Introduction
Esophageal cancer is the most common
gastrointestinal cancer, and is ranked sixth in terms of worldwide
cancer-associated mortality rate (1).
According to the World Health Organization histological
classification, esophageal cancer includes squamous cell carcinoma
(SCC) and adenocarcinoma of the esophagus, which account for 90 and
5% of cases, respectively (2). The
remaining 5% of cases are rare types of esophageal cancer,
including epidermal mucinous carcinoma, small cell carcinoma,
leiomyosarcoma and others (3). In the
past recent years, the incidence of esophageal adenocarcinoma in
the United States and Europe has increased significantly (4). The majority of esophageal cancer cases
are SCC, which is ranked fourth in terms of cancer-associated
mortality, and the current average annual incidence rate of SCC is
17/100,000 patients (5,6). The male incidence rate of SCC is
generally higher, compared with that of females (7). However, there are large differences
between different regions, and China is known to be an ‘esophageal
cancer-prone region’ (8).
As an important nuclear transcription factor,
nuclear factor (NF)-κB is able to mediate environmental stimuli,
and exerts numerous biological functions via control of gene
transcription, including regulation of cell proliferation and
apoptosis-mediated immunity, inflammation and tumor formation
(9). Han et al (10) indicated that neuregulin 1 was able to
promote the progression of gastric cancer via NF-κB inactivation.
In addition, Dai et al (11)
demonstrated that Golgi phosphoprotein 3 was able to promote
hepatocellular carcinoma cell aggressiveness via the NF-κB
signaling pathway. Wang et al (12) reported that andrographolide was able
to induce apoptosis of esophageal cancer cells via suppression of
the NF-κB signaling pathway.
Abnormal apoptosis is directly associated with the
occurrence and development of a number of diseases, including
cancer, viral diseases and a variety of degenerative diseases
(13). Previous studies have revealed
that constitutively activated phosphatidylinositol 3-kinase
(PI3K)/Akt signaling may lead to the development of disorders
classified within the human tumor spectrum, including ovarian,
breast, endometrial and nasopharyngeal cancer, as well as
glioblastoma, medulloblastoma and myeloproliferative abnormal
syndrome (14). Furthermore, Wang
et al (15) reported that
hypomethylation of the catalytic subunit alpha of PI3K has a
significant role in the activation of the PI3K/Akt signaling
pathway in esophageal cancer. Li et al (16) demonstrated that inhibitor of DNA
binding 1, dominant negative helix-loop-helix protein promoted
metastasis of human esophageal cancer cells via activation of the
PI3K/Akt signaling pathway.
Previous studies have identified that psoralidin
contains a variety of compounds, including coumarin, flavonoids,
monoterpenes and phenols, which may have immunomodulatory,
anti-inflammatory, antioxidant and antitumor effects (17–19). Yang
et al (17) reported that
psoralidin inhibited the proliferation of androgen-independent
prostate cancer cells via PI3K-mediated Akt signaling. Furthermore,
Hao et al (20) reported that
psoralidin was able to inhibit the proliferation of human lung
cancer A549 cells. The present study aimed to provide the first
evidence of the anticancer effect of psoralidin on esophageal
cancer, and render mechanistic insights into the antitumor action
of this compound against human esophageal carcinoma Eca9706
cells.
Materials and methods
Reagents
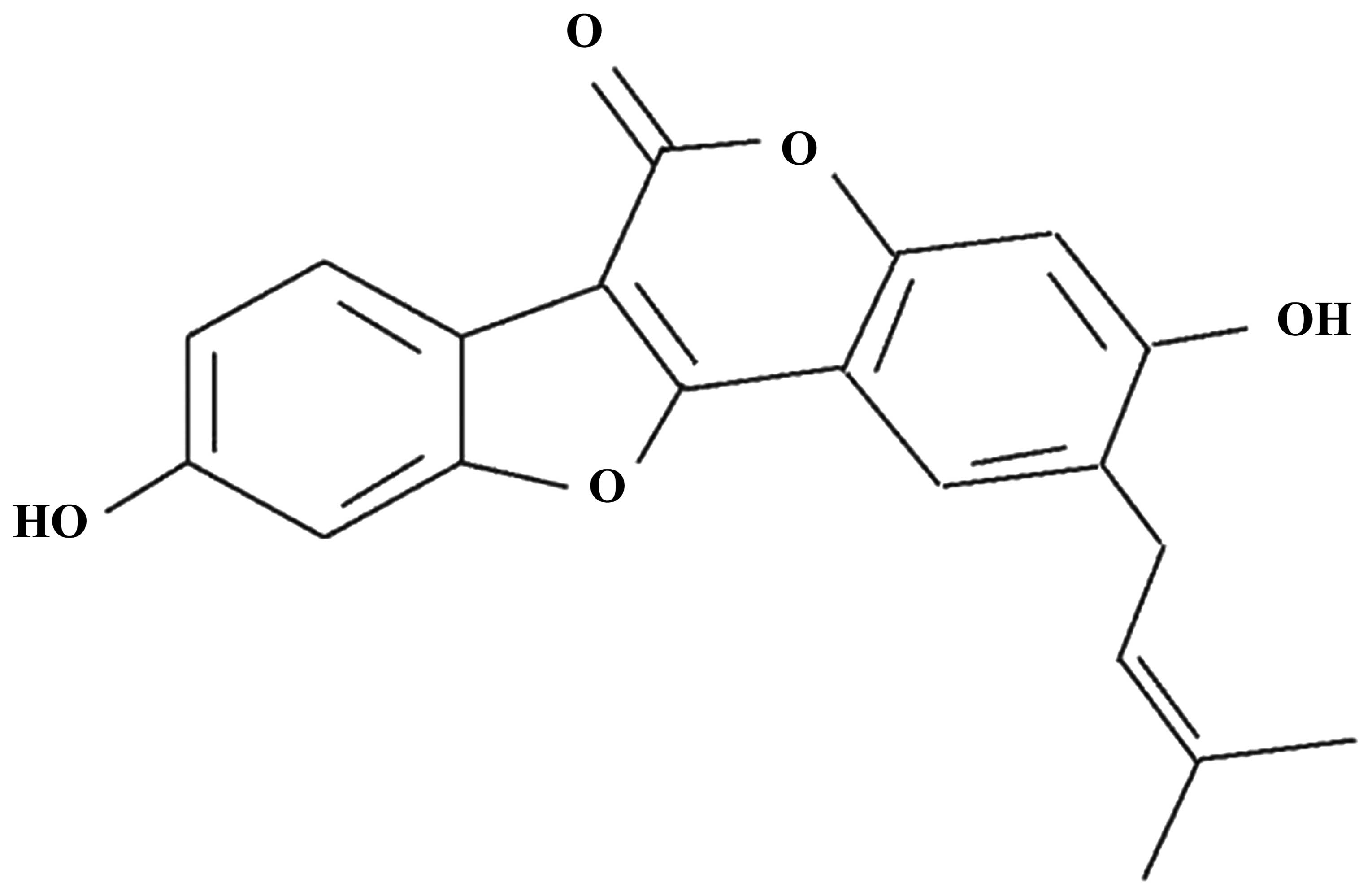
Psoralidin (purity, ≥98%), the chemical structure of
which is shown in Fig. 1, was
purchased from Sigma-Aldrich (St. Louis, MO, USA).
