Introduction
Gastric cancer has been a significant health problem
worldwide due to its poor prognosis and increasing incidence
(1). According to the latest
literature, 800,000 cancer-associated mortalities are caused by
gastric cancer each year globally, making it the second leading
cause of cancer-associated mortalities in the world (2,3). At
present, chemotherapy has become one of the major means for
treating gastric cancer of middle and advanced stages (4,5). As a
standard anticancer drug, paclitaxel (PTX) serves a significant
role in the treatment of a number of tumors. As reported in latest
studies, the efficiency of single anticancer drug-paclitaxel
reaches 11–23% in treating the gastric cancer of middle and
advanced stages, while that of drug combination therapy is 50–60%
(6). Harmine (HM), originally
isolated from the seeds of Peganum harmala, is a tricyclic
compound belonging to the β-carboline alkaloids. It inhibits the
proliferation of tumor cells and induces apoptosis, and it performs
well in reducing angiogenesis, tumor promotion and mutation
(7,8).
It has become a new focus in the chemoprevention study about cancer
in recent years. Recent studies have demonstrated that HM possessed
significant anti-tumor potential both in vitro and in
vivo (9,10). However, the synergistic anti-tumor
effect of a combination of HM and PTX on human gastric cancer
remains unknown.
Cyclooxygenase (COX), a key enzyme in the conversion
of arachidonic acid to prostaglandins (PGs) and other eicosanoids,
exists as two isoforms: Constitutive COX-1 and mitogen-inducible
COX-2. COX-2 is also constitutively expressed in gastric cancer and
is related to cell proliferation and apoptosis, tumor invasiveness
and metastasis (11). Our previous
studies have demonstrated that COX-2 inhibition by selective COX-2
inhibitors or small interfering RNA (siRNA) could suppress cell
proliferation and induces apoptosis in human gastric cancer cells
(12,13). Recently, we demonstrated that HM
induced apoptosis of gastric cancer cells by down-regulating COX-2
expression (14).
In the present study, a human gastric cancer cell
line SGC-7901, in which COX-2 was expressed (15), was selected to examine whether PTX in
combination with HM exerts synergistic anti-tumor effects on human
gastric cancer cells in vitro and in vivo and to
further explore the probable mechanism.
Materials and methods
Reagents
HM, PTX (purity >98%), dimethylsulfoxide (DMSO),
and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
chemical structures of HM and PTX are shown in Fig. 1. Stock solutions of HM were produced
in DMSO at a final concentration of DMSO (≤0.1%) and sterilized by
passage through a 0.22 µm pore size filter (Immobilon; EMD
Millipore, Bedford, MA, USA), diluted with culture media before
use. RPMI-1640 medium, fetal bovine serum (FBS) and
penicillin/streptomycin were purchased from Gibco BRL (Grand
Island, NY, USA). All other chemicals were of analytical grade and
used without further purification.
Cell lines and culture
Human moderately differentiated SGC-7901 gastric
cancer cell line was obtained from Shanghai Institute of Cell
Biology (Shanghai, China). The cells were cultured in RPMI-1640
medium supplemented with 10% FBS, 100 units/ml penicillin G and 100
µg/ml streptomycin at 37°C in a humidified incubator with 5%
CO2.
MTT assay
SGC-7901 cells (200 µl of cell suspension per well)
were seeded at a density of 5×103 cells/well in 96-well
plates and incubated overnight in 10% FBS medium. The cells were
then treated with different concentrations of HM or PTX in
serum-free conditions. Untreated cells in serum-free medium were
used as controls. After incubation for 48 h at 37°C, the cell
proliferation was determined by the MTT assay as described in our
previous study (15).
DAPI staining
Equal numbers of SGC-7901 cells (1×106
cells/well) were plated in 6-well plates and then incubated with 2
ng/ml PTX, 4 µg/ml HM, or a combination of these two drugs for 48 h
and then washed once in phosphate buffer saline (PBS) followed by
fixation in cold methanol: acetone (1:1) for 5 min. After washing
twice in PBS for 5 min, these cells were stained with 4 µg/ml DAPI
for 10 min at room temperature and subsequently examined by
fluorescence microscopy (Eclipse E-800; Nikon, Tokyo, Japan).
