Introduction
Prostate cancer (Pca) is the most common malignant
tumor in the male genitourinary system, and according to global
cancer epidemic statistics, Pca has become the second most common
cancer in men, being second only to lung cancer (1). However, there is lack of effective
imaging methods for early diagnosis, localized diagnosis of
metastases, choice of postoperative treatment and prevention of
postoperative recurrence.
Prostate-specific membrane antigen (PSMA) is a newly
discovered Pca-associated antigen. It has no or low expression in
normal prostate tissues, whereas it is significantly increased in
benign prostatic hyperplasia and Pca tissues, particularly in
certain Pca cell lines, including the Pca LNCap cell line (2). Therefore, PSMA may become a molecular
target for specific diagnosis of Pca. PSMA is a type 2 integrated
protein present in the prostate epithelial cell membrane (3), and contains 750 amino acids and has a
molecular weight of 100 kDa. PSMA has 3 known enzymes with
different biological effects (4), as
follows: Folyl polyglutamyl-γ-glutamate carboxypeptidase, also
known as folate hydrolase; N-acetylated-a-linked acidic
dipeptidase (NAALADase); and dipeptidyl peptidase IV.
Cyt-356 is the earliest monoclonal antibody to PSMA,
which was developed by Cytogen Corp. (Princeton, NJ, USA), and has
been used clinically (5). Cyt-356
binds to PSMA on the cell surface, and may be used for diagnosis
and imaging examination of Pca. Heston (6) previously cloned the cDNA of PSMA and
produced a series of other antibodies against the outer region of
the protein, and Bander et al (7,8) used these
antibodies in preclinical and clinical diagnosis and treatment of
Pca. In previous years, the implementation of specific small
molecules in combination with tumor markers to mediate tumor
imaging and surgical positioning has become a novel clinical
diagnosis and treatment method for tumors (9). Labeling tumor markers with small
molecules has an advantage of rapid serum clearance and good tumor
tissue permeability (10). However,
small molecular markers have certain limitations in affinity as
tumor markers.
In recent years, study on activity inhibitors of
PSMA enzymes is concentrated on the glutamic urea small molecule
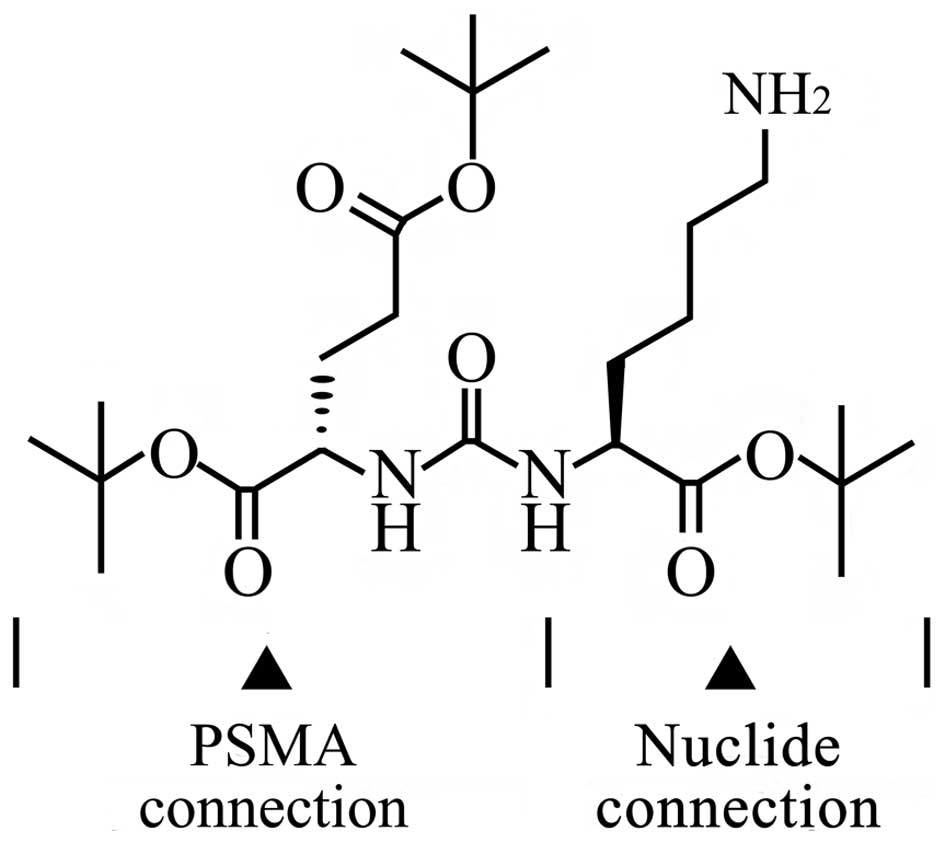
(Glu-urea-R) and its analogs. Glu-urea-R (Fig. 1) is a PSMA (folate hydrolase I) enzyme
inhibitor that efficiently and specifically binds to the PSMA on
the Pca cell surface, in which Glu-urea is the binding terminal to
PSMA and the R-group is the coupling terminal to other chemical
groups (8).
