therefore
grown in male mice (8,9,21,22). Castration of the mice leads to
apoptosis and tumor regression similar to that observed following
androgen withdrawal in patients with prostate cancer (8,21).
The effect of the GnRHm1-TT/Montanide ISA 51/VSSP
vaccine on anti-GnRH antibodies, testosterone levels and tumor size
in DD/S mice was evaluated in the present study. In all 5 mice,
100% of anti-GnRH seroconversion was achieved following the
administration of 2 immunizations (Table
I). The anti-GnRH immune response was investigated using an
in-house titration indirect ELISA method (6,12), which
rendered titers between 1:1,000 and 1:4,000 (Table I). Similarly to the anti-GnRH immune
response, the measurement of the testosterone levels by
radioimmunoassay demonstrated a decrease in the testosterone
concentration of the immunized animals, although the levels of
testosterone in these animals were higher than those detected in
the castrated mice (Table I).
 | Table I.Effects of various treatments on the
anti-GnRH seroconversion, testosterone levels, survival time and
anti-PABPN1 antibody titers of DD/S mice injected with
hormone-sensitive Shionogi carcinoma 115 tumor cells. |
Table I.
Effects of various treatments on the
anti-GnRH seroconversion, testosterone levels, survival time and
anti-PABPN1 antibody titers of DD/S mice injected with
hormone-sensitive Shionogi carcinoma 115 tumor cells.
| Animal no. | Experimental
group | Anti-GnRH,
titer | Testosterone
levels, nmol/l | Survival time,
days | Anti-PABPN1,
western blot signal |
|---|
| 1 | Placebo | 0 |
17.30±0.1c |
31c | ND |
| 2 | Placebo | 0 |
16.30±0.1c |
19c | ND |
| 3 | Placebo | 0 |
17.40±0.2c |
27c | − |
| 5 | Placebo | 0 |
12.90±0.0c |
19c | +++ |
| 8 | Placebo | 0 |
13.40±0.0c |
24c | + |
| 11 | Immunized |
1:1,000c |
1.97±0.1b |
30c | ND |
| 12 | Immunized |
1:4,000a |
1.63±0.1b | 100a | − |
| 13 | Immunized |
1:2,000b |
1.74±0.2b |
35c | − |
| 14 | Immunized |
1:4,000a |
0.89±0.0a | 100a | − |
| 17 | Immunized |
1:4,000a |
1.04±0.0a |
55b | +++ |
| 26 | Castrated | 0 |
0.07±0.1a |
90a | +++ |
| 27 | Castrated | 0 |
0.23±0.1a |
70b | − |
| 28 | Castrated | 0 |
0.74±0.2a |
90a | − |
| 29 | Castrated | 0 |
0.89±0.0a |
59b | +++ |
| 30 | Castrated | 0 |
0.47±0.0a |
90a | ND |
GnRHm1-TT/Montanide ISA 51/VSSP
produces tumor growth inhibition in SC 115 mice
The present study investigated the association
between testosterone ablation induced by anti-GnRH antibodies and
tumor growth rate. The present study observed that in the immunized
group, 3 out of 5 mice exhibited a significant decrease in tumor
size (P<0.01) (Fig. 1A). The tumor
volume reduced to >200 mm3 at day 30 (P<0.05).
These 3 slow-growing tumors exhibited a continuous decrease in size
over time, and completely regressed at day 65 following the
injection of SC 115 cells. There was no recurrence of the tumor by
the end of the study at day 100. In total, 1 of the 5 immunized
mice was sacrificed prior to the end of the present study (day 55),
since the mouse mutilated the tumor implanted region. The remaining
2 mice in the immunized group exhibited an accelerated tumor
growth, and had to be sacrificed on day 30 and 35, respectively
(Fig. 1A).