4′,6-diamidino-2-phenylindole (DAPI) was also obtained from
Sigma-Aldrich. RPMI-1640 was obtained from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China). Fetal bovine serum (FBS) was obtained
from HyClone (GE Healthcare Life Sciences, Logan, UT, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sangon Biotech Co., Ltd. (Shanghai, China).
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kit was obtained from BestBio Biotechnology
Co., Ltd. (Shanghai, China). Caspase-3 colorimetric assay kit and
NF-κB enzyme-linked immunosorbent assay (ELISA) kit were acquired
from Beyotime Institute of Biotechnology (Nanjing, China).
Cell culture
The Eca9706 human esophageal carcinoma cell line was
acquired from the Department of Oncology of Jingzhou Central
Hospital (Jingzhou, China). Cells were cultured in RPMI-1640 medium
supplemented with 10% (v/v) FBS, 100 U/ml penicillin (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 mg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.), at 37°C
in a humidified atmosphere of 5% CO2. The culture medium
was replaced every 2–3 days, and fresh complete medium was added to
cells.
MTT assay
The viability of Eca9706 cells (2.0×104
cells/well) was investigated following incubation with psoralidin
(0, 5, 10 and 20 µM). Cells were incubated at 37°C in a humidified
atmosphere of 5% CO2 for 0, 1, 2 and 3 days in 96-well
plates (Thermo Fisher Scientific). Eca9706 cells were washed twice
using phosphate-buffered saline (PBS; Sangon Biotech Co., Ltd.),
and 10 µl MTT was added to each well. Subsequently, Eca9706 cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2 for 4 h. Following incubation, the culture medium
was removed, and 150 µl dimethyl sulfoxide (Invitrogen; Thermo
Fisher Scientific, Inc.) was added into each well. Eca9706 cells
were incubated for 20 min at room temperature. The absorbance of
Eca9706 cells at 570 nm (iMark microplate reader; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was determined by MTT assay,
as previously described (21).
Flow cytometric detection of cellular
apoptosis
Eca9706 cells (2×106 cells/well) were
cultured in 6-well plates (Thermo Fisher Scientific) with
psoralidin (0, 5, 10 and 20 µM) at 37°C in a humidified atmosphere
of 5% CO2 for 2 days. Following incubation, Eca9706
cells were collected and washed twice with cold PBS. Subsequently,
annexin V binding buffer was used to resuspend Eca9706 cells
(1×106 cells/ml) in a test tube. A total of 10 µl
annexin V-FITC was added to the resuspended Eca9706 cells, and
incubated for 30 min in the dark. Following incubation, 5 µl PI was
added to the resuspended Eca9706 cells, and incubated for 10 min in
the dark. Apoptosis of Eca9706 cells was immediately measured using
flow cytometry (COULTER® EPICS® ALTRA™ Flow Cytometer; Beckman
Coulter, Inc., Brea, CA, USA).
DAPI staining assay
Eca9706 cells (2×106 cells/well) were
cultured in 6-well plates with psoralidin (0, 5, 10 and 20 µM), at
37°C in a humidified atmosphere of 5% CO2 for 2 days.
PBS was used to wash the cells, prior to the addition of 0.5
ml/well 4% paraformaldehyde (Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China). Cells were fixed for 30
min at 4°C, washed twice with PBS, and incubated for 5 min at 4°C
in the presence of sodium citrate (0.1%; Xinfan Biological
Technology Co., Ltd., Shanghai, China) containing 0.1% Triton X-100
(Biosharp, St. Louis, MO, USA). DAPI (5 µg/ml)was added to each
well, and incubated for 10–15 min at 4°C in the dark. Eca9706 cells
were observed and photographed under a fluorescence microscope
(Axio Observer A1; Zeiss AG, Oberkochen, Germany) at
excitation/emission ~340/450 nm.
Detection of caspase-3 activity
Eca9706 cells (2×106 cells/well) were
cultured in 6-well plates with psoralidin (0, 5, 10 and 20 µM) at
37°C in a humidified atmosphere of 5% CO2 for 2 days.
Caspase-3 activity was detected at a wavelength of 405/650 nm
(excitation/emission) using a caspase-3 colorimetric assay kit,
according to the manufacturer's protocol.
Measurement of NF-κB activity
Eca9706 cells (2×106 cells/well) were
cultured in 6-well plates with psoralidin (0, 5, 10 and 20 µM) at
37°C in a humidified atmosphere of 5% CO2 for 2 days.
NF-κB activity was analyzed using an ELISA kit, according to the
manufacturer's protocol.
Western blot analysis
Eca9706 cells (2×106 cells/well) were
cultured in 6-well plates with psoralidin (0, 5, 10 and 20 µM) at
37°C in a humidified atmosphere of 5% CO2 for 2 days.