Compared with PSMA monoclonal antibodies, Glu-urea-R
has the following advantages: Stable biological activity; short
half-life in vivo; good permeability to tumor parenchyma and
tumor metastases; and low side-effects in vivo (7,8). Kularatne
et al (11) coupled Glu-urea-R
with 99mTc-Dap-Asp-Cys chelate to obtain a molecular
imaging probe that may be used for single-photon emission computed
tomography diagnosis of Pca. In addition, Maresca et al
(12) designed and synthesized a
number of Glu-urea-R molecules that may easily be labeled with
radionuclide 123iodine and 131iodine. Those
authors hypothesized that the chemical properties of the R-group
and its coupling substrates may significantly impact the affinity
of the Glu-urea-R to PSMA. Zhang et al (10) coupled Glu-urea-R and 2,4-dinitrophenol
(DNP) to achieve targeted identification of Pca and intended to
obtain targeting immunotherapy of Pca by DNP recruiting immune
response-associated antibodies (10).
These previous studies suggest that Glu-urea-R has a high value in
positron emission tomography/computed tomography (PET/CT) diagnosis
of Pca. However, there remains a lack of research on positron
isotope labeling of Glu-urea-R as a PET/CT probe for Pca.
A previous study has revealed the properties of the
R-group in Glu-urea-R, and have demonstrated that the molecular
properties of its coupling groups may affect the affinity of a
Glu-urea-R molecular probe to PSMA (10). PET/CT molecular imaging probes require
radionuclide labeling and 18fluroine (18F)
possesses a relatively long half-life (t1/2 = 110 min),
allowing for an effective and sufficient drug labeling and imaging
time. When performing a prosthetic group label of Glu-urea-R using
18F, full consideration to the labeling efficiency and
radiochemical purity of the purified products must be provided. In
addition, consideration must be given to the affinity of the
labeled products to PSMA, the toxicity of 18F-Glu-urea-R
to normal cells and the impact of pH on the affinity of the probe
and PSMA. Successful development of a 18F-Glu-urea-R
PET/CT probe may provide a novel diagnostic method for Pca. The
present study aimed to develop 4 Glu-urea-R small molecular
precursor compounds, which is the first step for preparation of a
18F-Glu-urea-R PET/CT molecular probe.
Materials and methods
Synthesis of Glu-Urea-R
Background
The reaction was monitored using liquid
chromatography-MS (LC-MS) and the synthesized structures of
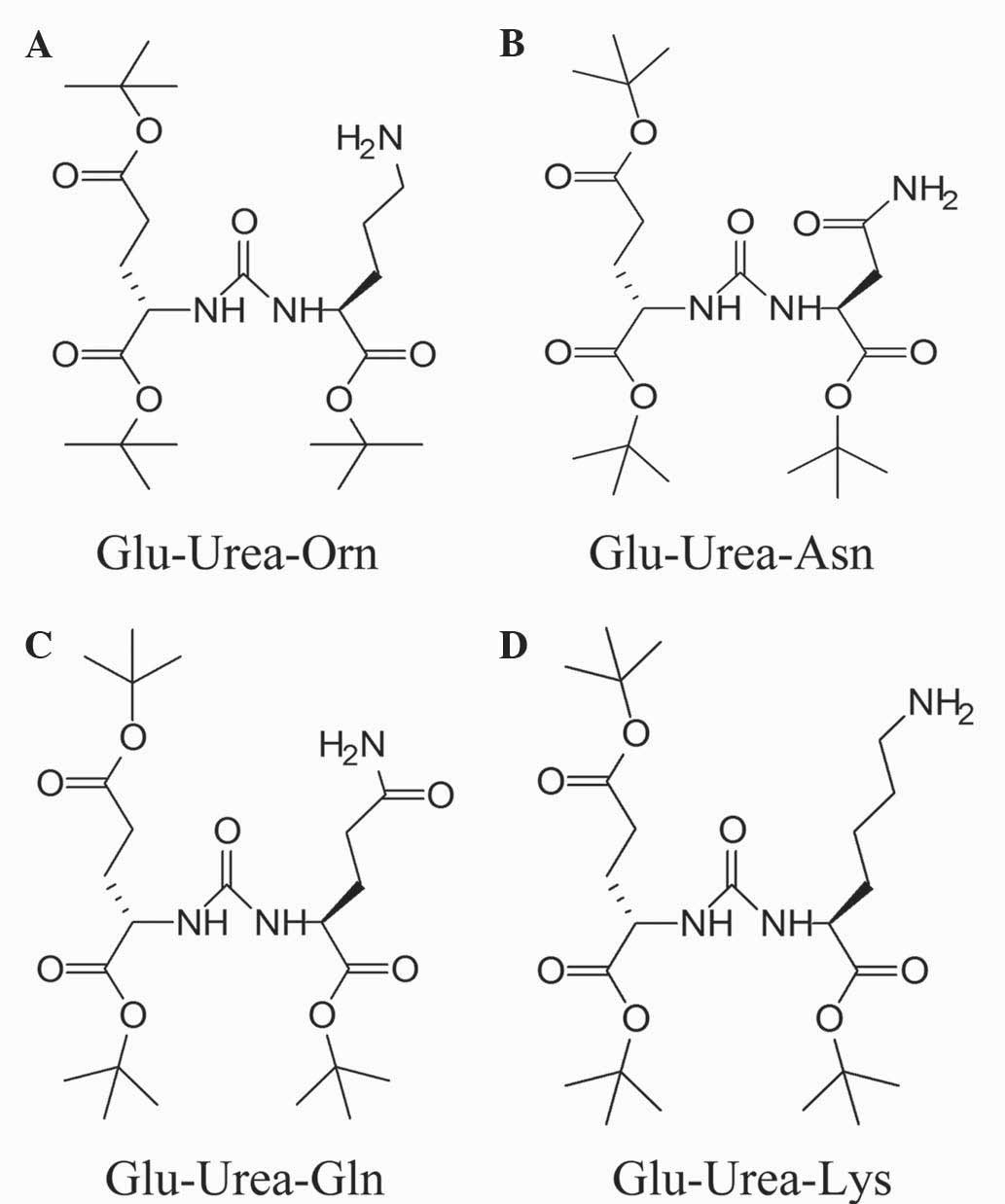
Glu-Urea-R are shown in Fig. 2.