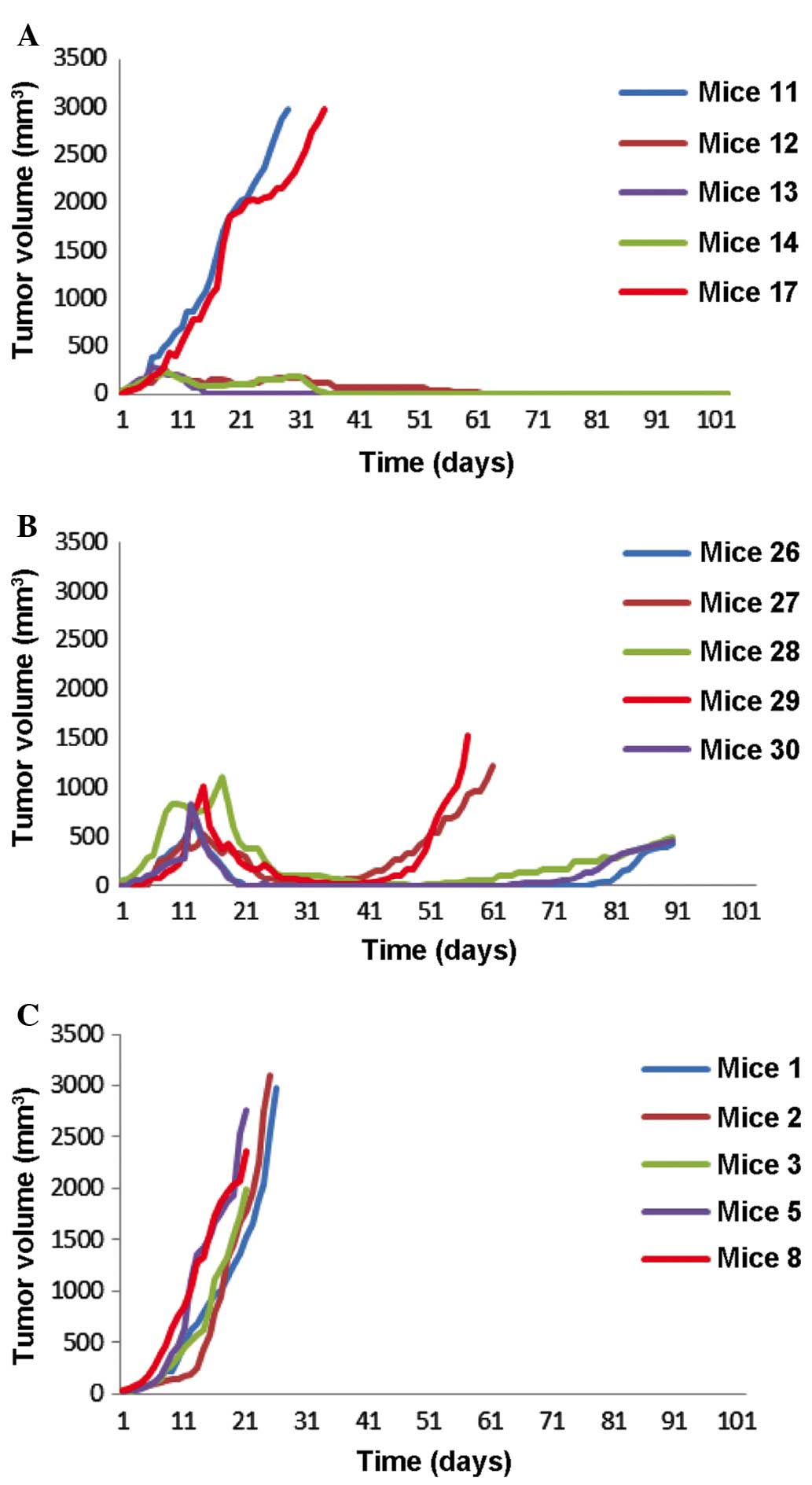 | Figure 1.Tumor growth rate of the Shionogi
carcinoma 115-implanted DD/S mice subjected to various treatments.