Subsequently, Eca9706 cells were incubated on ice for 30 min with
ice-cold lysis buffer (Biosharp), and centrifuged at 12,000 × g
(Biosharp) for 10 min at 4°C. The supernatant was collected in
order to determine the total protein concentration using Pierce BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc.). Equivalent
amounts of total protein were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Tiandz, Inc., Beijing,
China) and subsequently transferred onto a polyvinylidene
difluoride membrane (pore size, 0.22 µm; Roche Diagnostics GmbH,
Mannheim, Germany). The membrane was blocked with Tris-buffered
saline (TBS; Tiandz, Inc.) containing 5% skimmed milk for 2 h at
room temperature, and subsequently incubated overnight at 4°C with
goat polyclonl anti-PI3K (catalog no., sc-48637; dilution, 1:2,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal
anti-Akt (catalog no., sc-293125; dilution, 1:2,500; Santa Cruz
Biotechnology, Inc.) or mouse monclonal anti-β-actin (catalog no.,
sc-8432; dilution, 1:500; Santa Cruz Biotechnology, Inc.; control)
antibodies. The membrane was washed twice using TBS and 0.05% Tween
20 (Biosharp) for 2 h, and subsequently incubated at room
temperature for 2 h with horseradish peroxidase-conjugated rabbit
anti-mouse immunoglobulin G (catalog no., sc-358922; dilution,
1:1,000; Santa Cruz Biotechnology, Inc.). The specific protein
bands were detected using enhanced chemiluminescence (Beyotime
Institute of Biotechnology), FluorChem™(ProteinSimple, Santa Clara,
CA, USA) and AlphaEaseFC™ software (Alpha Innotech, San Leandro,
CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS version
17.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as the mean
± standard deviation of ≥3 independent experiments. Data was
analyzed using the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Psoralidin inhibits the viability of
Eca9706 cells
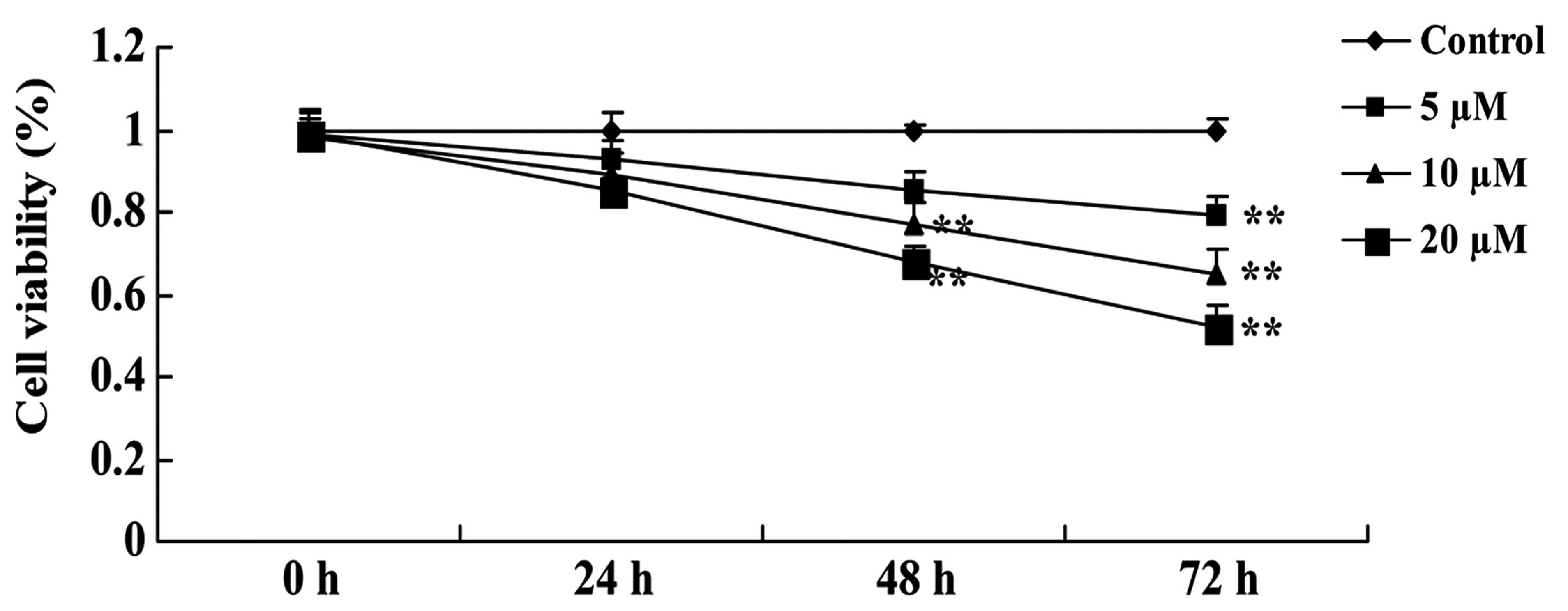
In order to clarify the anticancer effect of
psoralidin (0, 5, 10 and 20 µM) on the viability of Eca9706 cells,
cell viability was evaluated using an MTT assay. Treatment with
psoralidin (5, 10 and 20 µM) affected the viability of Eca9706
cells in a dose- and time-dependent manner. Notably, the anticancer
effect of psoralidin at 10 and 20 µM significantly inhibited the
proliferation of Eca9706 cells at 48 and 72 h (Fig. 2). Therefore, 48-h exposure to 10 µM
psoralidin was selected as the standard pretreatment in subsequent
experiments.