Synthesis of Glu-urea-Ornithine
(Glu-urea-Orn)
In total, 45 g H-Glu(OBut)-OBut HCl (Beijing
Bomaijie Technology Co., Ltd., Beijing, China) and 2,800 ml
tert-butyl acetate (Beijing Bomaijie Technology Co., Ltd.,) were
added to 31.3 g HClO4 (Shanghai Huayi Group Huayuan
Chemical Co., Ltd., Shanghai, China) while stirring for 16 h. NaOH
solution (5M; pH, 7–8) was added and extracted using ethyl acetate
(Shanghai Seebio Biotech, Inc., Shanghai, China). Organic facies
were dried using sodium sulfate for 30 min. Subsequently, the
organic phase was decompressed and distilled to dryness at 35°C to
obtain the intermediate. A total of 32 g H-Orn-OBut HCl and 36 g
triethylamine (Shanghai Seebio Biotech, Inc.) were dissolved in 570
ml dichloromethane (DCM; Shanghai Seebio Biotech, Inc.) under
anaerobic conditions (−78°C). Subsequently, 57 ml DCM solution
containing 11 g triphosgene (Shanghai Seebio Biotech, Inc.) was
added to the reaction liquid for 30 min at 20°C. The intermediate
(21 g) and 6.6 g triethylamine were added and stirred for 18 h at
20°C. In total, 100 ml DCM was added to the reaction liquid and the
organic phase was rinsed with water. The isolated organic phase was
dried with sodium sulfate for 30 min. Subsequently, the organic
phase was decompressed and distilled to dryness at 35°C, and the
residue underwent column chromatography (DCM:CH3OH in a
ratio of 50:1) to obtain the crude products. The obtained crude
products underwent catalytic hydrogenation for 16 h by dissolving
the crude products in 600 ml carbinol (Shanghai Seebio Biotech,
Inc.) with 10% wetted palladium (9.7 g; Shanghai Seebio Biotech,
Inc.). The organic phase was decompressed and distilled to dryness
at 35°C and the residue underwent column chromatography
(DCM:CH3OH at a ratio of 50:1) to obtain products
(oily).
Synthetic route of Glu-urea-Asparagine
(Glu-urea-Asn)
In total, 26 g H-Glu(OBut)-OBut HCl and 29 g
triethylamine were dissolved in 500 ml DCM under the conditions of
nitrogen protection (−78°C). Subsequently, 50 ml DCM solution
containing 8.9 g triphosgene was added to the reaction liquid for
30 min at 20°C. A total of 10 g H-Asn-OBut HCl (Sundia MediTech
Co., Ltd., Shanghai, China) and 5 g triethylamine were added and
stirred for 18 h at 20°C. DCM (100 ml) was added to the reaction
liquid and the organic phase was rinsed with water. The isolated
organic phase was dried with sodium sulfate for 30 min.
Subsequently, the organic phase was decompressed and distilled to
dryness at 35°C and the residue underwent column chromatography
(DCM:CH3OH at a ratio of 50:1) to obtain crude products,
which were beaten using n-heptane (Shanghai Seebio Biotech,
Inc.) to obtain the solid substance. The solid was dissolved in 60
ml methanol and slowly added dropwise to 120 ml water, during which
the solid was separated out. The mixture was stirred for 1 h and
filtrated, and the filtrate was rinsed and underwent vacuum drying
using an oil pump at 40°C for 6 h to obtain the product.
Synthetic route of Glu-urea-Glutamine
(Glu-urea-Gln)
In total, 21 g H-Glu(OBut)-OBut HCl and 23 g
triethylamine were dissolved in 500 ml DCM under the conditions of
nitrogen protection (−78°C). Subsequently, 50 ml DCM solution
containing 7.1 g triphosgene was added to the reaction liquid at
20°C for 30 min. H-Gln-OBut HCl (Sundia MediTech Co., Ltd.) (10 g)
and 4.3 g triethylamine were added and stirred for 18 h at 20°C.
Subsequently, 100 ml DCM was added to the reaction liquid and the
organic phase was rinsed with water. The isolated organic phase was
dried with sodium sulfate for 30 min, and decompressed and
distilled to dryness at 35°C. The residue underwent column
chromatography (DCM:CH3OH at a ratio of 50:1) to obtain
the crude products, which were beaten using n-heptane to
obtain the solid substance. The solid was dissolved in 15 ml
methanol and slowly added dropwise to 75 ml water, during which the
solids were separated out. Subsequently, the mixture was stirred
for 1 h and filtrated, and the filtrate was rinsed and underwent
vacuum drying (oil pump) at 40°C for 6 h to obtain the product.