(A) Mice that received vaccine candidate GnRHm1-TT/Montanide ISA
51/VSSP at days 0, 15, 30, 45 and 60 (n=5). It was observed that 2
out of 5 mice exhibited fast-growing tumors in the first 30 days,
and 2 out of 5 mice exhibited tumor growth inhibition until day
100. (B) Castrated mice, used as control (n=5). The mice were
castrated 15 days following tumor implantation. Tumor growth was
inhibited in all these mice, although tumor re-growth was observed
on days 59–90. (C) Placebo mice, which were immunized with a
mixture of Montanide ISA 51/VSSP (n=5). All the mice exhibited a
fast tumor growth, and had to be sacrificed prior to day 31
following tumor implantation. VSSP, very small size
proteoliposomes; GnRHm1-TT, gonadotropin-releasing hormone mimetic
peptide. |
In the castrated group, 4 out of 5 mice exhibited
tumor regression 10 days following castration. The maximum
reduction in tumor volume was observed at day 30 (180
mm3). In the remaining mice, ~40 days were required to
achieve a similar reduction in tumor size (Fig. 1B). However, there was tumor regrowth
in 2 mice at days 50 and 54. Therefore, the mice were sacrificed at
days 59 and 70, respectively (Table
I). The remaining 3 mice experienced tumor regrowth from day
70, and had to be sacrificed on day 90 (Fig. 1B). The entire placebo group had to be
sacrificed by ~day 30 post-tumor cell injection, since all the mice
exhibited an accelerated tumor growth (Fig. 1C).
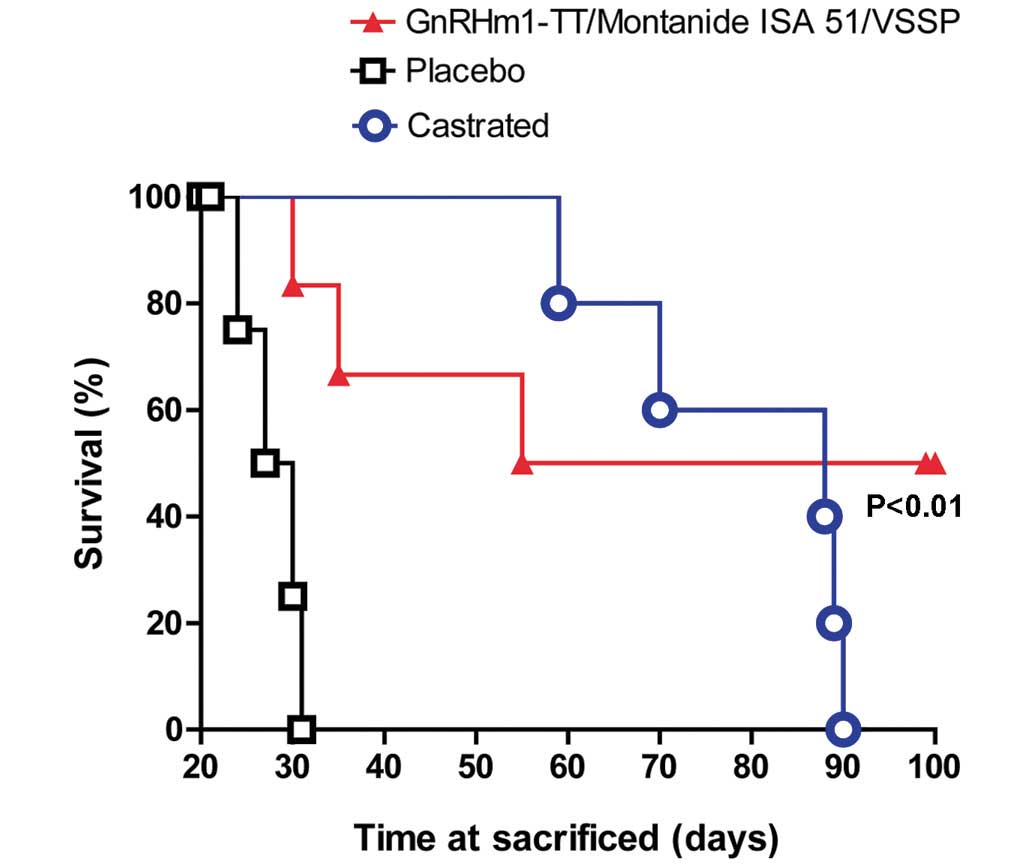
The comparison in survival time between the placebo,
surgically castrated and immunized mice was calculated using the
log-rank test. The results demonstrated that the immunized and
castrated animals survived for twice as long as the placebo group
(P<0.01). The surgically castrated and vaccinated mice did not
exhibit any significant differences in survival time (P>0.05).