Psoralidin induces apoptosis of
Eca9706 cells
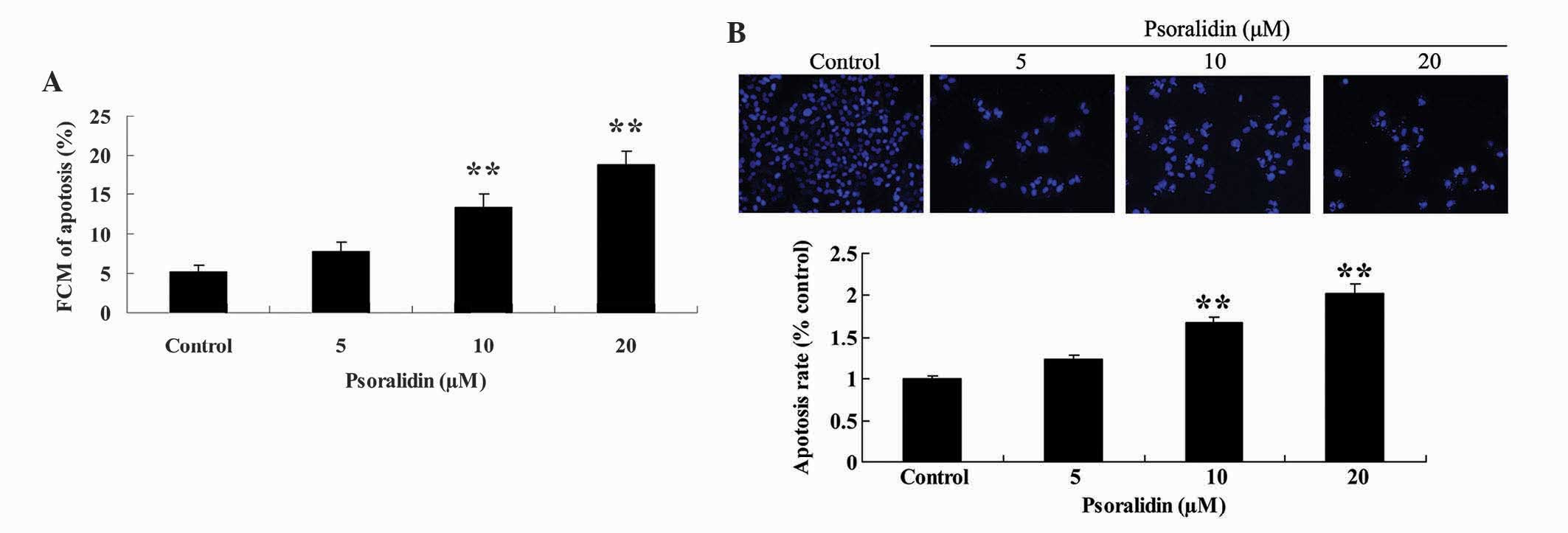
In order to reveal the anticancer effect of
psoralidin (0, 5, 10 and 20 µM) on the apoptosis of Eca9706 cells,
the apoptotic rate of Eca9706 cells exposed to psoralidin was
analyzed with flow cytometry and DAPI staining assay. Treatment
with psoralidin (5, 10 and 20 µM) induced apoptosis of Eca9706
cells in a dose-dependent manner. Notably, the apoptotic rate of
Eca9706 cells was significantly increased due to the anticancer
effect of psoralidin (10 and 20 µM) following 48 h of incubation
(Fig. 3A). Furthermore, apoptosis of
Eca9706 cells was augmented in psoralidin-treated (5, 10 and 20 µM)
cells, compared with the control group (Fig. 3B).
Psoralidin induces caspase-3 activity
in Eca9706 cells
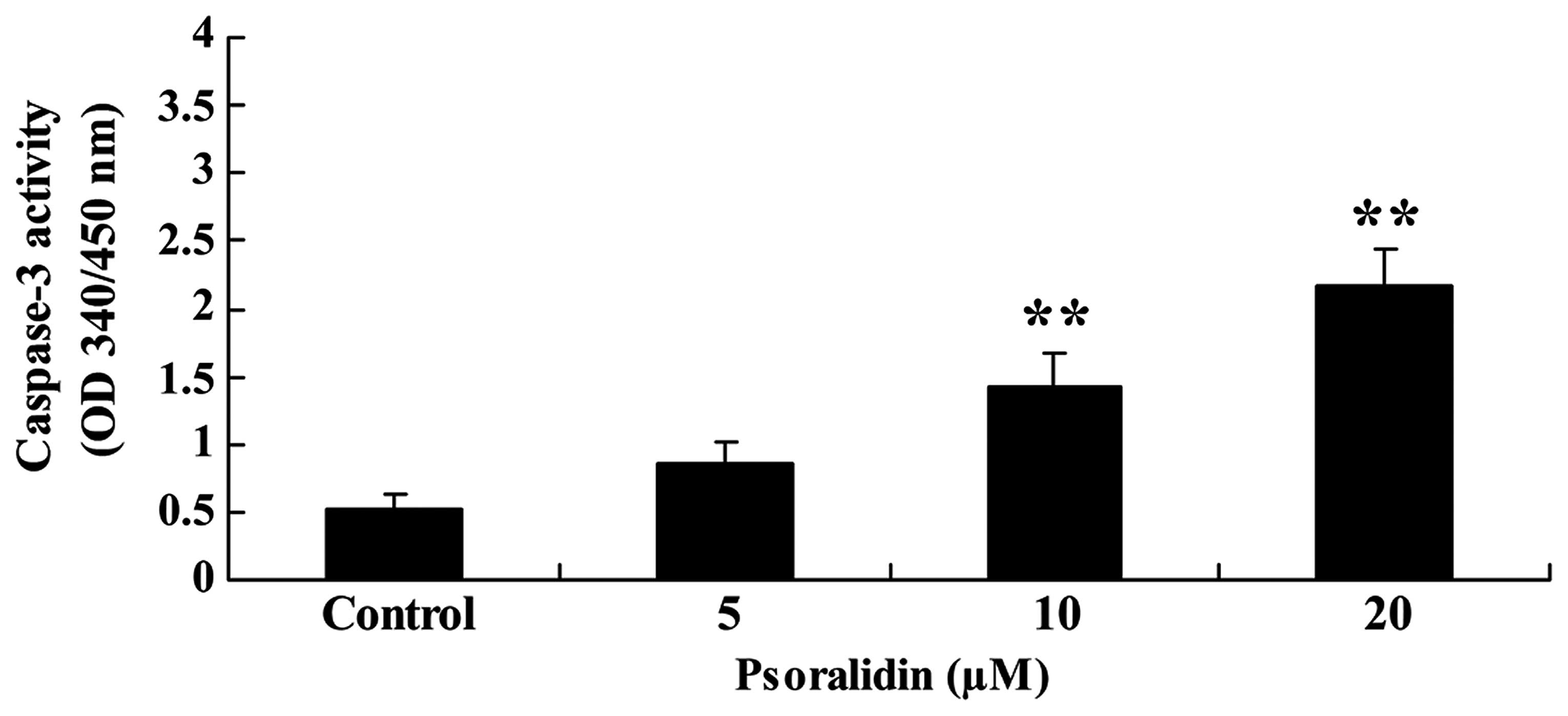
In order to investigate the anticancer effect of
psoralidin (0, 5, 10 and 20 µM) on the caspase-3 activity of
Eca9706 cells, caspase-3 activity was measured using a colorimetric
assay kit. Treatment with psoralidin (5, 10 and 20 µM) induced
caspase-3 activity in a dose-dependent manner. Notably, following
treatment with psoralidin (10 and 20 µM) for 48 h, caspase-3
activity was significantly augmented (Fig. 4).