Synthetic route of Glu-urea-Lysine
(Glu-urea-Lys)
In total, 26 g H-Glu(OBut)-OBut HCl and 30 g
triethylamine were dissolved in 500 ml DCM under the conditions of
nitrogen protection (−78°C). Subsequently, 50 ml DCM solution
containing 9 g triphosgene was added to the reaction liquid at 20°C
for 30 min. H-Lys(Z)-OBut HCl (Sundia MediTech Co., Ltd.) (20 g)
and 5.5 g triethylamine were added and stirred for 18 h at 20°C. In
total, 100 ml DCM was added to the reaction liquid and the organic
phase was rinsed with water. The isolated organic phase was dried
with sodium sulfate for 30 min and decompressed and distilled to
dryness at 35°C. The residue underwent column chromatography to
obtain the crude products (42 g). The obtained crude products were
dissolved in 680 ml methanol and 10 g 10% wetted palladium-carbon
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) was added. The
crude products were hydrogenated with H2 and stirred for
16 h at 18°C. The organic phase was decompressed and distilled to
dryness at 35°C, and the residue underwent column chromatography
(DCM:CH3OH at a ratio of 50:1) to obtain products
(oily). The products underwent vacuum drying (oil pump) at 40°C for
6 h and were subsequently placed at −18°C for 16 h to obtain the
solid product.
Hydrolysis of Glu-Urea-R molecule
Glu-urea-Orn, Glu-urea-Asn, Glu-urea-Gln, and
Glu-urea-Lys were separately added to 10 ml trifluoroacetic acid
(Shanghai Seebio Biotech, Inc.), stirred at 20–30°C for 2 h, and
the reaction liquids were decompressed to dryness to obtain S36886,
S36887, S36888 and S36889, respectively.
Affinity experiment of Glu-urea-R small
molecule
NAALADase, also known as glutamate carboxypeptidase
II, is a metallopeptidase dependent on zinc oxidation that
hydrolyzes N-acetyl-aspartyl-glutamate (NAAG), resulting in
N-acetyl aspartate and glutamate products. PSMA partially
hydrolyzes folic acid glutamate to generate glutamate, which is
similar to NAAG hydrolysation. PSMA is expressed by LNCap cells
(2). Human prostate cancer LNCaP cell
lines were obtained from the American Type Culture Collection
(Manassas, VA, USA) and maintained in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified incubator at 37°C with 5% CO2. Membrane and
cytosolic fractions were obtained from cultured LNCaP cells.
Inhibition constants were determined using a
fluorescence-based assay. Briefly, 25 µl lysates of LNCaP cell
extracts were incubated with 12.5 µl inhibitor (S36886, S36887,
S36888 and S36889) in the presence of 12.5 µl NAAG (4 µM; #A5930;
Sigma-Aldrich, St. Louis, MO, USA) for 120 min at 37°C. NAAG
hydrolysis, which is catalyzed by NAALADase, results in the release
of N-acetyl-aspartate and glutamate. The amount of released
glutamate was determined by incubating the cells with 50 µl working
solution from the Amplex Red Glutamic Acid kit (Invitrogen™; Thermo
Fisher Scientific, Inc.) for 60 min. Fluorescence was measured
using an EnSpire Multimode plate reader (PerkinElmer, Waltham, MA,
USA) with excitation and emission wavelengths at 490 and 640 nm,
respectively. The inhibitor concentration was derived using
2-phosphonomethyl pentanedioic acid (2-PMPA; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), which is a known PSMA
inhibitor, as a positive control group and Glu-urea-R was
formulated to a suitable concentration by exploring different
concentrations of 2-PMPA capable of inhibiting PSMA. Inhibition
curves were determined using semi-log plots and half maximal
inhibitory concentration (IC50) values were
calculated.
Statistical analysis
Enzyme inhibitory constants (Ki values) were
generated using the Cheng-Prusoff conversion. Data analysis was
performed using GraphPad Prism version 4.0 for Windows (GraphPad
Software, La Jolla, CA, USA). All experiments were repeated at
least three times and the results are expressed as the mean ±
standard deviation. SPSS version 17.0 software (SPSS Inc., Chicago,
IL, USA) was used for statistical analysis of the synthesis,
hydrolysis and affinity experiments of Glu-urea-R.
Results
Synthetic analogs of Glu-Urea-R
Overall
Purities and structural identification of the 4
synthesized compounds were performed using proton nuclear NMR
(1H NMR) spectroscopy and LC-MS.
Glu-Urea-Orn
Macroscopically, the compound was a white oily
substance with a chemical formula of
C23H43N3O7. The purity
of the compound was evaluated by high performance liquid
chromatography (HPLC) in Sundia MediTech Co., Ltd. The purity of
the compound was 97.0%. 1H NMR (400 MHz;
CDCl3): δ5.34–5.30 (m, 2H), 4.37–4.30 (m, 2H), 2.74–2.70
(m, 2H), 2.35–2.27 (m, 2H), 2.08–1.64 (m, 8H), 1.53–1.25 (m, 27H).
Electrospray mass spectrometry (ESMS; m/z): 474.3 (M + H)+.
Glu-Urea-Asn
Macroscopically, the compound was a white solid
substance with a chemical formula of
C22H39N3O8. The purity
of the compound was 98.6%. 1H NMR (400 MHz;
CDCl3): δ6.42–6.29 (m, 2H), 6.02–5.91 (m, 2H), 4.66–4.61
(m, 1H), 4.42–4.36 (m, 1H), 2.94–2.88 (m, 1H), 2.71–2.69 (m, 1H),
2.34–2.28 (m, 2H), 2.07–2.04 (m, 1H), 1.81–1.70 (m, 1H), 1.47–1.43
(m, 27H). ESMS (m/z): 474.3 (M + H)+.
