Introduction
Extranodal natural killer (NK)/T-cell lymphoma
(ENKTL), nasal type, is relatively more common in Asia and Latin
America compared with Western countries (1,2), and
accounts for 5–10% of all malignant lymphomas in China (3). ENKTL is characterized by
angiodestruction, obvious necrosis and association with the
Epstein-Barr virus (EBV). Considering its poor prognosis, extensive
clinical and pathological research has been conducted to
investigate possible prognostic markers in ENKTL. Clinically, two
major clinical prognostic models are applied in NK/T-cell lymphoma:
The International Prognostic Index (IPI) and the Korean Prognostic
Index (KPI). The IPI has been widely used for predicting prognosis
and selecting therapeutic strategies in patients with aggressive
non-Hodgkin's lymphoma. However, the IPI has not been approved for
use in ENKTL, as almost 60% of patients with ENKTL belong to the
low IPI risk group (score, 0–1), in which significant heterogeneity
exists. The KPI was developed in the era of anthracycline-based
chemotherapy and appears to be more useful than IPI for predicting
ENKTL prognosis (4,5). However, the prognostic value of KPI
could not be repeated in certain studies, particularly in the era
of asparaginase-based chemotherapy (6), suggesting that both the IPI and KPI
scoring systems should be further improved. These prognostic models
are also primarily based on pretreatment clinical
characteristics.
Numerous studies have demonstrated that histological
vascular invasion is associated with poor prognosis for various
types of solid tumor, such as thyroid carcinoma (7), nodular melanoma (8), colorectal cancer (9), gastric carcinoma (10), hepatocellular carcinoma (11), renal cell carcinoma (12) and breast cancer (13). However, the prognostic significance of
vascular invasion in ENKTL is unclear. ENKTL is a distinct and
heterogeneous histopathological subtype of non-Hodgkin's lymphoma
that shares the following characteristics with solid tumors: i)
Originates from local tissue (nasal cavity, skin or
gastrointestinal tract) outside the lymph nodes that contains
numerous blood vessels; and ii) as the disease progresses, lesions
spread to surrounding lymph nodes, and migrate to distant tissues
and organs. Based on the aforementioned characteristics, we
hypothesize that histological vascular invasion by the tumor is a
risk factor for disease progression and distant metastasis in
ENKTL. In the present study, the vascular invasion status of the
tumor in patients with untreated ENKTL was retrospectively examined
to investigate its association with clinical features, treatment
response and prognosis.
Patients and methods
Ethics statement
Written informed consent was obtained from all
patients for the use of patient tissue samples and other medical
information to be stored in our hospital database. The current
study was performed in accordance with the Declaration of Helsinki
and the institutional guidelines of the ethics committee of Sun
Yat-Sen University Cancer Center (Guangzhou, China). The study was
approved by the Institutional Review Board of the National Cancer
Institute and the ethics committees of Sun Yat-Sen University
Cancer Center.
Patient selection
A total of 214 patients with histologically
diagnosed ENKTL, nasal type, were selected for inclusion in the
present study between June 2002 and July 2013 at the Sun Yat-Sen
University Cancer Center. Patient selection was based on the
following criteria: i) Histologically confirmed diagnosis of ENKTL;
ii) NK/T-cell type proven by immunophenotypes and EBV status; iii)
no previous malignant tumor or second primary tumor; iv) previously
untreated; and v) adequate clinical information and follow-up data.
The primary site of the tumor was classified into two subtypes: i)
Upper aerodigestive tract ENKTL (UNKTL), including nasal cavity,
nasopharynx, paranasal sinuses, tonsils, hypopharynx and larynx;
and ii) extra UNKTL (EUNKTL), including all sites excluding the
upper aerodigestive tract (14).
Patients with primary lesions within the nasal cavity and with
secondary spread to other organs were also categorized as
UNKTL.
Clinical data of patients were obtained from the
hospital discharge database, mortality registry and electronic
medical records, which contained the following information: Patient
demographics, physical examination, Eastern Cooperative Oncology
Group (ECOG) performance status (PS) (15), B symptoms (including unexplained fever
with temperature >38°C, night sweating or weight loss of >10%
within 6 months), primary tumor site, involved sites, involvement
of regional lymph nodes, serum lactate dehydrogenase (LDH), serum
β2 microglobulin (β2 M), serum D-dimer (D-D), Ann Arbor stage
(16), IPI (age, stage, LDH,
extranodal sites and PS) and KPI (adverse factors: Stage >2,
>normal LDH, presence of B symptoms and involvement of regional
lymph nodes).
Pathological evaluation
Vascular invasion
Patient pathological records and original
histopathological slides were independently reviewed by two
pathologists (Professors Xin-Ke Zhang and Wan-Ming Hu) with
experience in lymphoma pathology. The pathologists were blinded to
the pathological diagnoses and outcome data. Discrepancies were
resolved by mutual consensus following simultaneous re-examination
of the slides by both pathologists using a BX41 double-headed
microscope (Olympus corporation, Tokyo, Japan). To assess vascular
invasion, 4-µm thick sections of paraffin-embedded tissues were
cut, placed on slides, deparaffinized in xylene, hydrated in a
graded alcohol series and hematoxylin and eosin-stained was
performed (C0105, Beyotime Insititute of Biotechnology, Inc.,
Shanghai, China). A mean of 4 (range, 2–8) conventional
histopathological sections per tumor sample were available for
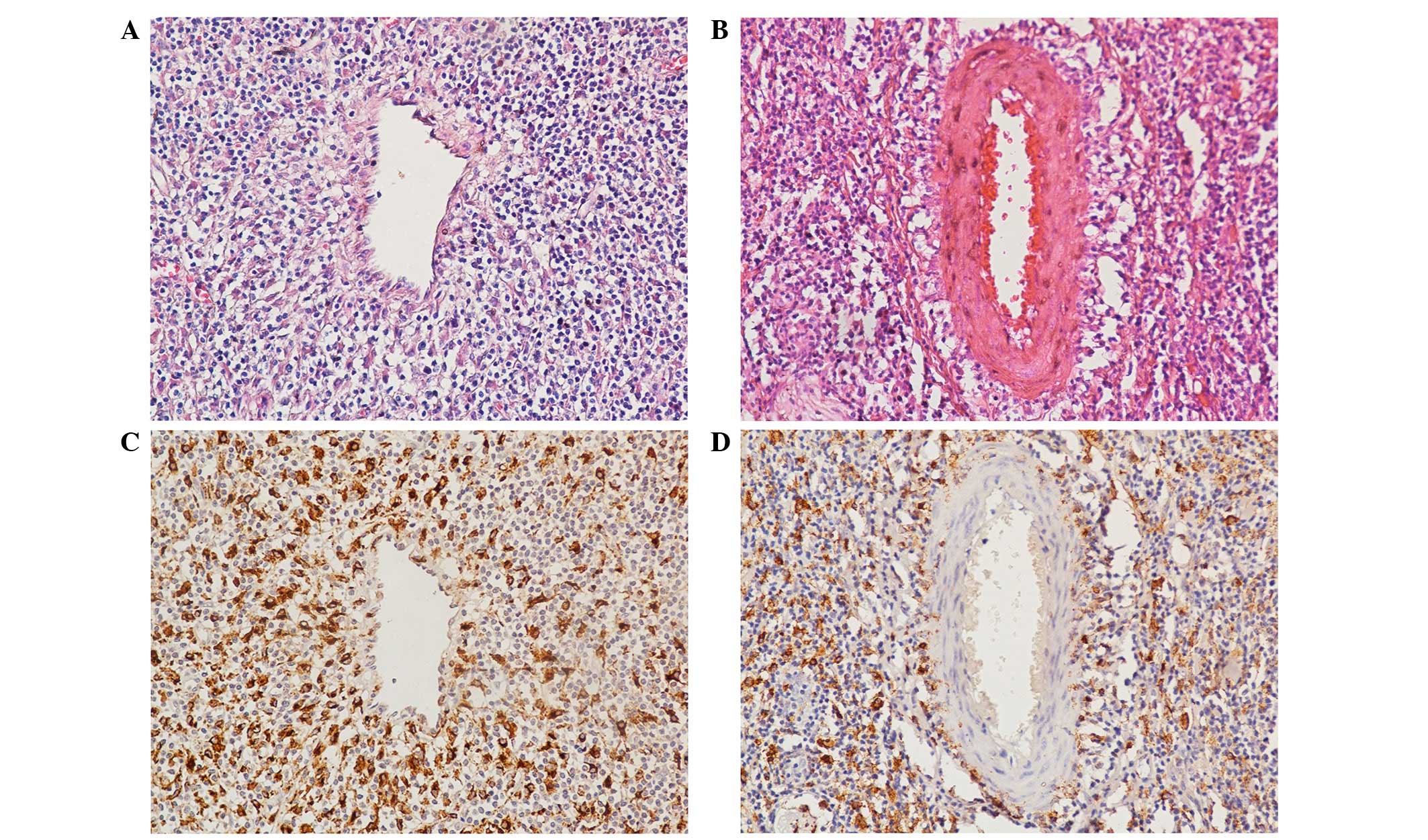
evaluation. Vascular invasion was defined as infiltration of vessel
walls or the existence of tumor emboli (Fig. 1A). Any equivocal focuses, in which
tumor cells merely encroached on a vascular lumen, were considered
negative (Fig. 1B).