However, it is notable that in the vaccinated group there were 2
animals in which tumors did not recur for the duration of the
present study (Fig. 2).
Anti-PABPN1 immune response in DD/S
mice implanted with SC 115 tumor cells is not associated with
improvements in tumor control or survival benefits
Previous studies have implicated anti-PABPN1
antibodies in the development of Shionogi carcinoma. Therefore, the
present study investigated whether the development of hormone
therapy-associated serological responses was associated with the
extent of tumor regression. The present study compared the SC 115
tumor-implanted DD/S mice that had received the standard castration
treatment with the GnRHm1-TT/Montanide ISA 51/VSSP vaccine and the
placebo groups. The results demonstrated that for the immunized
group, 1 out of 4 mice (25%) developed anti-PABPN1 antibodies
(mouse no., 17; Fig. 3A), compared
with 2 out of 4 mice (50%) in the castrated group (mice no., 26 and
29; Fig. 3B and C, respectively), and
2 out of 3 mice (66%) in the placebo group. (mice no., 5 and 8;
Fig. 3A and D, respectively).
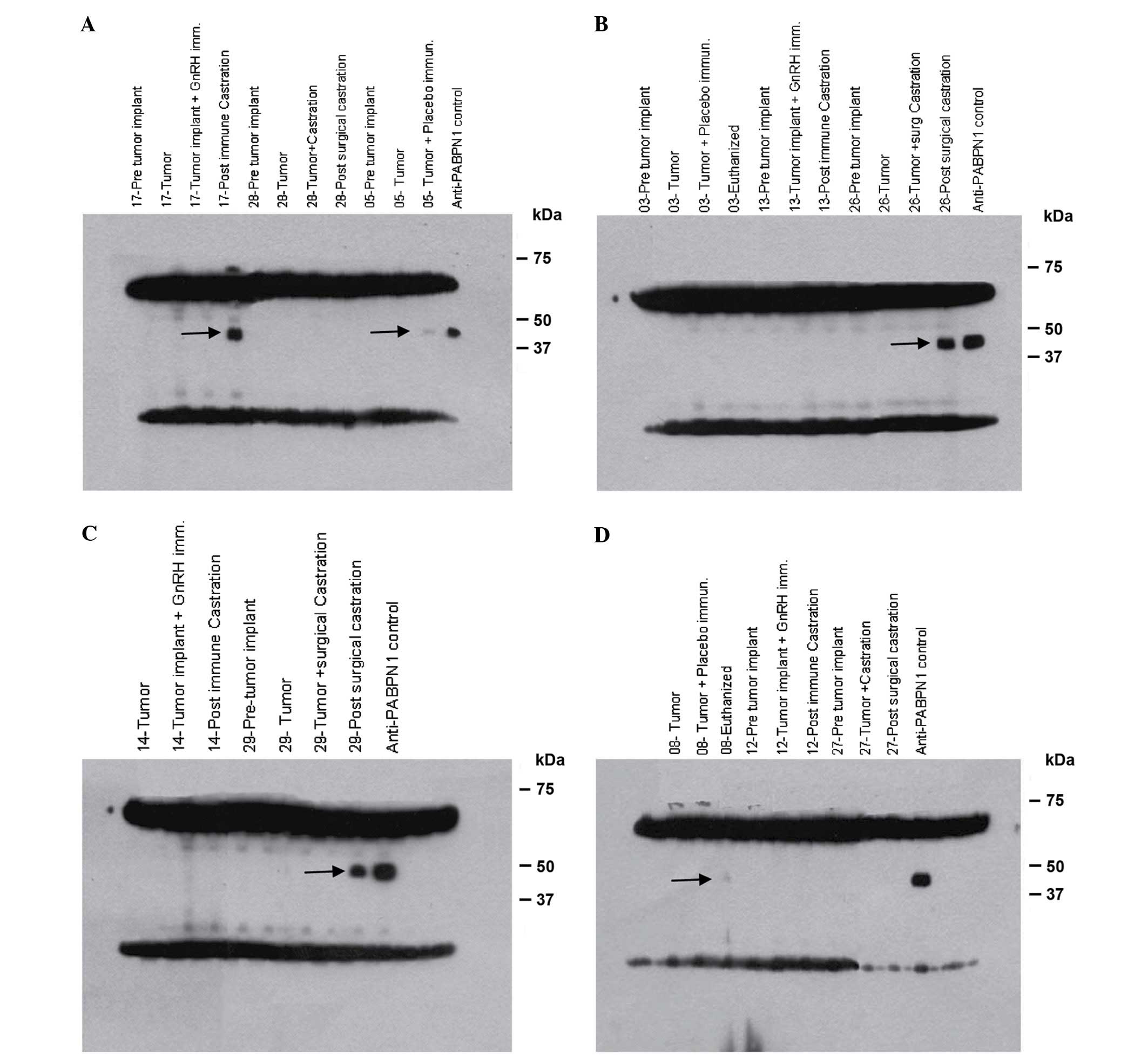 | Figure 3.Western blot analysis of serum from
mice injected with Shionogi carcinoma 115 cells, which was probed
against Shionogi tumor lysate to determine recognition of the
PABPN1 protein. Serum was collected from 5 mice from various
treatment groups at serial time points, including pre-tumor
implantation, tumor implantation, pre-castration, post-castration
and sacrifice. Western blot analysis demonstrated autoantibody
responses to a 44 kDa protein derived from the murine Shionogi
tumor model (shown by the black arrow) in mice numbers (A) 5,
placebo and 17, immunized, (B) 26, surgical castration, (C) 29,
surgical castration and (D) 8, placebo. Presence of autoantibodies
following treatment was observed, which appeared to be associated
with a poor prognosis. Intense bands at the top and bottom of the