Glu-Urea-Gln
Macroscopically, the compound was a white solid
substance with a chemical formula of
C23H41N3O8. The purity
of the compound was 97.8%. 1H NMR (400 MHz;
CDCl3): δ6.83 (m, 1H), 5.76–5.58 (m, 2H), 4.37–4.30 (m,
2H), 2.35–2.05 (m, 2H), 1.89–1.80 (m, 2H), 1.47–1.43 (m, 27H). ESMS
(m/z): 488.3 (M + H)+.
Glu-Urea-Lys
Macroscopically, the compound was a white oily
substance with a chemical formula of
C24H45N3O7. The purity
of the compound was 97.5%. 1H NMR (400 MHz;
CDCl3): δ5.34–5.30 (m, 2H), 4.36–4.31 (m, 2H), 2.72–2.68
(m, 2H), 2.35–2.28 (m, 2H), 2.08–2.06 (m, 1H), 1.93–1.84 (m, 2H),
1.77–1.76 (m, 2H), 1.63–1.61 (m, 1H), 1.50–1.34 (m, 31H). ESMS
(m/z): 488.4 (M + H)+.
Glu-Urea-R treatment
Overall
Prior to the affinity experiment, tert-butyl in
Glu-urea-R was hydrolyzed to expose the active carboxyl group, so
that the compound would bind to PSMA.
Hydrolytic products of Glu-Urea-Orn (S36886)
The chemical formula is
C13H20N3F3O9.
1H NMR (400 MHz): deuterated water (D2O),
δ4.213–4.189 (m, 1H), δ4.062–4.035 (m, 1H), δ3.219–3.211 (d, 2H),
δ2.458–2.423 (m, 2H), δ2.124–2.089 (m, 1H), δ2.024–2.000 (m, 1H),
δ1.926–1.698 (m, 4H). ESMS (m/z): 304 (M - H).
Glu-urea-Asn (S36887)
The chemical formula is
C10H15N3O8.
1H NMR (400 MHz): D2O, δ4.680–4.488 (m, 1H),
δ4.227–4.181 (m, 1H), δ2.773–2.739 (m, 2H), δ2.459–2.411 (m, 2H),
δ2.137–2.073 (m, 1H), δ1.929–1.881 (m, 1H). ESMS (m/z): 304 (M -
H).
Glu-urea-Gln (S36888)
The chemical formula is
C11H17N3O8.
1H NMR (400 MHz): D2O, δ4.193–4.152 (m, 2H),
δ2.444–2.415 (m, 2H), δ2.318–2.292 (m, 2H), δ2.105–2.072 (m, 2H),
δ1.906–1.882 (m, 2H). ESMS (m/z): 318 (M - H).
Glu-urea-Lys (S36889)
The chemical formula is
C14H22N3F3O9.
1H NMR (400 MHz): D2O, δ4.211–4.129 (m, 2H),
δ2.936–2.898 (t, 2H), δ2.458–2.422 (t, 2H), δ2.131–2.082 (m, 1H),
δ1.927–1.781 (m, 2H), δ1.683–1.573 (m, 3H), δ1.434–1.363 (m, 2H).
ESMS (m/z): 304 (M - H).
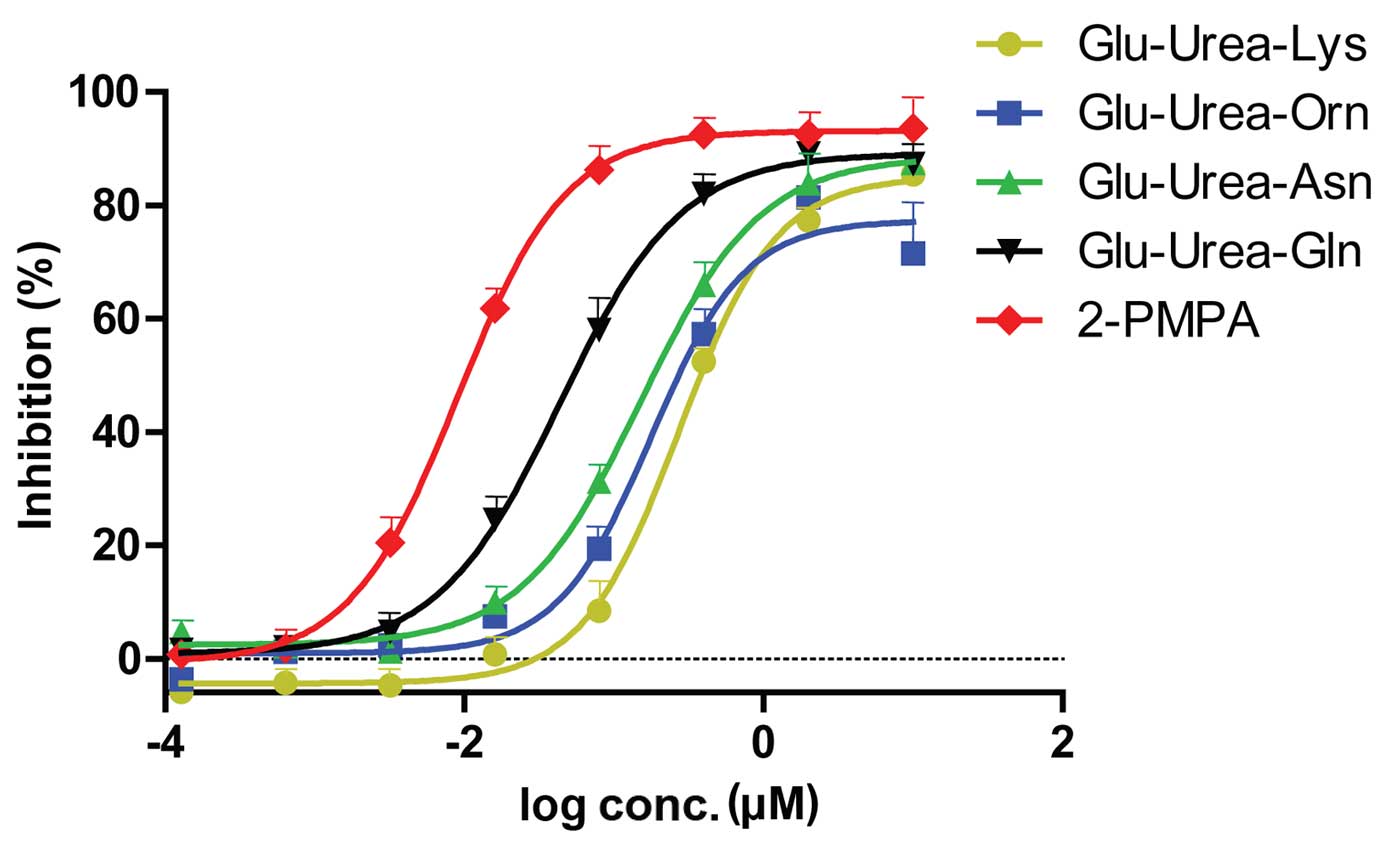
Affinity assays of Glu-Urea-R
Glu-urea-R of various concentrations were added to
the lysates of LNCaP cells, and fluorescence assays were performed.
The 2-PMPA group was considered to be the complete inhibition group
(positive control) and Tris was used as the negative control.
Therefore, the absorbance was obtained by subtracting the
absorbance of each compound from the absorbance of the Tris group.
The inhibition rate was calculated as follows: Inhibition rate = 1
- [(Avg X) × (Tris)] / [(Avg PSMA) (Avg Tris)], where X denotes a
generic compound. The inhibition rate of each group are shown in
Table I. The inhibition curves and
IC50 value of the 4 compounds obtained are revealed in
Fig. 3 and Table II, respectively. The compound with