Apoptotic cells were identified by chromatin condensation and
nuclear fragmentation.
Flow cytometry analysis
Equal numbers of SGC-7901 cells (1×106
cells/well) were plated in 6-well dishes and then incubated with 2
ng/ml PTX and/or 4 µg/ml HM or a combination of these two drugs for
48 h. The cells were washed with PBS, stained with annexinV-FITC
and propidium iodide (PI) using the AnnexinV-FITC kit (Bender
Medsystem, Vienna, Austria). The cells were then subjected to flow
cytometry according to the manufacturer's instructions and the
stained cells were analyzed by FACScan flow cytometer (BD
Biosciences, San Diego, CA, USA).
Western blot analysis
Proteins were extracted from cells or tumors and
western blot analyses were performed as described in our previous
reports (15). Cells
(1×106 cells/well) were treated with 2 ng/ml PTX, 4
µg/ml HM or a combination of these two drugs for 48 h at 37°C in a
humidified incubator with an atmosphere of 5% CO2. Cells
were then washed twice with ice cold PBS and protein extraction was
performed by lysis in RIPA buffer [150 mM NaCl, 1% (v/v) NP40, 0.5%
(w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS),
50 mM Tris HCl (pH 8), 10 mM EDTA and 1 mM phenyl methylsulfonyl
fluoride; Sigma-Aldrich] for 30 min at 4°C, followed by
centrifugation for 15 min at 12,000 × g. Protein concentrations
were determined using a bicinchoninic acid assay (Pierce
Biotechnology, Inc., Rockford, IL, USA), according to the
manufacturer's instructions. Subsequently, proteins (60 µg) were
loaded onto a 10% (w/v) SDS polyacrylamide gel, electrophoresed and
transferred onto a polyvinylidene fluoride membranes (EMD
Millipore) which was then blocked for 2 h at room temperature with
blocking buffer [Tris-buffered saline containing 0.1% (v/v)
Tween-20 (Sigma-Aldrich) and 5% (w/v) milk powder]. The following
primary antibodies were applied at a dilution of 1:1,000 for 1 h at
room temperature or overnight at 4°C: Polyclonal rabbit anti-mouse
COX-2 (cat no. 13314; Cell Signaling Technology, Inc., Beverly, MA,
USA), PCNA (cat no. sc53407; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), B-cell lymphoma-2 (Bcl-2; cat no. sc509; Santa
Cruz Biotechnology, Inc.), Bcl2-associated X protein (Bax; cat no.
sc20067; Santa Cruz Biotechnology, Inc.) and GAPDH (cat no. G5262;
Sigma-Aldrich). Membranes were then incubated for 2 h with
polyclonal goat anti-rabbit horseradish peroxidase-conjugated
secondary antibodies (1:20,000 diltion; cat no. BA1000; Vector
Laboratories, Inc., Burlingame, CA, USA) at 37°C in a humidified
incubator with an atmosphere of 5% CO2. Membranes were visualized
using an enhanced chemiluminescence kit and signals were quantified
by scanning densitometry (QuantityOne v4.6.2 software; Bio-Rad
Laboratories, Hercules, CA, USA). The relative expression levels of
COX-2, PCNA, Bax and Bcl-2 were normalized to that of GAPDH.