gel represents staining of the heavy and light chains of antibodies
contained in the tumors of the mice. GnRH, gonadotropin-releasing
hormone; PABN1, polyadenylate-binding protein nuclear 1; immun.,
immunization; implant, implantation. |
According to these results, there was no association
between the presence of anti-PABPN1 antibodies and the tumor growth
rate. However, a poor prognosis for tumor growth was observed in
mice that developed an immune response against PABPN1 (Fig. 3A-D). Notably, in mice treated with the
vaccine candidate GnRHm1-TT/Montanide ISA 51/VSSP, the mice that
exhibited the most accelerated tumor growth were those that
developed anti-PABPN1 antibodies, as measured by western blot
analysis (mouse no., 17; Fig. 3B).
Similarly, in the placebo group, which presented the highest tumor
growth rate, the highest anti-PABPN1 immune response was detected
(mouse no., 5 and 8; Fig. 3A and D,
respectively). For the surgically castrated mice, a similar
association between anti-PABPN1 immune response and survival time
was observed (mouse no., 26 and 29; Fig.
3B and C, respectively).
In order to determine the possible involvement of T
cells in the immunological recognition of PABPN1, in addition to
the analysis of anti-PABPN1 antibodies, the present study performed
an anti-IFN-γ ELISPOT using splenocytes collected from the
sacrificed mice. Lymphocytes were extracted at the same time to act
as controls. The results demonstrated a T-cell immune response
directed to PABPN1 in mice that had developed anti-PABPN1
antibodies (data not shown).
Discussion
GnRH-based vaccines represent a promising
anti-hormonal alternative treatment for patients with prostate
cancer, since they reduce serum testosterone levels, leading to the
generation of a memory immune response in vaccinated individuals
(6,10–12).
However, the poor immunogenicity of small and autologous peptides,
such as GnRH and its mimetics, is a challenging obstacle that must
be overcome by immunologists (10,11,24,25).