Tumor-associated macrophages (TAMs)
Immunohistochemical staining with a polyclonal
antibody against cluster of differentiation (CD)68 was performed on
4-µm-thick paraffin-embedded sections to identify and quantify TAMs
in ENKTL. CD68 was detected using a rabbit polyclonal antibody (cat
no. 11192-RP02; dilution, 1:1,000; final concentration, 1.08 µg/ml;
Sino Biological, Inc., Beijing, China). The secondary antibody used
was goat anti-rabbit IgG (polyclonal antibody (cat no. SSA018, Sino
Biological, Inc.; 1:1,000 dilution). The staining protocol and TAM
assessment were conducted according to previously described methods
(17). TAMs were detected in 71 cases
and, using a cut-off value of 56 TAMs per sample, 28 cases showed
high numbers of TAMs (Fig. 1C and D).
Sections were assessed on a BX41 microscope (Olympus
corporation).
Treatment and response evaluation
Patients received the following treatment
strategies: i) Patients with early-stage ENKTL received
chemotherapy followed by involved-field radiotherapy (IFRT); and
ii) patients with advanced stage ENKTL received chemotherapy alone.
The chemotherapy regimens were as follows: i) CHOP
(cyclophosphamide, doxorubicin, vincristine and prednisone) or
CHOP-like therapy (18); ii)
alternating triple therapy (ATT), consisting of CHOP-B
(cyclophosphamide 750 mg/m2, adriamycin 50
mg/m2, vincristine 1.4 mg/m2 and bleomycin 5
mg/m2;intravenously infused on day 1, prednisolone 100
mg was administered orally on days 1–5), IMVP-16 (ifosfamide,
methotrexate and etoposide) (19) and
DHAP (dexamethasone, cytarabine and cisplatin) (20); iii) EPOCH (etoposide, doxorubicin,
vincristine, cyclophosphamide and prednisone) (21); and iv) GELOX (gemcitabine, oxaliplatin
and L-asparaginase) (21). Following
a minimum of 2 cycles of chemotherapy, patients received IFRT. IFRT
of 36–60 Gy was delivered in daily fractions of 1.8–2.0 Gy (5
fractions per week). Computed tomography (CT) or
18F-fluorodeoxyglucose positron emission tomography/CT were
performed to investigate the curative effect every 2 courses of
chemotherapy. Routine follow-up imaging analyses, as well as
hematology and biochemical blood serum tests, were performed every
3 months for the first 2 years, then every 6 months for the next 3
years, and annually thereafter or when clinically indicated.
Statistical analysis
Treatment response was assessed according to the
International Working Group recommendations for response criteria
for non-Hodgkin's lymphoma (22).
Overall survival (OS) was measured from the date of diagnosis to
the date of mortality or last follow-up visit. Progression-free
survival (PFS) was calculated from the date of diagnosis to the
date of disease progression, relapse, mortality or last follow-up
visit. Local relapse-free survival (LRFS) was calculated from the
start of treatment to the date of locoregional relapse in patients
that responded completely. Distant metastasis-free survival (DMFS)
was calculated from the start of treatment to the date of distant
metastasis relapse in patients that responded completely. The
correlation between vascular invasion and clinicopathological
features was evaluated using the χ2 test. The
Kaplan-Meier method was used to calculate the probability of
survival and survival curves were compared by the log-rank test.
Univariate and multivariate analyses were performed using the Cox
proportional-hazards model. Two-sided P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS software (version 19.0; IBM SPSS,
Chicago, IL, USA).
Results
Patient characteristics
The clinical characteristics of the 214 patients are
summarized in Table I. The median age
was 41 years, with a range of 17–89 years. The majority of patients
were aged <60 years and the male:female ratio was 2:1. In
addition, 201 patients (93.9%) had a good PS (ECOG 0–1). The
majority of the patients initially presented with UNKTL tumors
(n=169, 79.0%) and localized diseases (stage I/II; n=164, 76.6%).
In EUNKTL patients (n=45, 21.0%), the primary lesion sites included
the small bowel, colon, lungs, skin, testes and soft tissues. Half
of the patients presented with B symptoms (n=106, 49.5%). A total
of 87 patients had elevated serum LDH levels. Serum β2-M was
detected in 123 cases; 74 of which were found be higher than normal
level. In 106 cases examined, 30 patients had increasing serum
D-dimer level. Furthermore, over half of the patients were
classified in the low-risk group according to the IPI (n=134,
62.6%) or KPI (n=109, 50.9%) scores.
 | Table I.Clinical characteristics according to
the presentation of vascular invasion at diagnosis. |
Table I.
Clinical characteristics according to
the presentation of vascular invasion at diagnosis.