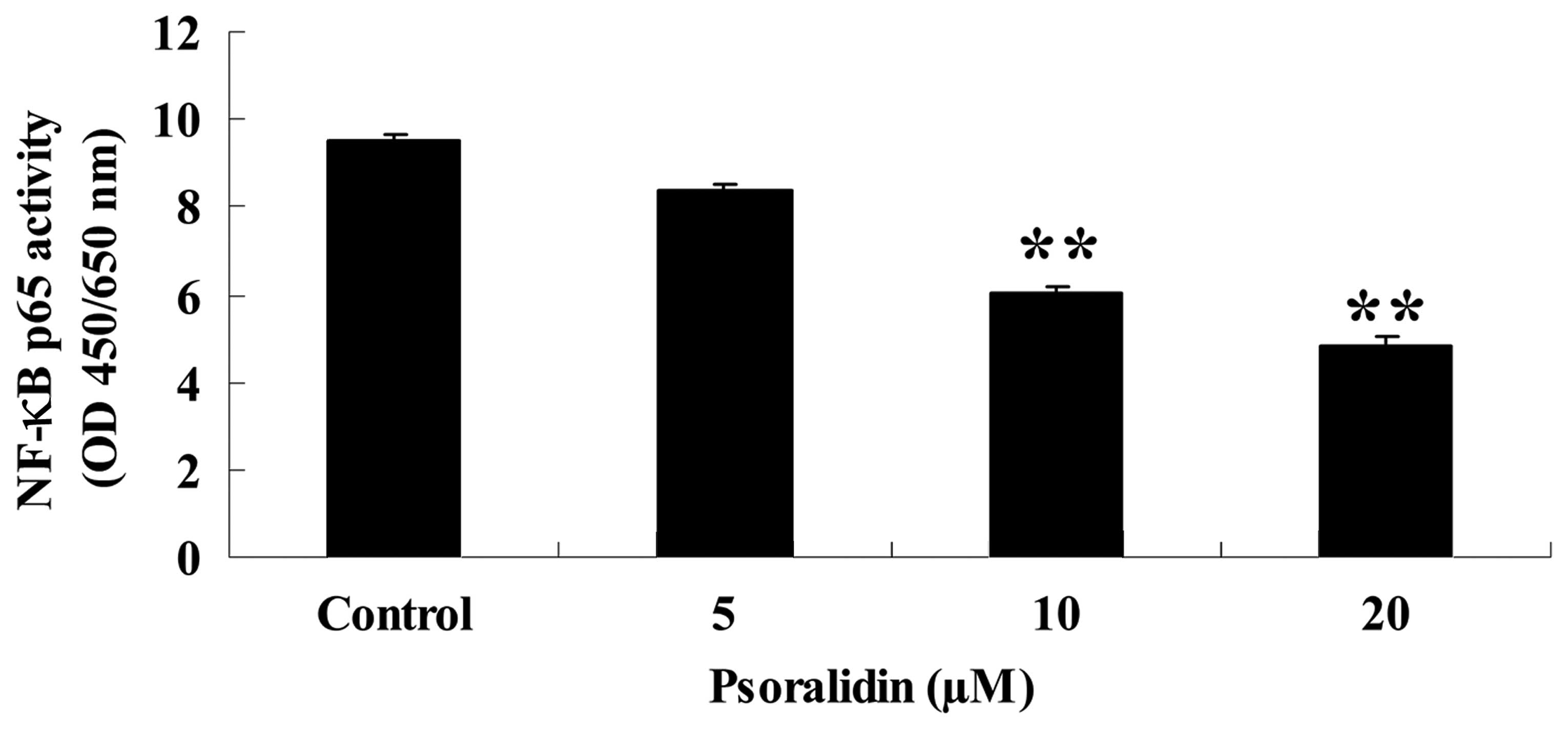
Psoralidin inhibits NF-κB activity in
Eca9706 cells
In order to investigate the effect on NF-κB activity
caused by treatment with psoralidin (0, 5, 10 and 20 µM) in Eca9706
cells, the activity of the p65 subunit of NF-κB was investigated
using an ELISA kit. Treatment with psoralidin (5, 10 and 20 µM)
inhibited the activity of NF-κB in Eca9706 cells (Fig. 5).
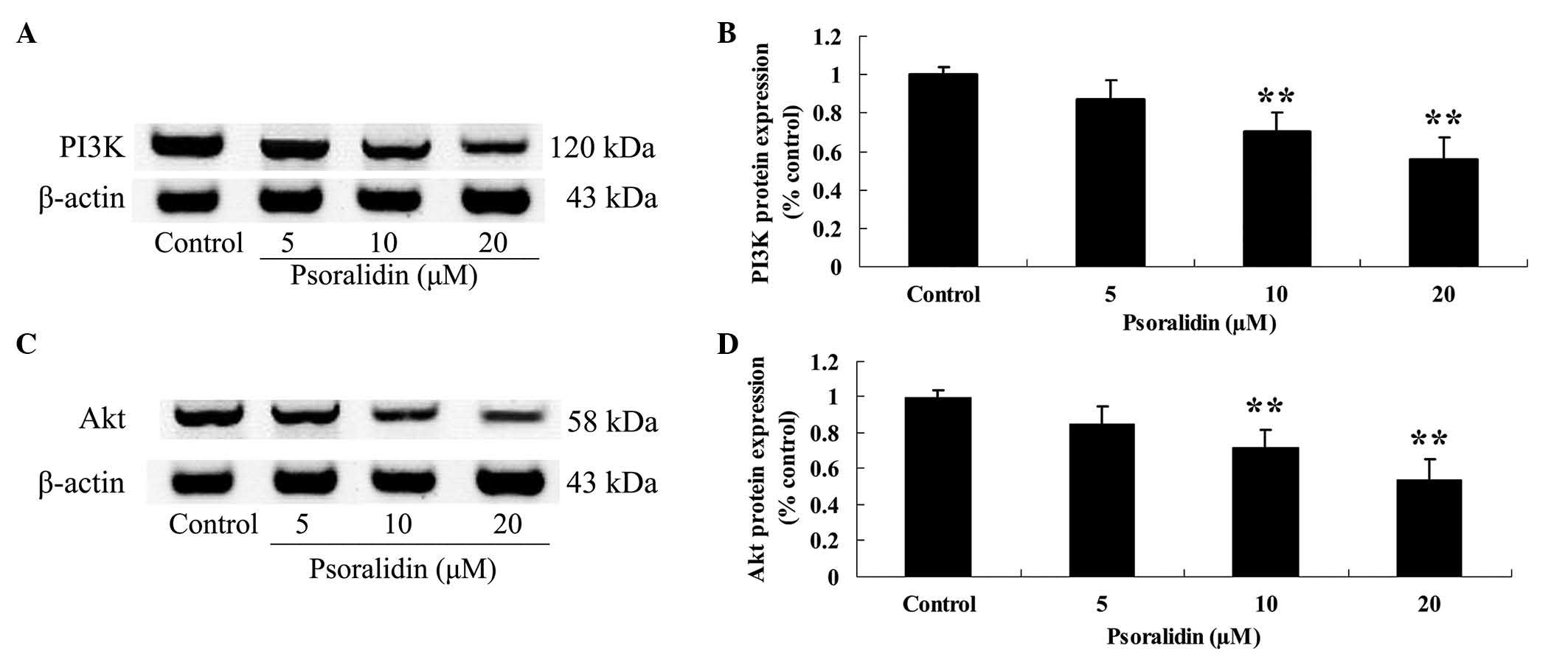
Psoralidin inhibits PI3K and Akt
protein expression in Eca9706 cells
In order to determine the effect of psoralidin (0,
5, 10 and 20 µM) on the PI3K/Akt signaling pathway in Eca9706
cells, PI3K and Akt protein expression in Eca9706 cells was
detected by western blot analysis. Treatment with psoralidin (5, 10
and 20 µM) reduced the protein expression levels of PI3K and Akt in
Eca9706 cells (Fig. 6).