The combination of potent adjuvants to emulsify poor
immunogenic antigens is an attractive approach that may promote an
inflammatory cell environment, and is useful for the development of
efficacious vaccines (26–28). A recent study demonstrated the
usefulness of the GnRHm1-TT peptide adjuvanted with Montanide ISA
51 and VSSP to promote 100% immunogenicity and testosterone
decrease in healthy adult animals in <60 days (12). However, the simulation of these
effects in a tumoral mouse model that mimics prostate cancer
represents an important challenge, which, to the best of our
knowledge, has not been investigated thus far.
The murine SC 115 mouse model mimics the initial
hormone-sensitive stage of prostate cancer and the development of
CRPC in humans, and is a useful model to evaluate the effect of
immunocastration (22). In order to
ensure the Shionogi tumor implantation in the mice and produce an
effective immune response to GnRH, the present study performed
three immunizations prior to tumor implantation, and three
additional immunizations following implantation.
As previously demonstrated in healthy rats (6,12), the
DD/S mice implanted with the SC 115 tumor in the present study
generated a rapid anti-GnRH seroconversion and high antibody titers
following the third injection of SC 115 cells. This humoral immune
response was associated with a significant decrease in testosterone
levels. In 1 mouse the decrease in testosterone levels was similar
to the decrease observed in the castration group. However, in the
other 4 mice, the levels of testosterone were significantly reduced
compared with the placebo mice, although not as reduced as in the
castrated mice.
The effective immune response observed in
tumor-implanted mice was similar to the immune response previously
reported in healthy animals (12),
and is possibly due to the use of a mixture of type 1 (Montanide
ISA 51) and type 2 (VSSP) adjuvants. This observation is in
agreement with previous studies describing the use of gram-negative
bacteria-derived adjuvants and VSSP to promote cell antigen
presentation in patients with cancer and immunodeficient patients
(24,25,29,30).
All the mice in the placebo group in the present
study exhibited rapid tumor growth, and had to be sacrificed at
19–31 days. All the castrated animals exhibited a reduction in
tumor volume, as expected in this hormone-sensitive prostate cancer
model. In total, 3 of the mice immunized against GnRH also
exhibited a reduction in tumor growth. However, 2 mice failed to
respond, and exhibited tumor growth similar to that observed in the
placebo group. Thus, they had to be sacrificed at days 30 and 35.
In contrast to the castrated animals, which all exhibited tumor
recurrence, the 3 remaining mice in the GnRH immunized group
exhibited no tumor recurrence. In total, 1 mouse had to be
sacrificed at day 55 due to self-mutilation, but the other 2 mice
had no regrowth up to day 100, when the present study was
terminated.
A previous study supports the role of testosterone
ablation as a promoter of the immune response to prostate antigens
and suggests that ADT stimulates quantitatively and qualitatively
the immune system, and mitigates prostate antigen tolerance
(23). To examine this hypothesis,
the present study performed western blot and ELISPOT analyses to
evaluate the humoral and cluster of differentiation 8+
T-cell immune response, respectively, in SC 115-implanted DD/S mice
vaccinated with the GnRHm1-TT/Montanide ISA 51/VSS candidate.
Notably, PABPN1, a ubiquitously expressed protein
that is involved in the polyadenylation of messenger RNA in
eukaryotes, was observed to be present in the serum of SC
115-implanted DD/S mice subjected to various treatments. Mice with
increased anti-PABPN1 antibody titers exhibited higher tumor growth
rates. These findings may be associated with the tumor suppression
mechanisms that operate in the host, which impede the immune
response against tumor antigens (9).
To associate these results with the cellular immune response, the
present study investigated whether the animals that generated
humoral anti-PABPN1 immune response also developed T-cell
recognition of the PABPN1 protein. A T-cell immune response was
revealed to be directed against the PABPN1 protein in mice that had
developed anti-PABPN1 antibodies. The authors of the present study
are currently investigating whether B- and T-cell responses to
PABPN1 actively promote tumor recurrence in the murine SC 115
model, or are markers of other biological processes associated with
tumor recurrence.