In vivo anti-tumor efficacy
Male athymic nude mice (6–8 weeks old and weighing
18–22 g) were purchased from Animal Center of Nanjing Medical
University (Nanjing, China). The mice were bred under specific
pathogen-free (SPF) conditions and all experimental procedures were
performed in accordance with the Guide for the Care and Use of
Laboratory Animals (NIH publication no. 80-23, revised 1996) and
the institutional ethical guidelines for animal experiments. The
mice were subcutaneously injected into the left axillary space with
0.1 ml of cell suspension containing 4–6×106 SGC-7901
cells. Seven days after implantation of tumor cells (when the tumor
size was ~0.1–0.2 cm3), mice were randomly divided into
four groups (n=6) to receive different treatments. The experimental
mice were injected intraperitoneally with PTX (10 mg/kg, daily, 3
days per week for 2 weeks), HM (30 mg/kg, daily, 5 days per week
for 2 weeks) and a combination of PTX 5 mg/kg and HM 20 mg/kg,
daily, 3 days per week for 2 weeks. Control mice were treated with
an equal volume of normal saline. Tumors were measured with
calipers at 3-day intervals and the volumes were calculated using
the following formula: (the shortest diameter)2 × (the
longest diameter) × 0.5.
Statistical analysis
Statistical analyses were performed using the SPSS
software package (version 11; SPSS Inc., Chicago, IL, USA). The
data are presented as mean ± standard deviation (SD), and were
analyzed using two-tailed Student's t-test or one-way analysis of
variance (ANOVA) with Dennett's multiple comparison tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of HM and PTX on the
proliferation of SGC-7901 cells
MTT assay was used to analyze metabolic activity in
proliferating cells. Either HM or PTX inhibited the cell
proliferation in a dose-dependent manner (Fig. 2A and B). The inhibitory effects on the