the highest affinity to PSMA was Glu-urea-Gln, followed by
Glu-urea-Asn, Glu-urea-Orn and Glu-urea-Lys.
 | Table I.Inhibition rate of 4 synthetic
analogs of Glu-urea-R to prostate-specific membrane antigen. |
Table I.
Inhibition rate of 4 synthetic
analogs of Glu-urea-R to prostate-specific membrane antigen.
|
| Inhibition, % |
|---|
|
|
|
|---|
| log concentration,
µM | Glu-Urea-Lys | Glu-Urea-Orn | Glu-Urea-Asn | Glu-Urea-Gln | 2-PMPA |
|---|
| 1 | 85.488469 | 71.517575 | 87.521311 | 87.316559 | 93.611272 |
| 0.3010 | 77.366075 | 81.439916 | 83.885526 | 89.210723 | 92.531223 |
| −0.3979 | 52.428683 | 57.259946 | 65.909721 | 82.020320 | 92.567931 |
| −1.0969 |
8.406071 | 19.438644 | 31.258820 | 58.004315 | 86.281376 |
| −1.7959 |
0.731113 |
7.434516 |
9.939268 | 24.502802 | 61.777146 |
| −2.4949 |
−4.746629 |
2.362200 |
1.307847 |
4.772733 | 20.431408 |
| −3.1938 |
−4.261259 |
1.230759 |
2.202313 |
1.983285 |
1.781796 |
| −3.8928 |
−5.881333 |
−3.697986 |
4.722972 |
1.554202 |
0.792702 |
 | Table II.IC50 value of synthetic
analogs of Glu-urea-R. |
Table II.
IC50 value of synthetic
analogs of Glu-urea-R.
| Compound | IC50,
µM |
|---|
| Glu-Urea-Lys | 0.381 |
| Glu-Urea-Orn | 0.271 |
| Glu-Urea-Asn | 0.184 |
| Glu-Urea-Gln | 0.054 |
| 2-PMPA | 0.010 |
Discussion
Individualized diagnosis is the main objective for
early Pca diagnosis, and it aims to achieve detection of Pca in
advanced stages, prevent local development and metastasis of Pca,
reduce Pca mortality and improve the quality of life of patients. A
study that took place in Tyrol in Austria revealed that early
screening and diagnosis may reduce the Pca mortality rate by 33%
(13). Another study in Canada
demonstrated that a lower mortality rate may be achieved in
individuals that actively cooperate with Pca examination (14). A study by the European Randomized
Study of Screening for Prostate Cancer and the Prostate, Lung,
Colorectal, and Ovarian cancer screening trial found that
performing the prostate-specific antigen (PSA) test for Pca once
every 4 year reduces the mortality of Pca by 20% (15). However, there are risks of
overdiagnosis using this method (15), and consequently, it is inappropriate
to perform large-scale screenings of Pca, but it remains necessary
to educate individuals about early examination. Although
conventional imaging methods have achieved considerable progress in
sensitivity and accuracy, there is a lack of effective imaging
measures for early diagnosis, localized diagnosis of metastases,
choice of postoperative treatment and prevention of postoperative
recurrence, and it is challenging to obtain a clear diagnosis of
early Pca (16,17).