|
| Patients, n
(%) |
|
|---|
|
|
|
|
|---|
| Characteristic | Vascular invasion
(n=70) | No vascular
invasion (n=144) | P-value |
|---|
| Age at diagnosis,
years |
|
| 0.075 |
|
≤60 | 66 (94.3) | 124 (86.1) |
|
|
>60 | 4 (5.7) | 20 (13.9) |
|
| Gender |
|
| 0.431 |
|
Male | 49 (70.0) | 93 (64.6) |
|
|
Female | 21 (30.0) | 51 (35.4) |
|
| ECOG PS |
|
| 0.011 |
|
0,1 | 61 (87.1) | 140 (97.2) |
|
| ≥2 | 9 (12.9) | 4 (2.8) |
|
| Subtype |
|
| 0.003 |
|
UNKTL | 47 (67.1) | 122 (84.7) |
|
|
EUNKTL | 23 (32.9) | 21 (15.3) |
|
| B-symptoms | 43 (61.4) | 63 (43.8) | 0.015 |
| Extranodal sites
≥2 | 48 (68.6) | 74 (51.4) | 0.017 |
| Regional
lymphadenopathy | 44 (62.9) | 78 (54.2) | 0.228 |
| Elevated serum
LDH | 37 (52.9) | 50 (34.7) | 0.011 |
| Elevated serum β2
Ma |
|
| 0.331 |
|
Yes | 29 (65.9) | 45 (57.0) |
|
| No | 15 (34.1) | 34 (43.0) |
|
| Elevated serum
D-dimerb |
|
| 0.003 |
|
Yes | 14 (50.0) | 16 (20.5) |
|
| No | 14 (50.0) | 62 (79.5) |
|
| CD68+
TAMsc |
|
High | 15 (40.0) | 13 (28.3) | 0.009 |
|
Low | 10 (60.0) | 33 (71.7) |
|
| Ann Arbor
stage |
|
| 0.001 |
|
I/II | 44 (62.9) | 120 (83.3) |
|
|
III/IV | 26 (37.1) | 24 (16.7) |
|
| IPI score |
|
| 0.079 |
|
0/1 | 38 (54.3) | 96 (66.7) |
|
| ≥2 | 32 (45.7) | 48 (33.3) |
|
| KPI score |
|
| 0.002 |
|
0/1 | 25 (35.7) | 84 (58.3) |
|
| ≥2 | 45 (64.3) | 60 (41.7) |
|
| Treatment |
|
| 0.199 |
|
Chemotherapy alone | 26 (37.1) | 41 (28.5) |
|
|
Chemotherapy +
radiotherapy | 44 (62.9) | 103 (71.5) |
|
| Chemotherapy
regimens |
|
| 0.153 |
|
CHOP | 18 (25.7) | 25.0 (17.4) |
|
|
ATT | 16 (22.9) | 40.0 (27.8) |
|
|
EPOCH | 21 (30.0) | 43.0 (29.8) |
|
|
GELOX | 15 (21.4) | 36.0 (25.0) |
|
Association between vascular invasion
and clinicopathological characteristics
Vascular invasion was observed in 70 patients
(32.7%). The majority of these patients were aged <60 years old,
excluding only 4 patients, and greater than two-thirds were male
(n=49, 70.0%). In addition, 45 (64.3%) and 32 (45.7%) patients in
the vascular invasion group were classified in the
high-intermediate and high-risk categories, respectively, based on
the KPI score and IPI score. Table I
indicates the association between vascular invasion and
clinicopathological characteristics. In brief, vascular invasion
was significantly associated with ECOG PS≥2 (P=0.011), EUNKTL
(P=0.003), B symptoms (P=0.015), ≥2 extranodal sites (P=0.017),
elevated serum LDH (P=0.011), elevated serum D-D (P=0.003),
increased numbers of CD68+ TAMs (P=0.009), stage III/IV
(P=0.001) and KPI score ≥2 (P=0.002). However, age, gender,
regional lymphadenopathy and elevated serum β2 M exhibited no
significant correlation with vascular invasion (Table I).
Treatment outcome and response
rate
Chemotherapy followed by IFRT was administered to
147 patients, while 67 patients received chemotherapy alone. No
statistical difference in vascular invasion at diagnosis was
identified between the different treatment modalities (P=0.199) or
chemotherapy regimens (P=0.153) (Table
I). The treatment response was evaluated in each patient.
Table II shows that 96 patients
(44.9%) achieved complete remission (CR) and 76 patients (35.5%)
achieved partial response following chemotherapy; thus, the overall
response rate (ORR) was 80.4% following chemotherapy. For the
non-vascular invasion group, CR and ORR rates following
chemotherapy were significantly higher than those in the vascular
invasion group (52.8 vs. 28.6%, P=0.001 for CR; 88.9 vs. 62.9%,
P<0.001 for ORR). At the end of treatment, the CR and ORR rates
for the non-vascular invasion group were also significantly higher
than those in the vascular invasion group (69.4 vs. 40.0%,
P<0.001 for CR; 89.6 vs. 62.9%, P<0.001 for ORR). The median
follow-up time was 26 months (range, 2–141 months), and the 5-year
OS and PFS rates were 63.5% [95% confidence interval (CI),
56.1–70.9%] and 47.5% (95% CI, 38.7–56.3%), respectively. Vascular
invasion was significantly associated with OS and PFS. The 5-year
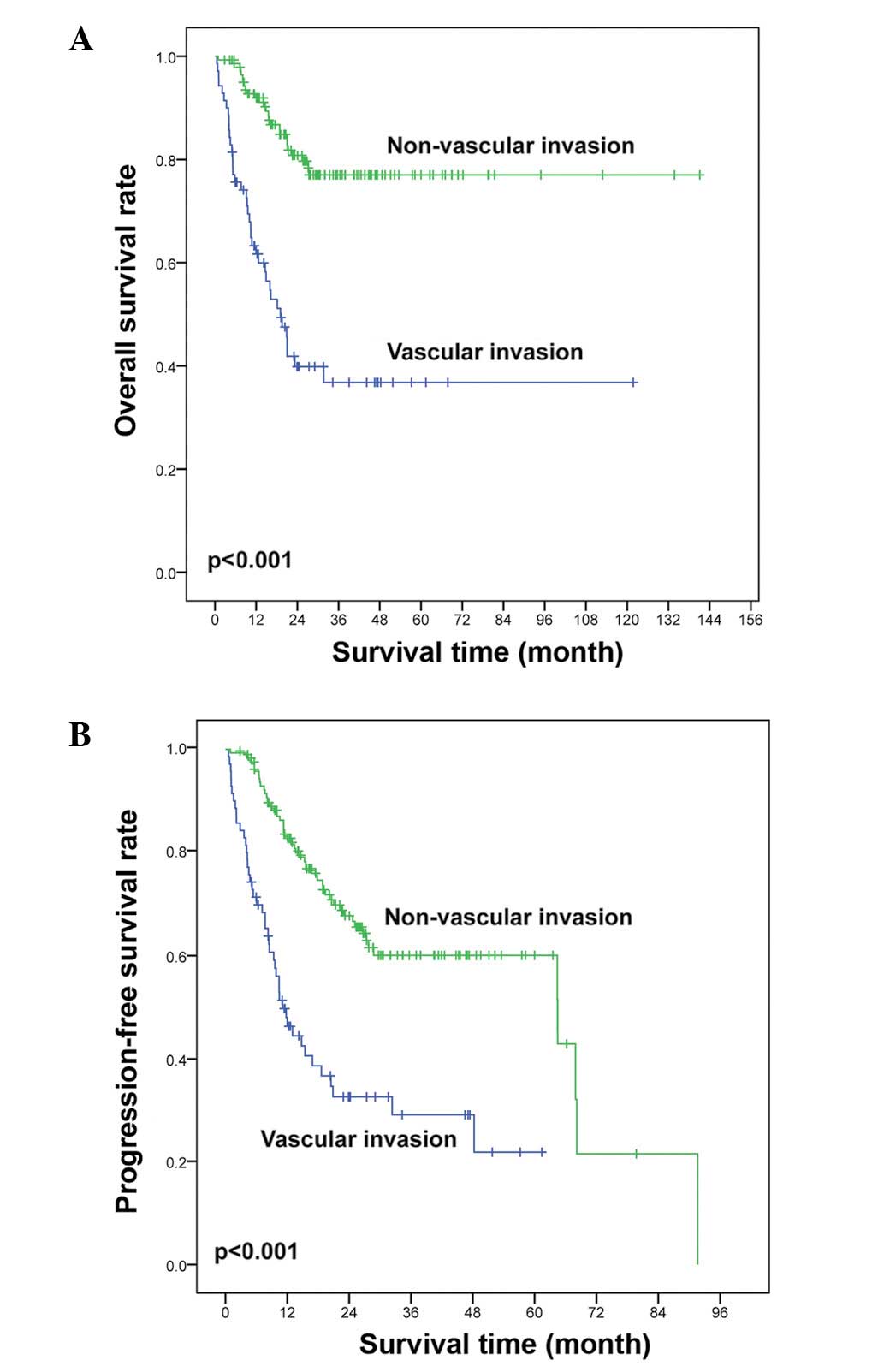
OS (36.8 vs. 77.0%, P<0.001; Fig.