cell proliferation were significantly enhanced when SGC-7901 cells
were treated with a combination of HM with PTX (Fig. 2C). As demonstrated in Fig. 2, the suppression rates of HM and PTX
were 18.0 and 38.7%, respectively. When HM and PTX were combined,
the suppression rate reached 74.5%, which was significantly higher
than HM or PTX alone (P<0.05; Fig.
2C). Furthermore, HM combined with PTX reduced PCNA expression
in gastric cancer cells (Fig.
2D).
Effects of HM and PTX on the apoptosis
of SGC-7901 cells
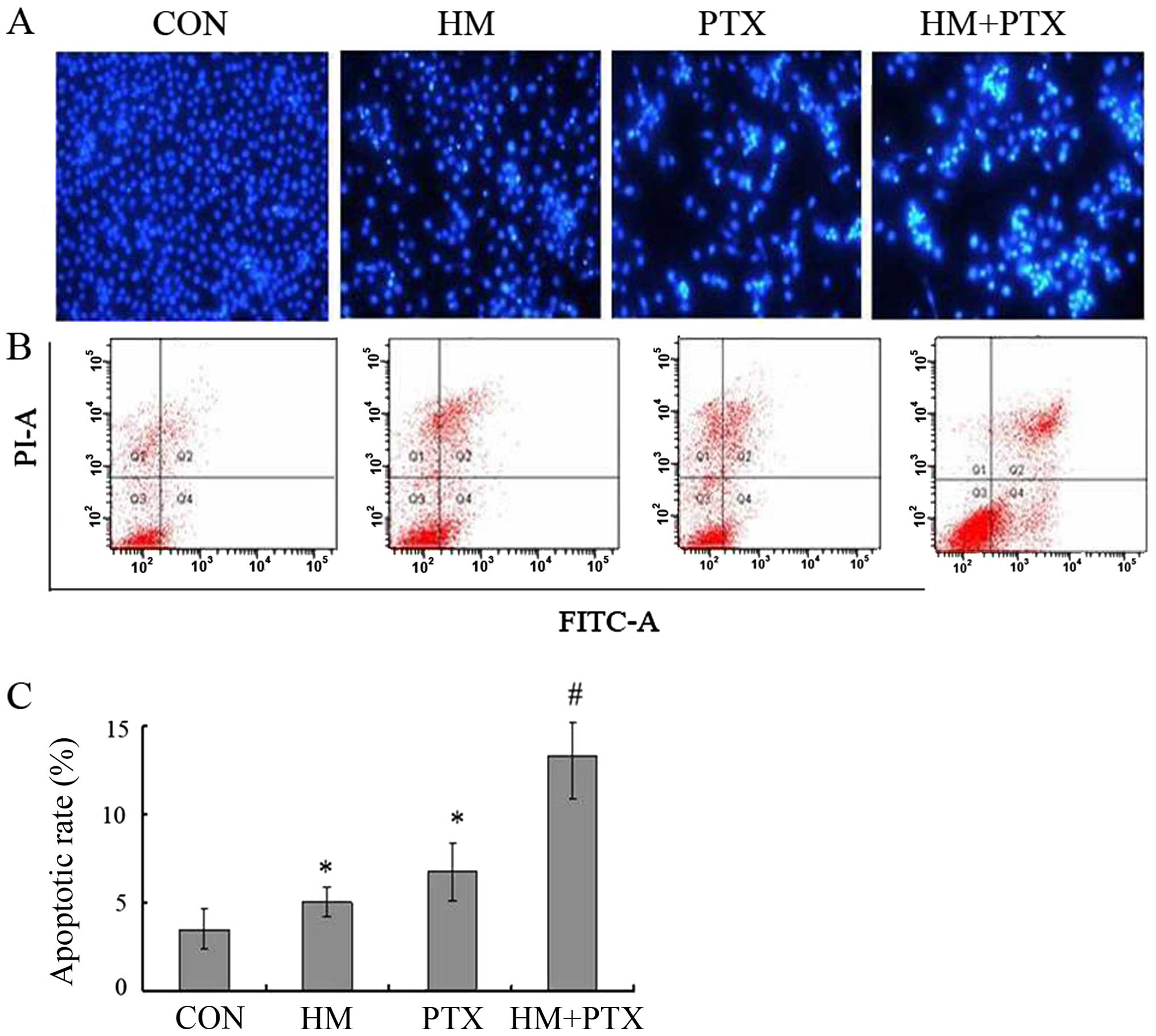
Morphological changes in apoptotic cells were
observed by DAPI staining. As shown in Fig. 3A, the nucleus of untreated control
cells were large and round without condensation or fragmentation,
whereas the nucleus from the HM or PTX treated cells were condensed
and fragmented, emitted bright fluorescence, which is indicative of
early apoptosis. In the combination group, changes in cellular
morphology were much stronger than that of either drug applied
individually.
To further confirm the apoptotic combined effects of
HM and PTX, fluorescent Annexin V-FITC/PI double staining was
performed. When cells undergo apoptosis, a phosphatidylserine
residue normally on the inside of the plasma membrane flips to the
outside and are specifically recognized by annexin V.
Counterstaining by PI allows the discrimination of apoptotic from
necrotic cells. Necrotic cells will be stained only with PI,
whereas early apoptotic cells will be stained with annexin V and
late apoptotic cells will be stained with both annexin V and PI. As
demonstrated in Fig. 3B, the lower
right panels correspond to apoptotic cells which have high FITC and
low PI signals. Both HM and PTX induced apoptosis in SGC-7901 cells
(P<0.05 vs. control for HM and PTX), the combination of two
drugs further enhanced the apoptosis ratio (P<0.05, HM+PTX vs.
HM and PTX) (Fig. 3C).