A digital rectal examination combined with a PSA
assay is widely recognized as the best screening method for early
Pca (18). With the development of
novel technologies, novel diagnostic methods have gradually been
identified. PCA3 is a novel biological marker that is
detected in the urine sediment, and it is one of the most specific
markers of Pca (19). Another
diagnostic method is gene detection; genome-wide association
studies revealed that at present >30 susceptible loci are
associated with Pca in various populations around the world
(19). Furthermore, it was
demonstrated that an increase in hypermorph susceptibility genes of
Pca leads to an increased risk for Pca, which meant that Pca
susceptibility genes have a clear cumulative effect (20).
PET/CT is a diagnostic imaging technique that
combines cellular molecular metabolism with morphology (21). Radionuclide-labeled tracer or
molecular probes targeting tumor cells effectively identify tumor
lesions that are challenging to detect with traditional imaging
methods, and PET/CT has become a powerful tool for the clinical
diagnosis of tumors (22). At
present, 11C-choline and other cell metabolic substrates
are used as PET/CT tracers, which have achieved a certain efficacy
in the diagnosis of Pca (23,24). However, these tracers lack specificity
to Pca, and it is challenging to distinguish between primary and
metastatic Pca, which is prone to false-positive results of benign
prostatic hyperplasia and prostatitis lesions (23,24).
Therefore, it is essential to identify an efficient and specific
molecular probe to improve the sensitivity and accuracy of PET/CT
in the diagnosis of Pca, which may be capable of effectively
providing early diagnosis, preoperative staging, postoperative
recurrence and restaging and postoperative efficacy evaluation and
detection.
PSMA as a target protein has become a major study
topic in recent years. Glu-urea-R inhibits the activity of PMSA,
and effectively and specifically binds to PSMA on the Pca cell
surface. Compared with the PSMA monoclonal antibody, Glu-urea-R is
characterized by stable biological activity, short in vivo
half-life, good permeability to tumor parenchyma and tumor
metastases and low side-effects in vivo (8). At present, studies have demonstrated
that Glu-urea-R PET/CT molecular probes may provide novel means for
the diagnosis of Pca (12).
The present study investigated the preparation of
Glu-urea-R analogs that target PSMA. Raw materials were employed to
obtain the desired compounds under anhydrous conditions, nitrogen
protection and a controlled reaction temperature. NMR spectroscopy
and LC-MS confirmed that the obtained products had the desired
structures, and their purities were identified to be >97% using
HPLC. Previous studies have confirmed that Glu-urea-R, which
contains a free carboxyl group III in its R-group, can bind with
high affinity to the active site of PSMA (11). Therefore, Glu-urea-R analogs are
capable of binding to PSMA. Glu-urea is the part of the molecule
that binds to PSMA, and the R-group is easily connected to other
chemical groups. Affinity experiments of the 4 compounds
synthesized by the present study, Glu-urea-Gln, Glu-urea-Lys,
Glu-urea-Asn and Glu-urea-Orn, to PSMA were performed to
preliminarily determine their binding activity with Pca cells and
compare their affinities, so as to provide data for future
experiments.
Overall, the present study revealed that these
analogs, particularly Glu-urea-Asn, provided an enriched choice for
positron isotope labeling, since they had a high affinity to PSMA.
Positron isotopes are used to label Glu-urea-R to investigate Pca
using PET/CT, and these compounds have high specificity and
sensitivity and low toxicity, therefore providing novel strategies
for early diagnosis, preoperative staging, postoperative recurrence
and restaging, targeted therapy of Pca as well as patient selection
for targeted therapy, efficacy evaluation and monitoring. In
conclusion, the present study demonstrated that Glu-urea-R
specifically binds to PSMA expressed in prostate cell line LNCap
and inhibits its activity, which is important for the provision of
novel diagnostic methods for Pca.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.,
30670544 and 81271603). The authors would like to thank Sundia
MediTech Company, Ltd. (Shanghai, China) for performing the
synthesis of and hydrolysis of Glu-urea-R and providing excellent
technical support.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Risk MC, Knudsen BS, Coleman I, Dumpit RF,
Kristal AR, LeMeur N, Gentleman RC, True LD, Nelson PS and Lin DW:
Differential gene expression in benign prostate epithelium of men
with and without prostate cancer: Evidence for a prostate cancer
field effect. Clin Cancer Res. 16:5414–5423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W and Mo ZN: Advances in
prostate-specific membrane antigen targeted therapies for prostate
cancer. Zhonghua Nan Ke Xue. 16:547–551. 2010.(In Chinese).