2A) and PFS (21.8 vs. 60.1%, P<0.001; Fig. 2B) rates were lower in the vascular
invasion group compared with the non-vascular invasion group
(Table II).
 | Table II.Treatment outcomes according to the
presentation of vascular invasion. |
Table II.
Treatment outcomes according to the
presentation of vascular invasion.
| Vascular
invasion | PR after CT
(%) | P | CR after CT, n
(%) | P | ORR after CT, n
(%) | P | CR at end, n
(%) | P | ORR at end, n
(%) | P | 5-year PFS rate,
% | P | 5-year OS rate,
% | P |
|---|
|
Presenta | 24 (34.3) | 0.879 | 20 (28.6) | 0.001 | 44
(62.9) | <0.001 | 28
(40.0) | <0.001 | 44
(62.9) | <0.001 | 21.8 | <0.001 | 36.8 | <0.001 |
| Absentb | 52 (36.1) |
| 76 (52.8) |
| 128 (88.9) |
| 100 (69.4) |
| 129 (89.6) |
| 60.1 |
| 77.0 |
|
| Total | 76 (35.5) |
| 96 (44.9) |
| 172 (80.4) |
| 128 (59.8) |
| 173 (80.8) |
| 47.5 |
| 63.5 |
|
In stage I/II patients (n=164), CR rates were
significantly higher for the non-vascular invasion group than those
for the vascular invasion group following chemotherapy alone (58.3
vs. 40.9%, P=0.047). The non-vascular invasion group also had a
higher CR rate than the vascular invasion group at the end of
treatment (76.7 vs. 59.1%), however, no statistical difference
existed (P=0.152). In stage I/II patients, vascular invasion was
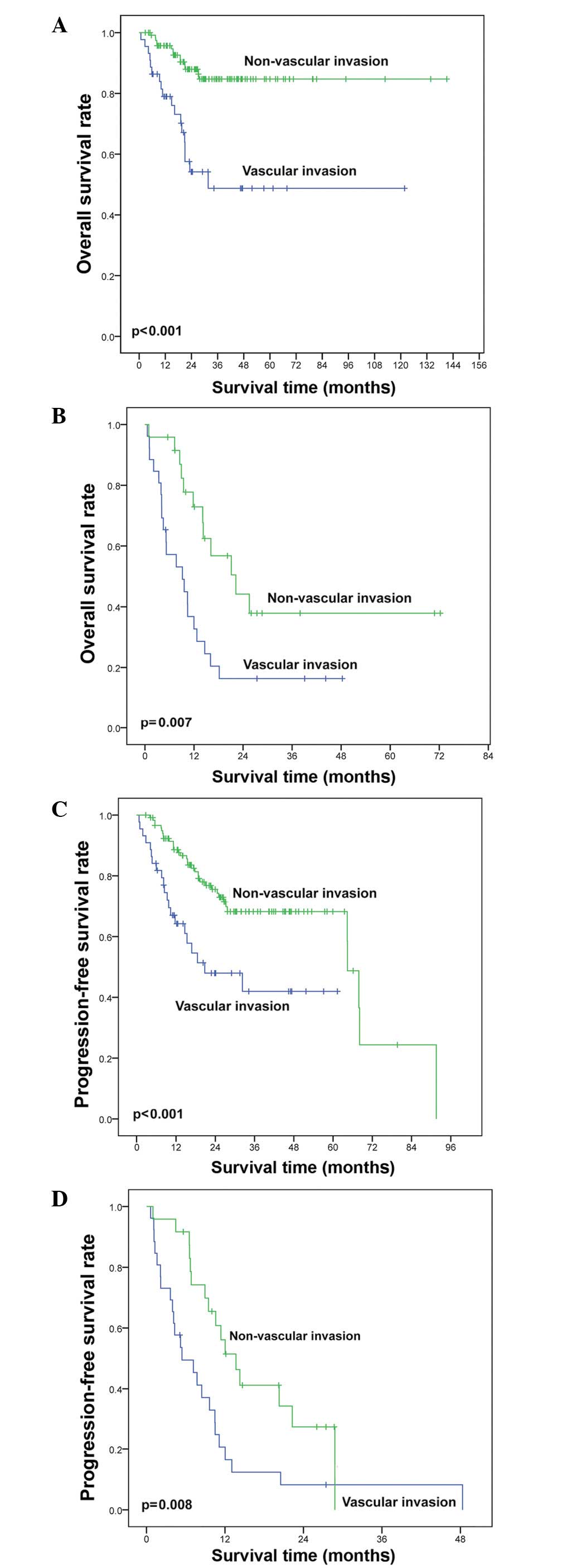
associated with shorter OS (48.7 vs. 87.9%, P<0.001; Fig. 3A) rates compared with non-vascular
invasion. In stage III/IV patients (n=50), vascular invasion at
diagnosis was also associated with inferior OS (Fig. 3B). Similarly, vascular invasion was
associated with shorter PFS rates in stage I/II (42.0 vs. 68.2%,
P<0.001; Fig. 3C) and stage III/IV
(Fig. 3D) patients compared with
non-vascular invasion (Table
III).
 | Table III.Treatment outcome and response rate
for stage I/II patients. |
Table III.
Treatment outcome and response rate
for stage I/II patients.
| Vascular
invasion | CR after CT, n
(%) | P-value | CR at the end of
treatment, n (%) | P-value | 5-year PFS rate,
% | P-value | 5-year OS rate,
% | P-value |
|---|
| Present (n=44) | 18 (40.9) | 0.047 | 26
(59.1) | 0.152 | 42.0 | <0.001 | 48.7 | <0.001 |
| Absent (n=120) | 70 (58.3) |
| 92
(76.7) |
| 68.2 |
| 87.9 |
|
| Total (n=164) | 88 (53.7) |
| 118 (72.0) |
| 61.3 |
| 75.0 |
|
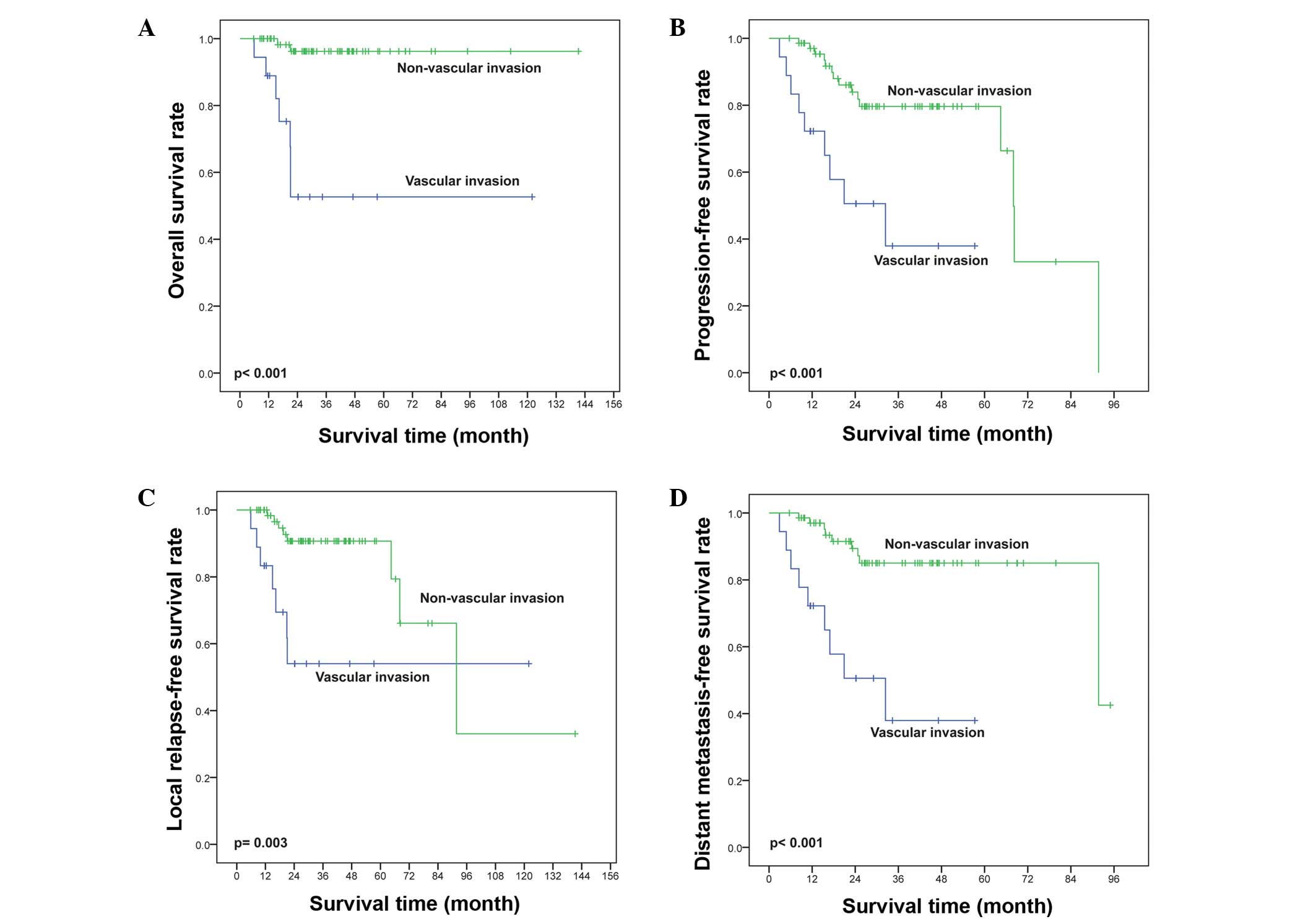
In stage I/II patients that achieved CR at the end
of treatment (n=118), vascular invasion resulted in a high distant
metastatic relapse (DMR) rate (42.3 vs. 15.2%, P=0.035) but did not
significantly effect the locoregional relapse (LR) rate (15.4 vs.