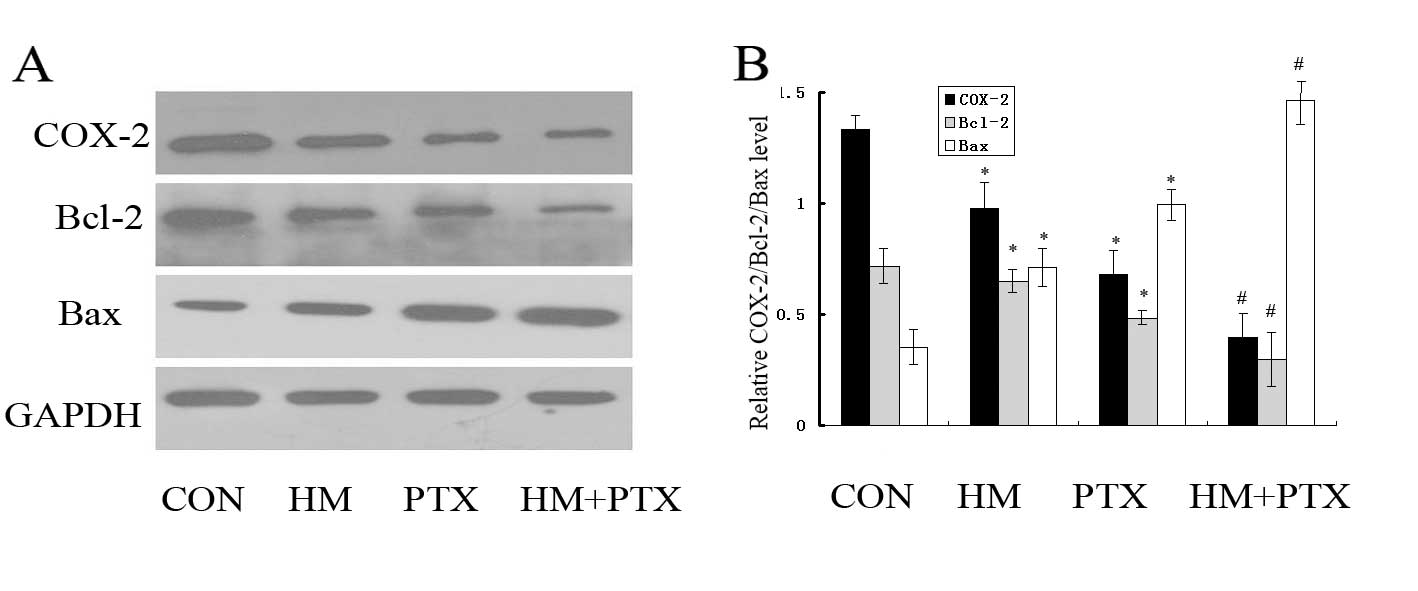
Effects of HM and PTX on the
expression levels of COX-2, Bcl-2 and Bax in SGC-7901 cells in
vitro
To determine which genes are regulated by HM and PTX
during apoptosis, the expression of COX-2, Bcl-2 and Bax were
measured using western blot analysis. Exposure of gastric cancer
cells to HM and PTX reduced the expression levels of COX-2 and
Bcl-2, while the Bax expression levels were increased (Fig. 4). In addition, the combined
application of HM and PTX resulted in a reduction in the COX-2 and
Bcl-2 expression levels with a simultaneous increase in the Bax
expression compared with the effects of either of drugs alone.
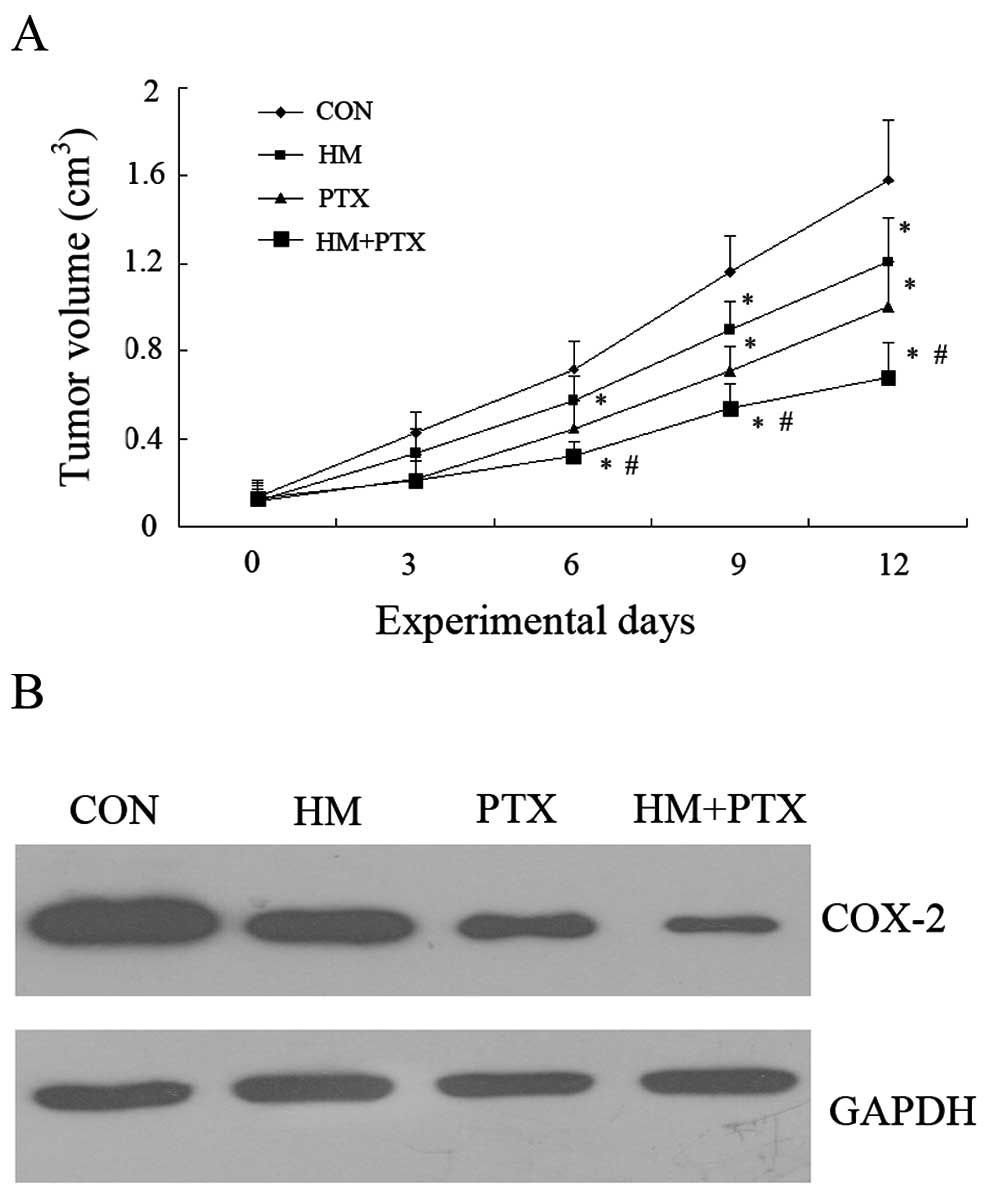
Effects of HM and PTX on the tumor
growth in vivo
To further explore the effects of HM and PTX on
tumor growth, a gastric xenograft tumor model was established. As
shown in Fig. 5A, both HM and PTX
effectively inhibited tumor growth compared to control mice. The
combination groups exhibited an improved inhibition on tumor growth
compared with either of the drugs used alone. In addition, the
combined application of HM and PTX resulted in a statistically
significant decrease in the COX-2 expression compared with the
effects of either of drugs alone (Fig.
5B).
Discussion
Gastric cancer is one of the common malignant tumors
in China which severely threatens human health due to its high
morbidity and low early diagnosis rate (16). PTX is an important drug for treating
gastric cancer, for it can effectively prolong the survival time of
late gastric cancer patients and improve their living quality
(17). However, a number of clinical
and experimental studies in recent years have discovered that
certain types of malignant tumors, including breast cancer, lung
cancer, ovarian cancer and gastric cancer would primarily or
secondarily resist the paclitaxe (18). It is, therefore, of great significance
to explore a novel method to enhance the anticancer effect of PTX
and to produce medicines which can reverse the resistance against
PTX.
The present study reveals the combined
administration of HM and PTX is significantly more efficient than
the administration of a single drug on inhibiting proliferation
inhibition and apoptosis induction. It preliminarily demonstrates
that HM and PTX have synergistic effects on anticancer treatment.
Therefore, in order to reach the same inhibiting effect, HM can
reduce the dosage of PTX, strengthen the gastric cancer cells'
sensitivity to the PTX, and weaken the toxic side effects of
high-dose chemotherapeutical medicine on multiple systems of
body.