In conclusion, the present study has demonstrated
that gradually lowering testosterone to moderate levels using
immunization against GnRH appears to protect against prostate
cancer recurrence, which is in agreement with the rapid and
complete inhibition observed in castration and GnRH analogue
therapy. More detailed and extensive studies are required to
confirm this possibility of immune neutralization of GnRH as an
improved treatment to reduce the rate of tumor recurrence in
patients with androgen-insensitive prostate cancer.
Acknowledgements
The authors would like to thank the Union for
International Cancer Control (Geneva, Switzerland; grant no.,
YY1/09/008/2009) for the fellowship received to support the present
study. The authors would also like to thank the researchers at the
Trev and Joyce Deeley Research Centre (Victoria, Canada), British
Columbia Cancer Research Centre (Vancouver, Canada) and University
of Victoria (Victoria, Canada), particularly Professor Brad Nelson,
Dr Julian Lum and Dr John Webb, for purchasing the mice and
providing the laboratory facilities required to conduct the present
study. The authors would also like to thank Mrs. Angela Lum and
Mrs. Melanie Mawer at the Deeley Research Centre (Vancouver,
Canada) for their contribution, and Mr. Orestes Padrón Yordi (Cuban
Center for Genetic Engineering and Biotechnology, Havana, Cuba),
Ms. Victoria Mazo Gray (University of Victoria; retired) and Mrs.
Jean Howell (University of Victoria; retired) for correcting the
manuscript. The present study was also supported by grants from the
National Research Foundation (Pretoria, South Africa), University
of Pretoria (Pretoria, South Africa) and University of Cape Town
(Cape Town, South Africa).
References
|
1
|
Schröder FH: Prostate cancer around the
world. An overview. Urol Oncol. 28:663–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malvezzi M, Arfé A, Bertuccio P, Levi F,
La Vecchia C and Negri E: European cancer mortality predictions for
the year 2011. Ann Oncol. 22:947–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirano D, Nagane Y, Satoh K, Mochida J,
Sugimoto S, Ichinose T, Takahashi S, Maebayashi T and Saitoh T:
Neoadjuvant LHRH analog plus estramustine phosphate combined with
three-dimensional conformal radiotherapy for intermediate- to
high-risk prostate cancer: A randomized study. Int Urol Nephrol.
42:81–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Labrie F: Keyrole of endocrinology in the
victory against prostate cancer. Bull Cancer. 93:949–958. 2006.(In
French). PubMed/NCBI
|
|
6
|
Junco JA, Peschke P, Zuna I, Ehemann V,
Fuentes F, Bover E, Pimentel E, Basulto R, Reyes O, Calzada L, et
al: Immunotherapy of prostate cancer in a murine model using a
novel GnRH based vaccine candidate. Vaccine. 25:8460–8468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyake H, Nelson C, Rennie PS and Gleave
ME: Testosterone-repressed prostate message-2 is an antiapoptotic
gene involved in progression to androgen independence in prostate
cancer. Cancer Res. 60:170–176. 2000.PubMed/NCBI
|
|
8
|
So AI, Bowden M and Gleave M: Effect of
time of castration and tumour volume on time to
androgen-independent recurrence in Shionogi tumours. BJU Int.
93:845–850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hahn S, Nesslinger NJ, Drapala RJ, Bowden
M, Rennie PS, Pai HH, Ludgate C and Nelson BH: Castration induces
autoantibody and T cell responses that correlate with inferior
outcomes in an androgen-dependent murine tumor model. Int J Cancer.
125:2871–2878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Talwar GP, Raina K, Gupta JC, Ray R,
Wadhwa S and Ali MM: A recombinant
luteinising-hormone-releasing-hormone immunogen bioeffective in
causing prostatic atrophy. Vaccine. 22:3713–3721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Talwar GP, Vyas HK, Purswani S and Gupta
JC: Gonadotropin-releasing hormone/human chorionic gonadotropin
beta based recombinant antibodies and vaccines. J Reprod Immunol.