10.9%, P=0.087) or the simultaneous DMR and LR rate (11.5 vs. 3.3%,
P=0.344; Table IV). The OS and PFS
were reduced in patients with vascular invasion compared with those
without vascular invasion (P<0.001 for OS, Fig. 4A; P=0.001 for PFS, Fig. 4B). The LRFS and DMFS were
significantly shorter for patients with vascular invasion compared
with those without vascular invasion (P=0.015, Fig. 4C and P<0.001, Fig. 4D, respectively). CR was achieved in 88
stage I/II patients following chemotherapy alone. The OS (52.6 vs.
96.2%, P<0.001; Fig. 5A), PFS
(37.9 vs. 79.6%, P<0.001; Fig.
5B), LRFS (54.0 vs. 90.7%, P=0.003; Fig. 5C), and DMFS (37.9 vs. 85.0%,
P<0.001; Fig. 5D) of patients with
vascular invasion were all significantly shorter than those without
vascular invasion.
 | Table IV.Relapse rate for stage I/II patients
that achieved CR at the end of treatment. |
Table IV.
Relapse rate for stage I/II patients
that achieved CR at the end of treatment.
| Vascular
invasion | LR rate, n (%) | P-value | DMR rate, n
(%) | P-value | LR and DMR rate, n
(%) | P-value |
|---|
| Present (n=26) | 4
(15.4) | 0.087 | 11 (42.3) | 0.035 | 3
(11.5) | 0.344 |
| Absent (n=92) | 10 (10.9) |
| 14 (15.2) |
| 3 (3.3) |
|
| Total (n=118) | 14 (11.9) |
| 25 (21.2) |
| 6 (5.1) |
|
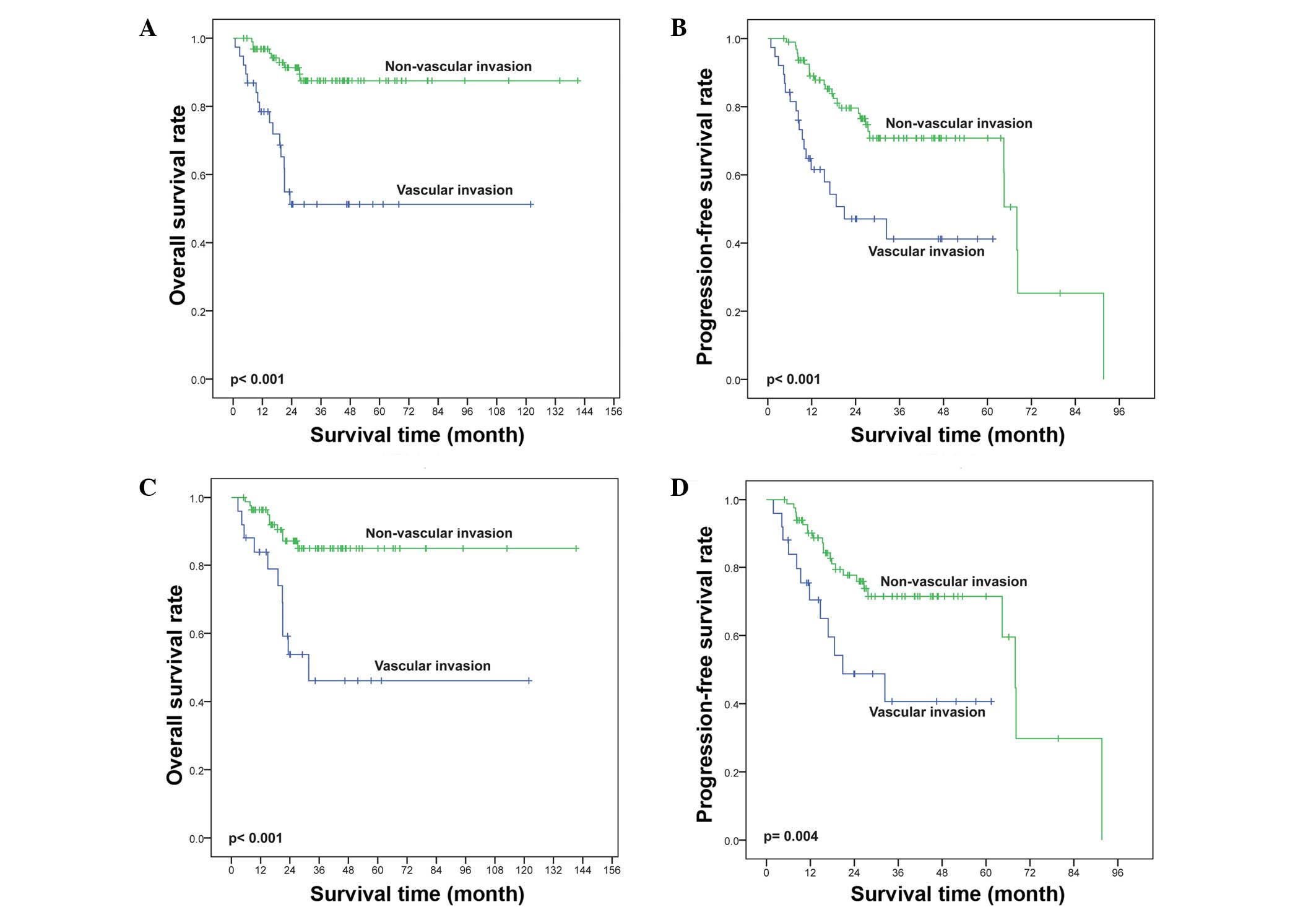
The effects of vascular invasion on OS and PFS in
patients with IPI 0–1 (n=134) were also verified in subgroup
analysis. IPI 0–1 patients were further categorized into different
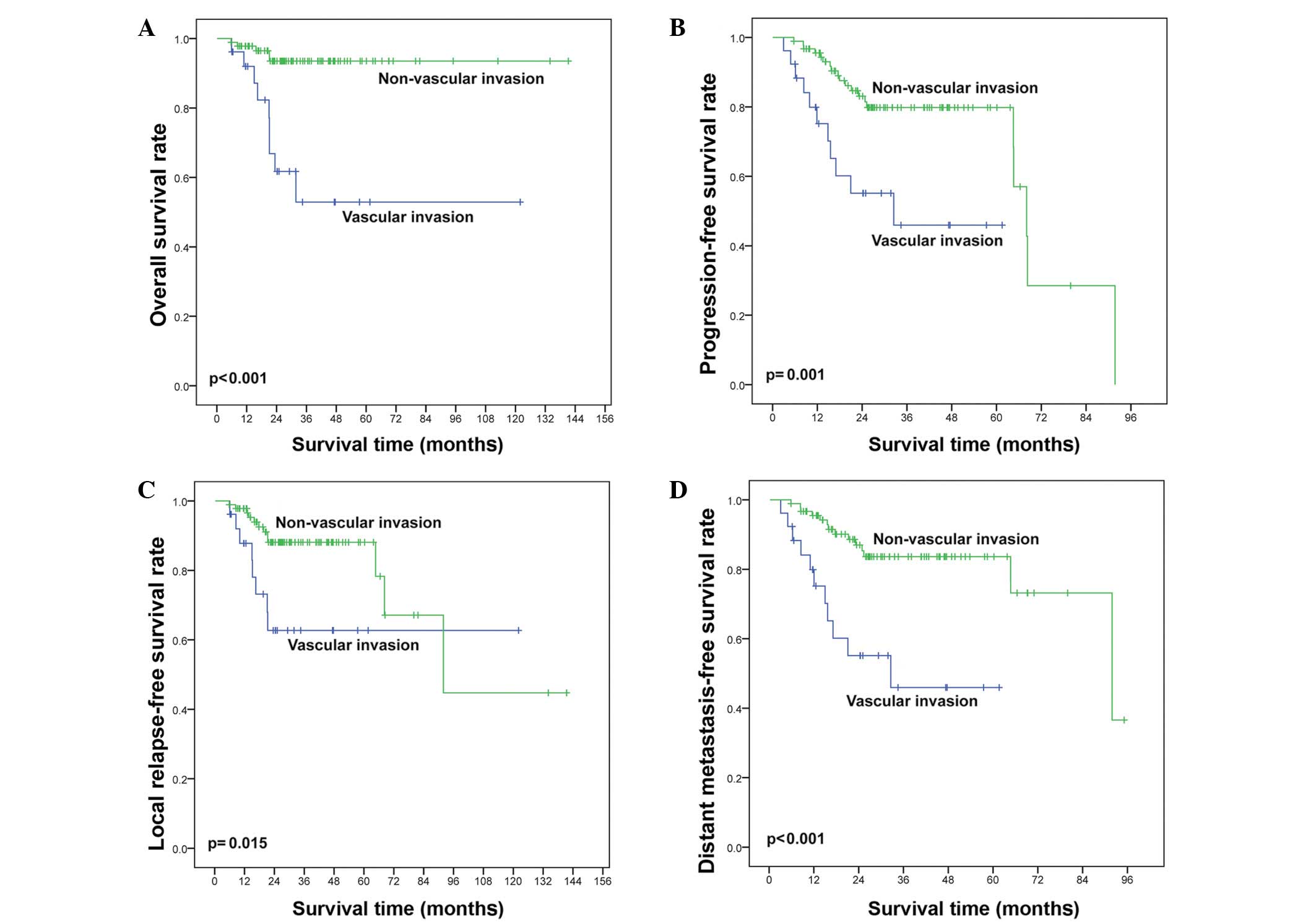
prognostic groups by vascular invasion. Vascular invasion was
significantly associated with shorter OS (P<0.001; Fig. 6A) and PFS (P<0.001; Fig. 6B). Similarly, in the KPI 0–1 subgroup
patients (n=109), vascular invasion at diagnosis was significantly
associated with inferior OS (P<0.001; Fig. 6C) and PFS (P=0.004; Fig. 6D).
Univariate and multivariate
analyses
Univariate analysis revealed that ECOG PS >2,
EUNKTL, B symptoms, ≥2 extranodal sites, regional lymphadenopathy,
elevated serum LDH, vascular invasion, advanced stage (III/IV) and
CR after CT could significantly predict shorter OS and PFS.
Clinical factors that were statistically significant predictors of
OS and PFS (P<0.05) were included in the multivariate analysis.
IPI and KPI values were not included in the univariate and
multivariate analyses due to their overlap with several other
clinical variables. Multivariate analysis revealed that vascular