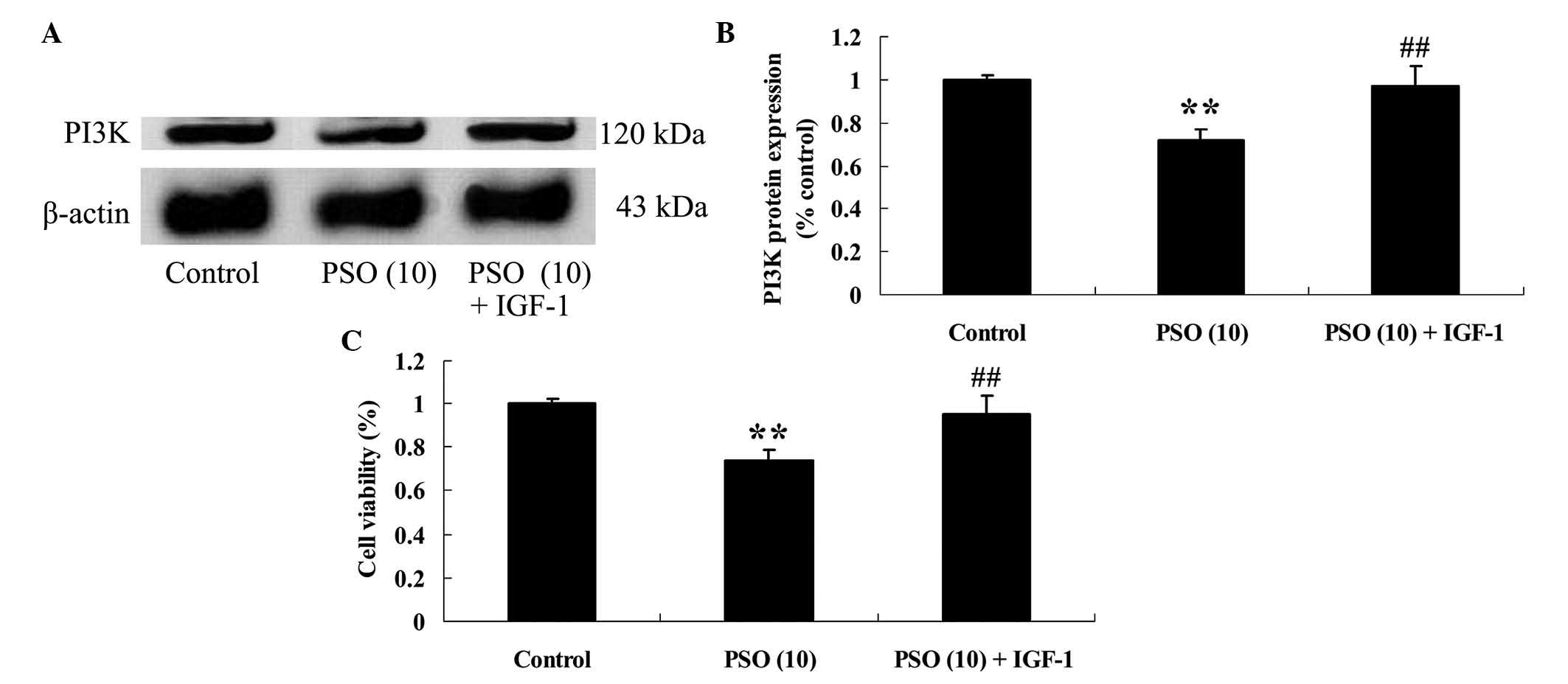
PI3K agonist is able to reverse the
effect of psoralidin on Eca9706 cells
In order to investigate the potential association
between upregulation of PI3K protein expression and the effect of
psoralidin on Eca9706 cells, Eca9706 cells were incubated with a
PI3K agonist, namely insulin-like growth factor 1 (1 µg/10 µl) for
48 h. Notably, the PI3K agonist markedly increased PI3K protein
expression in Eca9706 cells (Fig. 7A and
B), and increased the viability of Eca9706 cells (Fig. 7C), compared with the
psoralidin-treated (10 µM) group.
Discussion
Esophageal cancer is a common type of human cancer,
and is ranked second and third in terms of cancer-associated
mortality in men and women, respectively (22). The treatment of esophageal cancer may
include surgery, radiation therapy and comprehensive treatment,
among which, surgical treatment is preferred (23). However, only 1/4 of patients are able
to tolerate radical surgery (24).