PubMed/NCBI
|
|
4
|
Cheng L, Song SY, Pretlow TG, Abdul-Karim
FW, Kung HJ, Dawson DV, Park WS, Moon YW, Tsai ML, Linehan WM, et
al: Evidence of independent origin of multiple tumors from patients
with prostate cancer. J Natl Cancer Inst. 90:233–237. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhao X, Chen L, Guo H, Lv F, Jia K,
Yv K, Wang F, Li C, Qian J, et al: Safety and pharmacokinetics of
novel selective vascular endothelial growth factor receptor-2
inhibitor YN968D1 in patients with advanced malignancies. BMC
Cancer. 10:5292010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heston WD: Significance of
prostate-specific membrane antigen (PSMA). A neurocarboxypeptidase
and membrane folate hydrolase. Urologe A. 35:400–407. 1996.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bander NH, Nanus DM, Milowsky MI,
Kostakoglu L, Vallabahajosula S and Goldsmith SJ: Targeted systemic
therapy of prostate cancer with a monoclonal antibody to
prostate-specific membrane antigen. Semin Oncol. 30:667–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bander NH, Trabulsi EJ, Kostakoglu L, Yao
D, Vallabhajosula S, Smith-Jones P, Joyce MA, Milowsky M, Nanus DM
and Goldsmith SJ: Targeting metastatic prostate cancer with
radiolabeled monoclonal antibody J591 to the extracellular domain
of prostate specific membrane antigen. J Urol. 170:1717–1721. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elsässer-Beile U, Reischl G, Wiehr S,
Bühler P, Wolf P, Alt K, Shively J, Judenhofer MS, Machulla HJ and
Pichler BJ: PET imaging of prostate cancer xenografts with a highly
specific antibody against the prostate-specific membrane antigen. J
Nucl Med. 50:606–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang AX, Murelli RP, Barinka C, Michel J,
Cocleaza A, Jorgensen WL, Lubkowski J and Spiegel DA: A remote
arene-binding site on prostate specific membrane antigen revealed
by antibody-recruiting small molecules. J Am Chem Soc.
132:12711–12716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kularatne SA, Wang K, Santhapuram HK and
Low PS: Prostate-specific membrane antigen targeted imaging and
therapy of prostate cancer using a PSMA inhibitor as a homing
ligand. Mol Pharm. 6:780–789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maresca KP, Hillier SM, Femia FJ, Keith D,
Barone C, Joyal JL, Zimmerman CN, Kozikowski AP, Barrett JA,
Eckelman WC and Babich JW: A series of halogenated heterodimeric
inhibitors of prostate specific membrane antigen (Psma) as
radiolabeled probes for targeting prostate cancer. J Med Chem.
52:347–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartsch G, Horninger W, Klocker H,
Reissigl A, Oberaigner W, Schönitzer D, Severi G, Robertson C and
Boyle P: Tyrol Prostate Cancer Screening Group: Prostate cancer
mortality after introduction of prostate-specific antigen mass
screening in the federal state of tyrol, Austria. Urology.
58:417–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Labrie F, Candas B, Dupont A, Cusan L,
Gomez JL, Suburu RE, Diamond P, Lévesque J and Belanger A:
Screening decreases prostate cancer death: First analysis of the
1988 Quebec prospective randomized controlled trial. Prostate.
38:83–91. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schroder FH, Hugosson J, Roobol MJ,
Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H,
Zappa M, et al: Screening and prostate-cancer mortality in a
randomized European study. N Engl J Med. 360:1320–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajasekaran AK, Anilkumar G and
Christiansen JJ: Is prostate-specific membrane antigen a
multifunctional protein? Am J Physiol Cell Physiol. 288:C975–C981.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosh A and Heston WD: Tumor target
prostate specific membrane antigen (PSMA) and its regulation in
prostate cancer. J Cell Biochem. 91:528–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohler JL, Kantoff PW, Armstrong AJ,
Bahnson RR, Cohen M, D’Amico AV, Eastham JA, Enke CA, Farrington
TA, Higano CS, et al: National comprehensive cancer network:
Prostate cancer, version 1.2014. J Natl Compr Canc Netw.
11:1471–1479. 2013.PubMed/NCBI
|
|
19
|
Auprich M, Bjartell A, Chun FK, de la
Taille A, Freedland SJ, Haese A, Schalken J, Stenzl A, Tombal B and
van der Poel H: Contemporary role of prostate cancer antigen 3 in
the management of prostate cancer. Eur Urol. 60:1045–1054. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng SL, Sun J, Wiklund F, Smith S,
Stattin P, Li G, Adami HO, Hsu FC, Zhu Y, Bälter K, et al:
Cumulative association of five genetic variants with prostate
cancer. N Engl J Med. 358:910–919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Wever W, Coolen J and Verschakelen JA:
Integrated PET/CT and cancer imaging. JBR-BTR. 92:13–19.
2009.PubMed/NCBI
|
|
22
|
Del Vecchio S, Zannetti A, Fonti R,
Iommelli F, Pizzuti LM, Lettieri A and Salvatore M: PET/CT in
cancer research: From preclinical to clinical applications.
Contrast Media Mol Imaging. 5:190–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel CN, Goldstone AR, Chowdhury FU and
Scarsbrook AF: FDG PET/CT in oncology: ‘Raising the bar’. Clin
Radio. 165:522–535. 2010. View Article : Google Scholar
|
|
24
|
Picchio M, Briganti A, Fanti S,
Heidenreich A, Krause BJ, Messa C, Montorsi F, Reske SN and
Thalmann GN: The role of choline positron emission
tomography/computed tomography in the management of patients with
prostate-specific antigen progression after radical treatment of
prostate cancer. Eur Urol. 59:51–60. 2011. View Article : Google Scholar : PubMed/NCBI
|