invasion, stage III/IV and CR after chemotherapy were independent
prognostic factors for OS (P<0.001, P=0.016 and P=0.002,
respectively) and PFS (P<0.001, P=0.019 and P=0.005,
respectively) (Table V).
 | Table V.Results of univariate and
multivariate analyses of prognostic factors for PFS and OS in
patients with ENKTL. |
Table V.
Results of univariate and
multivariate analyses of prognostic factors for PFS and OS in
patients with ENKTL.
|
| PFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, >60
years | 0.865 | 0.652 |
|
| 1.623 | 0.159 |
|
|
| Gender, male | 1.222 | 0.347 |
|
| 1.101 | 0.711 |
|
|
| ECOG PS ≥2 | 5.816 | <0.001 |
|
| 5.300 | <0.001 |
|
|
| Subtype,
EUNKTL | 2.614 | <0.001 |
|
| 2.727 | <0.001 |
|
|
| B symptoms | 2.415 | <0.001 |
|
| 2.427 | 0.001 |
|
|
| ≥2 extranodal
sites | 2.532 | <0.001 |
|
| 2.577 | 0.001 |
|
|
| Regional
lymphadenopathy | 2.618 | <0.001 |
|
| 2.398 | 0.002 |
|
|
| Elevated serum
LDH | 2.803 | <0.001 |
|
| 3.225 | <0.001 |
|
|
| Vascular
invasion | 3.125 | <0.001 | 2.141
(1.354–3.347) | <0.001 | 4.274 | <0.001 | 3.042
(1.743–5.162) | <0.001 |
| Stage III/IV | 4.895 | <0.001 | 2.032
(1.089–3.731) | 0.019 | 5.434 | <0.001 | 2.411
(1.141–5.493) | 0.016 |
| CR after CT | 0.293 | <0.001 | 2.043
(1.192–3.328) | 0.005 | 0.206 | <0.001 | 2.768
(1.265–5.412) | 0.002 |
Discussion
ENKTL is an aggressive type of non-Hodgkin's
lymphoma that is characterized by poor survival. Pathological and
molecular markers for predicting the outcome of ENKTL have yet to
be established, although Ki-67 proliferation rate was correlated
recently with shorter disease-free survival and OS (P<0.05)
(23). Loss of granzyme B protease
inhibitor, cyclooxygenase-2 expression and decreased quantity of
tumor-infiltrating forkhead box P3-positive regulatory T-cells have
also been associated with poor prognosis of ENKTL, nasal type or
UNKTL (24–26). Furthermore, tumor cell nuclear
diameter and CD30 expression may be potential prognostic parameters
in patients with ENKTL, nasal type (27). Vascular invasion is an associated
prognostic factor for various types of malignant tumor. For
example, Cuadra-Garcia et al reported that easily
recognizable vascular invasion and occlusion by tumor cells exists
in 20% (12/58) of patients with ENKTL (28). However, reports regarding the
association between histological vascular invasion and ENKTL
prognosis are limited (29).