Radiation therapy is a safe and effective method for the treatment
of esophageal cancer (25). As cancer
possesses the characteristics of recurrence and metastasis, a
review of the efficacy of radiation therapy for patients with
esophageal cancer prior to discharge from hospital is important for
consolidation and reduction of the recurrence rate (26). In the present study, the anticancer
effect of psoralidin resulted in significant inhibition of cell
proliferation and increased apoptosis of Eca9706 cells in a
dose-dependent manner. Furthermore, psoralidin was additionally
able to decrease the caspase-3 activity of Eca9706 cells in a
dose-dependent manner. Hao et al (20) reported that psoralidin was able to
inhibit the proliferation of A549 human lung cancer cells via
generation of reactive oxygen species. Yang et al (17) reported that psoralidin was able to
inhibit cell viability and induce apoptosis in human prostate
cancer PC-3 and DU-145 cells. Das et al (27) suggested that psoralidin promoted
growth arrest via activation of caspase-3 and caspase-9 in prostate
cancer cells.
Loss of control of NF-κB activity is associated with
the occurrence of mammalian tumors (28). Activation and abnormal expression of
NF-κB is observed in numerous tumors (29). In esophageal cancer tissue, NF-κB gene
amplification is common in the form of multiple internal and
external factors acting on the body, which encode proteins that are
expressed in the cytoplasm and nucleus (30). A number of intracellular signal
transduction pathways are activated, and malignant cells abnormally
proliferate, eventually leading to cancer (31). The present study demonstrated that
treatment with psoralidin inhibited the NF-κB activity of Eca9706
cells. Chiou et al (32)
reported that psoralidin inhibited lipopolysaccharide-induced
expression of nitric oxide via the NF-κB signaling pathway. Yang
et al (17) suggested that
psoralidin regulated ionizing radiation-induced pulmonary
inflammation via modulation of the NF-κB signaling pathway.
Previous studies have demonstrated that the PI3K/Akt
signaling pathway has a significant role in the development of a
number of tumors (33). PI3K/Akt
signaling primarily exerts anti-apoptotic effects by affecting
multiple downstream effector molecules (34). Knockout of PI3K, Akt and associated
genes by genetic intervention, or inhibition by small molecule
drugs, which blocks the activation of downstream anti-apoptotic
effector molecules and promotes apoptosis, has become a key focus
of research on cancer treatment (35). The results of the present study
suggested that psoralidin was able to reduce the protein expression
levels of PI3K and Akt in Eca9706 cells; however, upregulation of
PI3K protein expression reduced the viability of Eca9706 cells.
Previous studies have demonstrated that psoralidin is able to
regulate ionizing radiation-induced pulmonary inflammation via
regulation of the PI3K/Akt signaling pathway (17), and is also capable of inhibiting
lipopolysaccharide-induced nitric oxide expression via the
activation of PI3K/Akt-mediated signaling (32).
In conclusion, the results of the present study
suggested that psoralidin may have significant therapeutic effects
on esophageal cancer, via the NF-κB and PI3K/Akt signaling
pathways. The results of the present study additionally suggest the
potential benefits of the use of psoralidin in clinical
practice.
References
|
1
|
He B, Yin B, Wang B, Xia Z, Chen C and
Tang J: MicroRNAs in esophageal cancer (Review). Mol Med Rep.
6:459–465. 2012.PubMed/NCBI
|
|
2
|
Yano T, Muto M, Yoshimura K, Niimi M, Ezoe
Y, Yoda Y, Yamamoto Y, Nishisaki H, Higashino K and Iishi H: Phase
I study of photodynamic therapy using talaporfin sodium and diode
laser for local failure after chemoradiotherapy for esophageal
cancer. Radiat Oncol. 7:1132012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kano Y, Konno M, Ohta K, Haraguchi N,
Nishikawa S, Kagawa Y, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T,
et al: Jumonji/Arid1b (Jarid1b) protein modulates human esophageal
cancer cell growth. Mol Clin Oncol. 1:753–757. 2013.PubMed/NCBI
|
|
4
|
Su S, Scott WJ, Allen MS, Darling GE,
Decker PA, McKenna RJ and Meyers BF: Patterns of survival and
recurrence after surgical treatment of early stage non-small cell
lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac
Cardiovasc Surg. 147:747–752; discussion 752–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Furusaka T, Matsuda A, Tanaka A, Matsuda H
and Ikeda M: Superselective intra-arterial chemoradiation therapy
for functional laryngeal preservation in advanced squamous cell
carcinoma of the glottic larynx. Acta Otolaryngol. 133:633–640.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chak A, Buttar NS, Foster NR, Seisler DK,
Marcon NE, Schoen R, Cruz-Correa MR, Falk GW, Sharma P, Hur C, et
al: Metformin does not reduce markers of cell proliferation in
esophageal tissues of patients with Barrett's esophagus. Clin
Gastroenterol Hepatol. 13:665–672, e661-664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang YC, Chang PM, Chen MH, Chu PY, Tzeng
CH, Chang SY and Yang MH: A study using ifosfamide and etoposide in
patients with cisplatin - refractory recurrent or metastatic head
and neck squamous cell carcinoma. Jpn J Clin Oncol. 41:630–636.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeno S, Yamashita SI, Yamamoto S,
Takahashi Y, Moroga T, Kawahara K, Shiroshita T, Yamana I, Maki K
and Yamashita Y: Number of metastasis-positive lymph node stations
is a simple and reliable prognostic factor following surgery in
patients with esophageal cancer. Exp Ther Med. 4:1087–1091.
2012.PubMed/NCBI
|
|
9
|
Yang JJ, Li WH, Liu BJ, Tang RH and Zhang
YH: Influence of pentylenetetrazol and NF-κB decoy
oligodeoxynucleotides on p38 expression in neuron-like cells. Exp
Ther Med. 8:395–400. 2014.PubMed/NCBI
|
|
10
|
Han ME, Kim HJ, Shin DH, Hwang SH, Kang CD
and Oh SO: Overexpression of NRG1 promotes progression of gastric
cancer by regulating the self-renewal of cancer stem cells. J
Gastroenterol. 50:645–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai T, Zhang D, Cai M, Wang C, Wu Z, Ying
Z, Wu J, Li M, Xie D, Li J and Song L: Golgi phosphoprotein 3
(GOLPH3) promotes hepatocellular carcinoma cell aggressiveness by
activating NF-κB pathway. J Pathol. 235:490–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZM, Kang YH, Yang X, Wang JF, Zhang
Q, Yang BX, Zhao KL, Xu LP, Yang LP, Ma JX, et al: Andrographolide
radiosensitizes human esophageal cancer cell line ECA109 to
radiation in vitro. Dis Esophagus. Jul 25–2014.(Epub ahead of
print).
|
|
13
|
Yang B, Rice TW, Adelstein DJ, Rybicki LA
and Goldblum JR: Overexpression of p53 protein associates decreased
response to chemoradiotherapy in patients with esophageal
carcinoma. Mod Pathol. 12:251–256. 1999.PubMed/NCBI
|
|
14
|
Yang Y, Hui L, Yuqin C, Jie L, Shuai H,
Tiezhu Z and Wei W: Effect of saw palmetto extract on PI3K cell
signaling transduction in human glioma. Exp Ther Med. 8:563–566.