83:158–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aguilar FF, Barranco JJ, Fuentes EB,
Aguilera LC, Sáez YL, Santana MD, Vázquez EP, Baker RB, Acosta OR,
Pérez HG and Nieto GG: Very small size proteoliposomes (VSSP) and
Montanide combination enhance the humoral immuno response in a GnRH
based vaccine directed to prostate cancer. Vaccine. 30:6595–6599.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferro VA and Stimson WH: Investigation
into suitable carrier molecules for use in an anti-gonadotrophin
releasing hormone vaccine. Vaccine. 16:1095–1102. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko EC, Wang X and Ferrone S: Immunotherapy
of malignant diseases. Challenges and strategies. Int Arch Allergy
Immunol. 132:294–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lage A, Perez R and Fernandez LE:
Therapeutic cancer vaccines: At midway between immunology and
pharmacology. Curr Cancer Drug Targets. 5:611–627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clements CJ and Griffiths E: The global
impact of vaccines containing aluminium adjuvants. Vaccine.
20(Suppl 3): S24–S33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lell B, Agnandji S, von Glasenapp I,
Haertle S, Oyakhiromen S, Issifou S, Vekemans J, Leach A, Lievens
M, Dubois MC, et al: A randomized trial assessing the safety and
immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine
candidates in children in Gabon. PLoS One. 4:e76112009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giannini SL, Hanon E, Moris P, Van
Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J,
Losonksy G, et al: Enhanced humoral and memory B cellular immunity
using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium
salt combination (AS04) compared to aluminium salt only. Vaccine.
24:5937–5949. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Didierlaurent AM, Morel S, Lockman L,
Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O,
Vanderheyde N, Schiavetti F, et al: AS04, an aluminum salt- and
TLR4 agonist-based adjuvant system, induces a transient localized
innate immune response leading to enhanced adaptive immunity. J
Immunol. 183:6186–6197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwarz TF: Clinical update of the
AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine,
Cervarix. Adv Ther. 26:983–998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rennie PS, Bruchovsky N, Buttyan R, Benson
M and Cheng H: Gene expression during the early phases of
regression of the androgen-dependent Shionogi mouse mammary
carcinoma. Cancer Res. 48:6309–6312. 1988.PubMed/NCBI
|
|
22
|
Nesslinger NJ, Sahota RA, Stone B, Johnson
K, Chima N, King C, Rasmussen D, Bishop D, Rennie PS, Gleave M, et
al: Standard treatments induce antigen-specific immune responses in
prostate cancer. Clin Cancer Res. 13:1493–1502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nesslinger NJ, Ng A, Tsang KY, Ferrara T,
Schlom J, Gulley JL and Nelson BH: A viral vaccine encoding
prostate-specific antigen induces antigen spreading to a common set
of self-proteins in prostate cancer patients. Clin Cancer Res.
16:4046–4056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carr A, Mazorra Z, Alonso DF, Mesa C,
Valiente O, Gomez DE, Perez R and Fernandez LE: A purified GM3
ganglioside conjugated vaccine induces specific, adjuvant-dependent
and non-transient antitumour activity against B16 mouse melanoma in
vitro and in vivo. Melanoma Res. 11:219–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matzinger P: Tolerance, danger, and the
extended family. Annu Rev Immunol. 12:991–1045. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ambrosino DM, Bolon D, Collard H, Van
Etten R, Kanchana MV and Finberg RW: Effect of Haemophilus
influenzae polysaccharide outer membrane protein complex
conjugate vaccine on macrophages. J Immunol. 149:3978–3983.
1992.PubMed/NCBI
|
|
27
|
Udono H and Srivastava PK: Heat shock
protein 70-associated peptides elicit specific cancer immunity. J
Exp Med. 178:1391–1396. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andersen BM: Endotoxin release from
Neisseria meningitidis. Relationship between key bacterial
characteristics and meningococcal disease. Scand J Infect Dis
Suppl. 64(S64): 1–43. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zollinger WD: New and improved vaccines
against meningococal disease. New Generation Vaccines. Woodrow GC
and Levine MM: Marcel Dekker, Inc. (New York, NY). 325–348.
1990.
|