In the current study, the histological vascular
invasion status was investigated in tumor samples from 214 patients
with untreated ENKTL. The present study is the first to report on
the prognostic role of vascular invasion in hematopoietic
malignancies. The results showed a significant difference in
clinical behavior between the vascular invasion and non-vascular
invasion groups. Patients with vascular invasion had more adverse
clinical features, such as poor PS, B symptoms, bulky disease and
advanced stage. Notable among these features was elevated serum
D-D. Blood vessels were compressed and partially filled by tumor
cells during tumor cell invasion. Under these circumstances,
thrombosis is more likely to occur. Consequently, serum D-D levels
were markedly higher in ENKTL tumor samples with histological
vascular invasion than in those without vascular invasion. Wróbel
et al identified that elevated serum D-D levels are
associated with poor prognosis in non-Hodgkin's lymphoma (30). Similarly, vascular invasion was
associated with poor responses to chemotherapy in the present
study. According to the Cox regression model, which included ECOG
PS, B symptoms, local tumor invasion, elevated serum LDH, advanced
stage (III/IV), histological vascular invasion and CR after CT,
vascular invasion was an independent prognostic factor for both OS
and PFS.
In the present study, the CR and ORR rates of the
vascular invasion group were significantly lower following
chemotherapy and at the end of treatment. Patients with vascular
invasion have a distinctive tumor microenvironment with an elevated
number of TAMs (31). Numerous
studies have indicated that TAMs produce a vast diversity of growth
factors, including proteolytic enzymes, pro-angiogenic cytokines
and inflammatory mediators, which not only directly stimulate tumor
cell growth and/or facilitate tumor metastatic invasion but also
induce immune suppression of host defenses against tumors (32,33). We
propose that the aforementioned factors may be attributed to the
responses to radiation and chemotherapy.
In the present study, survival analysis indicated
that the 5-year OS and PFS rates in the vascular invasion group
were significantly lower than those in the non-vascular invasion
group (36.8 vs. 77.0% for OS, P<0.001; 21.8 vs. 60.1% for PFS,
P<0.001). Further analysis identified that, following treatment,
stage I/II UNKTL patients with histological vascular invasion were
significantly more likely to metastasize distally than patients
without vascular invasion (DMR rate: 42.3 vs. 15.2%, P=0.035).
Notably, the DMFS of patients with vascular invasion was inferior
to that of non-vascular invasion patients (37.9 vs. 85.0%,
P<0.001). Several large retrospective studies have shown that
radiotherapy is an important treatment modality for ENKTL (34–37).
Radiotherapy is beneficial in controlling local lesions but may
lead to distant dissemination. Although chemotherapy combined with
radiotherapy can reduce the risk of recurrence, distant metastasis
still commonly occurs, which is a fatal sign in patients with ENKTL
following the completion of treatment (38,39).
Intravascular invasion of a tumor is a prerequisite for the
occurrence of metastasis. The current results identified more stage
III/IV patients in the vascular invasion group than the
non-vascular invasion group (P<0.001). A higher proportion of
EUNKTL patients also existed in the vascular invasion group,
indicating strong invasiveness and easy spread of disease through
the body. Thus, the histological vascular invasion status of a
tumor may result in distant metastasis, leading to shorter patient
survival. The multiple factor analysis performed in the present
study revealed CR following chemotherapy as a significant favorable
prognostic factor in patients with ENKTL. However, even if the
stage I/II ENKTL patients with vascular invasion achieved CR
following chemotherapy alone, their prognosis remained worse than
that for patients without vascular invasion at the same stage. This
finding indicates that vascular invasion is a poor independent
prognostic factor of patients' response to chemotherapy.
Two major clinical prognostic models, termed IPI and
KPI, were applied in NK/T-cell lymphoma. The distribution of
patients within risk groups based on IPI and KPI scores is
presented in Table I. Based on IPI
scores, >60% of the cases belonged to the low-risk category
(with 0 or 1 adverse factor). The KPI model balanced the
distribution of patients into different risk groups better than IPI
did. However, both prognostic models failed to differentiate
patients with different outcomes in the low-risk group. The
vascular invasion group can be divided based on IPI or KPI scores
of 0–1 into two subgroups with significant differences in OS and
PFS (IPI: P<0.001 and P<0.001, respectively; KPI: P<0.001
and P<0.004, respectively) (Fig.
6). Thus, vascular invasion may be a good independent
prognostic factor for determining OS and PFS in the whole group of
ENKTL patients, as well as in those with low-risk IPI or KPI
scores.
In conclusion, histological vascular invasion was an
independent prognostic factor for OS and PFS in patients with
ENKTL, nasal type. Further investigation is required to gain a
better understanding of the mechanisms underlying the association
between vascular invasion and clinical outcomes. UNKTL derived from
the nasal cavity and its surroundings is also characterized by
metastasis to the cervical lymph nodes or distant organs, and is
commonly accompanied by histological vascular invasion. This
distinction may be useful for encouraging clinicians to refer to
nasopharyngeal carcinoma research methods to explore diagnostic and
therapeutic methods for ENKTL.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81272620).
References
|
1
|
Vose J, Armitage J and Weisenburger D:
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: Pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Au WY, Ma SY, Chim CS, et al:
Clinicopathologic features and treatment outcome of mature T-cell
and natural killer-cell lymphomas diagnosed according to the World
Health Organization classification scheme: A single center
experience of 10 years. Ann Oncol. 16:206–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li YX, Liu QF, Fang H, Qi SN, Wang H, Wang
WH, Song YW, Lu J, Jin J, Wang SL, et al: Variable clinical
presentations of nasal and Waldeyer ring natural killer/T-cell
lymphoma. Clin Cancer Res. 15:2905–2912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T,
Tse E, Leung AY and Chim CS: SMILE for natural killer/T-cell
lymphoma: Analysis of safety and efficacy from the Asia Lymphoma
Study Group. Blood. 120:2973–2980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Xia ZJ, Huang HQ, Lu Y and Zhang
YJ: Cyclophosphamide, doxorubicin, vincristine and prednisone
(CHOP) in the treatment of stage IE/IIE extranodal natural killer/T
cell lymphoma, nasal type: 13-year follow-up in 135 patients. Int J
Hematol. 96:617–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee
JH, Lee DH, Huh J, Oh SY, Kwon HC, et al: Extranodal natural killer
T-cell lymphoma, nasal-type: A prognostic model from a
retrospective multicenter study. J Clin Oncol. 24:612–618. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishida T, Katayama S and Tsujimoto M: The
clinicopathological significance of histologic vascular invasion in
differentiated thyroid carcinoma. Am J Surg. 183:80–86. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Straume O and Akslen LA: Independent
prognostic importance of vascular invasion in nodular melanomas.
Cancer. 78:1211–1219. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouchi K, Sugawara T, Ono H, Fujiya T,
Kamiyama Y, Kakugawa Y, Mikuni J and Tateno H: Histologic features
and clinical significance of venous invasion in colorectal
carcinoma with hepatic metastasis. Cancer. 78:2313–2317. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maehara Y, Kabashima A, Koga T, Tokunaga
E, Takeuchi H, Kakeji Y and Sugimachi K: Vascular invasion and
potential for tumor angiogenesis and metastasis in gastric
carcinoma. Surgery. 128:408–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chau GY, Lui WY and Wu CW: Spectrum and
significance of microscopic vascular invasion in hepatocellular
carcinoma. Surg Oncol Clin N Am. 12:25–34, viii. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Poppel H, Vandendriessche H, Boel K,
Mertens V, Goethuys H, Haustermans K, Van Damme B and Baert L:
Microscopic vascular invasion is the most relevant prognosticator
after radical nephrectomy for clinically nonmetastatic renal cell
carcinoma. J Urol. 158:45–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schoppmann SF, Bayer G, Aumayr K, Taucher
S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant
M, et al: Prognostic value of lymphangiogenesis and lymphovascular
invasion in invasive breast cancer. Ann Surg. 240:306–312. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang JJ, Jiang WQ, Lin TY, Huang Y, Xu
RH, Huang HQ and Li ZM: Absolute lymphocyte count is a novel
prognostic indicator in extranodal natural killer/T-cell lymphoma,
nasal type. Ann Oncol. 22:149–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conill C, Verger E and Salamero M:
Performance Status Assessment in Cancer Patients. Cancer.