2014.PubMed/NCBI
|
|
15
|
Wang WF, Xie Y, Zhou ZH, Qin ZH, Wu JC and
He JK: PIK3CA hypomethylation plays a key role in activation of the
PI3K/AKT pathway in esophageal cancer in Chinese patients. Acta
Pharmacol Sin. 34:1560–1567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung AL: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang HJ, Youn H, Seong KM, Yun YJ, Kim W,
Kim YH, Lee JY, Kim CS, Jin YW and Youn B: Psoralidin, a dual
inhibitor of COX-2 and 5-LOX, regulates ionizing radiation
(IR)-induced pulmonary inflammation. Biochem Pharmacol. 82:524–534.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi LT, Li YC, Pan Y, Li JM, Xu Q, Mo SF,
Qiao CF, Jiang FX, Xu HX, Lu XB, et al: Antidepressant-like effects
of psoralidin isolated from the seeds of Psoralea corylifolia in
the forced swimming test in mice. Prog Neuropsychopharmacol Biol
Psychiatry. 32:510–519. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao G, Li G, Chen L, Zhang Z, Yin JJ, Wu
T, Cheng Z, Wei X and Wang Z: Isolation of antioxidants from
Psoralea corylifolia fruits using high-speed counter-current
chromatography guided by thin layer chromatography-antioxidant
autographic assay. J Chromatogr A. 1217:5470–5476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao W, Zhang X, Zhao W and Chen X:
Psoralidin induces autophagy through ROS generation which inhibits
the proliferation of human lung cancer A549 cells. PeerJ.
2:e5552014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015.PubMed/NCBI
|
|
22
|
Wang S, Liu H, Wang Z and Chen HX: Effects
of 5-azacytidine on RUNX3 gene expression and the biological
behavior of esophageal carcinoma cells. Mol Med Rep. 9:1259–1265.
2014.PubMed/NCBI
|
|
23
|
Kataoka K, Nakamura K, Mizusawa J, Fukuda
H, Igaki H, Ozawa S, Hayashi K, Kato K, Kitagawa Y and Ando N:
Variations in survival and perioperative complications between
hospitals based on data from two phase III clinical trials for
oesophageal cancer. Br J Surg. 102:1088–1096. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiozaki A, Fujiwara H, Konishi H,
Morimura R, Komatsu S, Murayama Y, Kuriu Y, Ikoma H, Kubota T,
Nakanishi M, et al: Middle and lower esophagectomy preceded by
hand-assisted laparoscopic transhiatal approach for distal
esophageal cancer. Mol Clin Oncol. 2:31–37. 2014.PubMed/NCBI
|
|
25
|
Yu L, Ge X, Huang S, Wang Y and Shen P:
Primary squamous cell carcinoma of the esophagus initially
presenting as a large retroperitoneal mass: A case diagnosed as
cancer of unknown primary site. Mol Clin Oncol. 1:503–506.
2013.PubMed/NCBI
|
|
26
|
Chen H, Wang Z, Yang Z, Shang B, Liu X and
Chen G: Prospective study of adjuvant radiotherapy on preventing
lymph node metastasis after Ivor-lewis esophagectomy in esophageal
cancer. Ann Surg Oncol. 20:2721–2726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das TP, Suman S and Damodaran C: Induction
of reactive oxygen species generation inhibits
epithelial-mesenchymal transition and promotes growth arrest in
prostate cancer cells. Mol Carcinog. 53:537–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cras A, Politis B, Balitrand N,
Darsin-Bettinger D, Boelle PY, Cassinat B, Toubert ME and Chomienne
C: Bexarotene via CBP/p300 induces suppression of NF-κB-dependent
cell growth and invasion in thyroid cancer. Clin Cancer Res.
18:442–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rengarajan T, Nandakumar N, Rajendran P,
Haribabu L, Nishigaki I and Balasubramanian MP: D-pinitol promotes
apoptosis in MCF-7 cells via induction of p53 and Bax and
inhibition of Bcl-2 and NF-κB. Asian Pac J Cancer Prev.
15:1757–1762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hasan R, Chauhan SS, Sharma R and Ralhan
R: siRNA-mediated downregulation of TC21 sensitizes esophageal
cancer cells to cisplatin. World J Gastroenterol. 18:4127–4135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoon JH, Cho ML, Choi YJ, Back JY, Park
MK, Lee SW, Choi BJ, Ashktorab H, Smoot DT, Nam SW, et al:
Gastrokine 1 regulates NF-κB signaling pathway and cytokine
expression in gastric cancers. J Cell Biochem. 114:1800–1809. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiou WF, Don MJ, Liao JF and Wei BL:
Psoralidin inhibits LPS-induced iNOS expression via repressing
Syk-mediated activation of PI3K-IKK-IκB signaling pathways. Eur J
Pharmacol. 650:102–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wheler JJ, Moulder SL, Naing A, Janku F,
Piha-Paul SA, Falchook GS, Zinner R, Tsimberidou AM, Fu S, Hong DS,
et al: Anastrozole and everolimus in advanced gynecologic and
breast malignancies: Activity and molecular alterations in the
PI3K/AKT/mTOR pathway. Oncotarget. 5:3029–3038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pang XL, He G, Liu YB, Wang Y and Zhang B:
Endoplasmic reticulum stress sensitizes human esophageal cancer
cell to radiation. World J Gastroenterol. 19:1736–1748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Y and Li LP: Progress of cancer
research on astrocyte elevated gene-1/Metadherin (Review). Oncol
Lett. 8:493–501. 2014.PubMed/NCBI
|