A molecular target for cancer prevention and
treatment that has been studied extensively in the last decade is
COX-2. Our previous study demonstrated that HM significantly
inhibits COX-2 expression in BGC-823 and SGC-7901 cells (14). The western blot results in the present
study indicated that the combined application of HM and PTX
resulted in a significant reduction in the expression of COX-2
compared with the effects of either of drugs alone in vitro
and in vivo for the first time.
It is well known that tumorigenesis is due to an
imbalance between cell proliferation and apoptosis (19). PCNA is a nuclear protein that is
synthesized in late G1 and S phases of the cell cycle and it is
used to monitor changes in cellular growth status (18). The modulation of PCNA expression is an
important indicator of early changes in cellular proliferation and
provides a potential mechanism by which HM and PTX may inhibit cell
proliferation. Our previous study also demonstrated that treatment
with NS-398, which is a selective COX-2 inhibitor, significantly
reduced PCNA expression in human pancreatic carcinoma cells
(20). Therefore, PTX combined with
HM suppressed cell proliferation through inhibition of
COX-2-associated PCNA expression in gastric cancer cells.
It has previously been shown that apoptosis is in
part modulated by the Bcl-2 family (19). In the present study, it was observed
that both HM and PTX down-regulated Bcl-2 expression as well as
up-regulating Bax expression, while the combination of the two
drugs generated an improved effect. Data from both in vivo
and in vitro studies have demonstrated that up-regulation of
COX-2 expression reduces the apoptosis rate by upregulating Bcl-2
protein (21,22). Our previous study demonstrated that
NS-398 significantly decreases Bcl-2 protein level but increases
Bax protein level in human gastric cancer cells (13). These results indicate that the
combination of HM and PTX inducing apoptosis may be due to the
down-regulation of COX-2 expression in gastric cancer cells.
The results of the present study indicate that the
combination of HM and PTX inhibits gastric cancer development more
effectively than each drug alone. PTX in combination with HM may
inhibit tumor proliferation and induces apoptosis through
down-regulation of cyclooxygenase-2 expression in gastric cancer.
These findings may provide theoretical and experimental basis for
the treatment of gastric cancer with single Chinese medicine HM and
chemotherapeutic drug PTX in clinical application.
References
|
1
|
Liu SZ, Wang B, Zhang F, Chen Q, Yu L,
Cheng LP, Sun XB and Duan GC: Incidence, survival and prevalence of
esophageal and gastric cancer in linzhou city from 2003 to 2009.
Asian Pac J Cancer Prev. 14:6031–6034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwarz Re and Smith DD: Clinical impact
of lymphadenectomy extent in resectable gastric cancer of advanced
stage. Ann Surg Oncol. 14:317–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu W, Yang Q, Liu B and Zhu Z: Serum
proteomics for gastric cancer. Clin Chim Acta. 431:179–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Oliveira R, Deschoemaeker S, Henze AT,
Debackere K, Finisguerra V, Takeda Y, Roncal C, Dettori D, Tack E,
Jönsson Y, et al: Gene-targeting of Phd2 improves tumor response to
chemotherapy and prevents side-toxicity. Cancer Cell. 22:263–277.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wani WA, Saleem K and Haque A: Platinum
compounds: A hope for future cancer chemotherapy. Anticancer Agents
Med Chem. 13:296–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakamoto J, Matsui T and Kodera Y:
Paclitaxel chemotherapy for the treatment of gastric cancer.
Gastric Cancer. 12:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cao MR, Li Q, Liu ZL, Liu HH, Wang W, Liao
XL, Pan YL and Jiang JW: Harmine induces apoptosis in HepG2 cells
via mitochondrial signaling pathway. Hepatobiliary Pancreat Dis
Int. 10:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao X, Che X, Zhao W, Zhang D, Bi T and
Wang G: The β-adrenoceptor antagonist, propranolol, induces human
gastric cancer cell apoptosis and cell cycle arrest via inhibiting
nuclear factor κB signaling. Oncol Rep. 24:1669–1676.