65:1864–1866. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Musshoff K and Schmidt-Vollmer H:
Proceedings: Prognosis of non-Hodgkin's lymphomas with special
emphasis on the staging classification. Cancer Res Clin Oncol.
83:323–341. 1975.
|
|
17
|
Lin ZX, Bai B, Cai QC, Cai QQ, Wang XX, Wu
XY and Huang HQ: High numbers of tumor-associated macrophages
correlate with poor prognosis in patients with mature T- and
natural killer cell lymphomas. Med Oncol. 29:3522–3528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Xia ZJ, Huang HQ, Lu Y and Zhang
YJ: Cyclophosphamide, doxorubicin, vincristine and prednisone
(CHOP) in the treatment of stage IE/IIE extranodal natural killer/T
cell lymphoma, nasal type: 13 year follow up in 135 patients. Int J
Hematol. 96:617–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huijgens PC, Ossenkoppele GJ, Van Der
Lelie J, Thomas LL, Wijngaarden MJ and Slaper CM: IMVP-16 followed
by high dose chemotherapy and autologous bone marrow
transplantation as salvage treatment for malignant lymphoma.
Hematol Oncol. 9:245–251. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velasquez WS, Cabanillas F, Salvador P,
McLaughlin P, Fridrik M, Tucker S, Jagannath S, Hagemeister FB,
Redman JR and Swan F: Effective salvage therapy for lymphoma with
cisplatin in combination with high-dose Ara-C and dexamethasone
(DHAP). Blood. 71:117–122. 1988.PubMed/NCBI
|
|
21
|
Wang H, Wuxiao ZJ, Zhu J, Wang Z, Wang KF,
Li S, Chen X, Lu Y and Xia ZJ: Comparison of gemcitabine,
oxaliplatin and L-asparaginase and etoposide, vincristine,
doxorubicin, cyclophosphamide and prednisone as firstline
chemotherapy in patients with stage IE to IIE extranodal natural
killer/Tcell lymphoma: A multicenter retrospective study. Leuk
Lymphoma. 56:971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin's lymphomas. NCI sponsored international
working group. J Clin Oncol. 17:12441999.PubMed/NCBI
|
|
23
|
Jiang L, Li P, Wang H, Liu J, Zhang X, Qiu
H and Zhang B: Prognostic significance of Ki-67 antigen expression
in extranodal natural killer/T-cell lymphoma, nasal type. Med
Oncol. 31:2182014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bossard C, Belhadj K, Reyes F,
Martin-Garcia N, Berger F, Kummer JA, Brière J, Baglin AC, Cheze S,
Bosq J, et al: Expression of the granzyme B inhibitor PI9 predicts
outcome in nasal NK/T-cell lymphoma: Results of a Western series of
48 patients treated with first-line polychemotherapy within the
Groupe d'Etude des Lymphomes de l'Adulte (GELA) trials. Blood.
109:2183–2189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim WY, Jeon YK, Kim TM, Kim JE, Kim YA,
Lee SH, Kim DW, Heo DS and Kim CW: Increased quantity of
tumor-infiltrating FOXP3-positive regulatory T cells is an
independent predictor for improved clinical outcome in extranodal
NK/T-cell lymphoma. Ann Oncol. 20:1688–1696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shim SJ, Yang WI, Shin E, Koom WS, Kim YB,
Cho JH, Suh CO, Kim JH and Kim GE: Clinical significance of
cyclooxygenase-2 expression in extranodal natural killer
(NK)/T-cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys.
67:31–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong J, Park S, Baek HL, Jung JH, Kang IG,
Sym SJ, Park J, Ahn JY, Cho EK, Kim ST, et al: Tumor cell nuclear
diameter and CD30 expression as potential prognostic parameter in
patients with extranodal NK/T-cell lymphoma, nasal type. Int J Clin
Exp Pathol. 5:939–947. 2012.PubMed/NCBI
|
|
28
|
Cuadra-Garcia I, Proulx GM, Wu CL, Wang
CC, Pilch BZ, Harris NL and Ferry JA: Sinonasal lymphoma: A
clinicopathologic analysis of 58 cases from the Massachusetts
General Hospital. Am J Surg Pathol. 23:1356–1369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seki D, Ueno K, Kurono Y and Eizuru Y:
Clinicopathological features of Epstein-Barr virus-associated nasal
T/NK cell lymphomas in southern Japan. Auris Nasus Larynx.
28:61–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wróbel T, Poreba M, Mazur G, Poreba R,
Pyszel A, Beck B, Steinmetz-Beck A, Andrzejak R and Kuliczkowski K:
Angiogenic and coagulation-fibrinolysis factors in non Hodgkin's
lymphoma. Neoplasma. 53:253–258. 2006.PubMed/NCBI
|
|
31
|
Tan KL, Scott DW, Hong F, Kahl BS, Fisher
RI, Bartlett NL, Advani RH, Buckstein R, Rimsza LM, Connors JM, et
al: Tumor-associated macrophages predict inferior outcomes in
classic Hodgkin lymphoma: A correlative study from the E2496
intergroup trial. Blood. 120:3280–3287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siveen KS and Kuttan G: Role of
macrophages in tumour progression. Immunol Lett. 123:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YX, Yao B, Jin J, Wang WH, Liu YP, Song
YW, Wang SL, Liu XF, Zhou LQ, He XH, et al: Radiotherapy as primary
treatment for stage IE and IIE nasal natural killer/T-cell
lymphoma. J Clin Oncol. 24:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim GE, Cho JH, Yang WI, Chung EJ, Suh CO,
Park KR, Hong WP, Park IY, Hahn JS, Roh JK and Kim BS: Angiocentric
lymphoma of the head and neck: Patterns of systemic failure after
radiation treatment. J Clin Oncol. 18:54–63. 2000.PubMed/NCBI
|
|
36
|
Koom WS, Chung EJ, Yang WI, Shim SJ, Suh
CO, Roh JK, Yoon JH and Kim GE: Angiocentric T-cell and NK/T-cell
lymphomas: Radiotherapeutic viewpoints. Int J Radiat Oncol Biol
Phys. 59:1127–1137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li CC, Tien HF, Tang JL, Yao M, Chen YC,
Su IJ, Hsu SM and Hong RL: Treatment outcome and pattern of failure
in 77 patients with sinonasal natural killer/T-cell or T-cell
lymphoma. Cancer. 100:366–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Wuxiao ZJ, Zhu J, Wang Z, Wang KF,
Li S, Chen X, Lu Y and Xia ZJ: Comparison of gemcitabine,
oxaliplatin and L-asparaginase and etoposide, vincristine,
doxorubicin, cyclophosphamide and prednisone as first-line
chemotherapy in patients with stage IE to IIE extranodal natural
killer/T-cell lymphoma: A multicenter retrospective study. Leuk
Lymphoma. 56:971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS,
Hong DS, Won JH, Lee JH, Yoon DH, Cho J, et al: Concurrent
chemoradiotherapy followed by L-asparaginase-containing
chemotherapy, VIDL, for localized nasal extranodal NK/T cell
lymphoma: CISL08-01 phase II study. Ann Hematol. 93:1895–1901.
2014. View Article : Google Scholar : PubMed/NCBI
|