2010.PubMed/NCBI
|
|
9
|
Abe A and Yamada H: Harmol induces
apoptosis by caspase-8 activation independently of Fas/Fas ligand
interaction in human lung carcinoma H596 cells. Anticancer Drugs.
20:373–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai F, Chen Y, Song Y, Huang L, Zhai D,
Dong Y, Lai L, Zhang T, Li D, Pang X, et al: A natural small
molecule harmine inhibits angiogenesis and suppresses tumour growth
through activation of p53 in endothelial cells. PLoS One.
7:e521622012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun WH, Sun YL, Fang RN, Shao Y, Xu HC,
Xue QP, Ding GX and Cheng YL: Expression of cyclooxygenase-2 and
matrix metalloproteinase-9 in gastric carcinoma and its correlation
with angiogenesis. Jpn J Clin Oncol. 35:707–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan MW, Wong CY, Cheng AS, Chan VY, Chan
KK, To KF, Chan FK, Sung JJ and Leung WK: Targeted inhibition of
COX-2 expression by RNA interference suppresses tumor growth and
potentiates chemosensitivity to cisplatin in human gastric cancer
cells. Oncol Re. 18:1557–1562. 2007.
|
|
13
|
Sun WH, Zhu F, Chen GS, Su H, Luo C, Zhao
QS, Zhang Y, Shao Y, Sun J, Zhou SM, et al: Blockade of
cholecystokinin-2 receptor and cyclooxygenase-2 synergistically
induces cell apoptosis, and inhibits the proliferation of human
gastric cancer cells in vitro. Cancer Lett. 263:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang
K, Li X and Sun W: Harmine induces apoptosis and inhibits tumor
cell proliferation, migration and invasion through down-regulation
of cyclooxygenase-2 expression in gastric cancer. Phytomedicine.
15:348–355. 2014. View Article : Google Scholar
|
|
15
|
He XP, Shao Y, Li XL, Xu W, Chen GS, Sun
HH, Xu HC, Xu X, Tang D, Zheng XF, et al: Downregulation of miR-101
in gastric cancer correlates with cyclooxygenase-2 overexpression
and tumor growth. FEBS J. 279:4201–4212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neugut AI, Hayek M and Howe G:
Epidemiology of gastric cancer. Semin Oncol. 23:281–291.
1996.PubMed/NCBI
|
|
17
|
Tuan TF, Tsai ML, Yeh KC, Huang HC, Chung
CT, Huang CL, Han CH, Chen CP, Wang MH, Shen CC, et al: Intravenous
paclitaxel against metastasis of human gastric tumors of diffuse
type. Cancer Chemother Pharmacol. 66:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadaki C, Mavroudis D, Trypaki M,
Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki E,
Georgoulias V and Souglakos J: Tumoral expression of TXR1 and TSP1
predicts overall survival of patients with lung adenocarcinoma
treated with first-line docetaxel-gemcitabine regimen. Clin Cancer
Res. 15:3827–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams GT and Smith CA: Molecular
regulation of apoptosis: Genetic controls on cell death. Cell.
74:777–779. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun WH, Chen GS, Ou XL, Yang Y, Luo C,
Zhang Y, Shao Y, Xu HC, Xiao B, Xue YP, et al: Inhibition of COX-2
and activation of peroxisome proliferators activated receptor γ
synergistically inhibits proliferation and induces apoptosis of
human pancreatic carcinoma cells. Cancer Lett. 275:247–255. 2013.
View Article : Google Scholar
|
|
21
|
Tsujii M and DuBois RN: Alterations in
cellular adhesion and apoptosis in epithelial cells overexpression
prostaglandin endoperoxide synthase-2. Cell. 83:493–501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawaoka H, Tsuji S, Tsujii M, Gunawan ES,
Sasaki Y, Kawano S and Hori M: Cyclooxygenase inhibitors suppress
angiogenesis and reduce tumor growth in vivo. Lab Invest.
79:1469–1477. 1999.PubMed/NCBI
|