Introduction
Lung cancer is the most common cause of
cancer-associated mortalities worldwide (1). The American Cancer Society estimated
that there would be ~221,200 novel cases and ~158,040 mortalities
caused by lung cancer in the USA in 2015 (1,2). Lung
cancer is the second most common malignancy and the most common
cause of cancer-associated mortality in American men and women
(1). In China, novel cases and lung
cancer-associated mortalities were estimated to be 536,407 and
475,768, respectively, in 2005 (2).
Globally, it has been estimated that there were 1,824,700 novel
cases and 1589,900 lung cancer-associated mortalities in 2012
(3).
Currently, surgical resection remains the standard
of care for the majority of patients with non-metastatic non-small
cell lung cancer (NSCLC). Cancer immunotherapy has recently
received attention (4), since the
United States Food and Drug Administration (FDA) approved
Provenge® (sipuleucel-T) for the treatment of metastatic
castration-resistant prostate cancer and Yervoy®
(ipilimumab) for the treatment of metastatic melanoma (5,6).
Inhibitors of the programmed cell death protein 1 (PD-1), an
immunosuppressive checkpoint protein, and the programmed cell death
protein 1 ligand 1 (PD-L1) and ligand 2 (PD-L2), have demonstrated
positive outcomes in the treatment of cancers, including lung
cancer, in clinical trials (7).
A phase I clinical trial reported objective
responses in approximately 1/4 to 1/5 of patients with NSCLC,
melanoma and renal-cell cancer, who were treated with anti-PD-1
antibodies (8). Another phase I
clinical trial reported objective response rates of 6–17% and a
stabilization of disease at rates of 12–41% at 24 weeks in patients
with advanced cancers, including NSCLC, melanoma and renal-cell
cancer, who were treated with anti-PD-L1 antibodies (9). Three patients sustained long-term
partial or complete response in 16 months to 3 years following
treatment (10). Subsequent studies
showed that anti-PD-1 antibody (lambrolizumab) produced a response
rate of ~38% in melanoma patients, with or without prior ipilimumab
treatment (11). A combination of
anti-PD-1 antibody (nivolumab) and ipilimumab produced a 53%
objective response in the patients with advanced melanoma (12). A phase III trial showed that anti-PD-1
antibody (pembrolizumab, also called lambrolizumab or MK-3475)
produced a significantly better response rate (~33%) compared with
ipilimumab (11.9%; P<0.001) in the treatment of advanced
melanoma (13). A recent phase I
trial showed that pembrolizumab produced an objective response rate
of 19.4% in 495 patients with NSCLC. The median duration of
progression-free survival was 3.7 months and the median duration of
overall survival was 12.0 months (14). Therefore, on September 4, 2014, the
FDA granted accelerated approval to the anti-PD-1 antibody
pembrolizumab (Keytruda®; Merck & Co, Inc.,
Whitehouse Station, NJ, USA) for the treatment of patients with
unresectable or metastatic melanoma and disease progression
following ipilimumab and, if B-Rapidly Accelerated Fibrosarcoma
(BRAF) V600 mutation positive, a BRAF inhibitor such as
vemurafenib, sorafenib or dabrafenib. The FDA also approved
nivolumab (Opdivo®; Bristol-Myers Squibb Company,
Princeton, NJ, USA) for the treatment of patients with unresectable
or metastatic melanoma and disease progression following ipilimumab
and, if BRAF V600 mutation positive, a BRAF inhibitor for the
treatment of patients with metastatic squamous NSCLC with
progression during or following platinum-based chemotherapy, on
December 22, 2014 and March 4, 2015, respectively. FDA assigned a
priority review designation to pembrolizumab (Keytruda®)
as a treatment for patients with advanced NSCLC and a final
approval decision will be made in the future. Anti-PD-L1 antibody
(MPDL3280A; Genentech; Roche, South San Francisco, CA, USA) showed
responsive rates of 13–26% in solid tumors, including NSCLC
(15). On February 2, 2015, the FDA
gave MPDL3280A a breakthrough therapy designation for the treatment
of PD-L1-positive NSCLC that has progressed during or following
platinum-based chemotherapy, as well as a targeted therapy for
patients with epidermal growth factor receptor (EGFR)-positive or
anaplastic lymphoma kinase (ALK)-positive tumors. MPDL3280A is
currently undergoing phase II and III trials to obtain FDA approval
(16).
PD-1 was originally identified by Ishida et
al (17) in search of genes
responsible for programmed cell death. The study cloned a gene
encoding a protein with 288 amino acids, which was activated during
programmed cell death; therefore, the protein was named PD-1
(17). Disruption of the PD-1 gene
led to development of lupus-like arthritis and glomerulonephritis,
indicating that PD-1 is a negative regulator of immune responses
(18,19). Honjo and Freeman et al
(20) collaboratively identified
PD-L1, which is identical to B7-H1 reported by Dong et al
(21). Latchman et al
(22) further identified a second
PD-1 ligand PD-L2, which is identical to B7-DC (23). The binding of PD-1 by PD-L1 and PD-L2
is now known to inhibit T cell receptor-mediated lymphocyte
proliferation and cytokine secretion, thus suppressing immune
responses (24). In the tumor
microenvironment, the PD-1-PD-L1/L2 pathway is upregulated,
resulting in the immune evasion of tumor cells (22,25).
Therefore, the antibodies against PD-1, PD-L1 and likely PD-L2 may
block the immune evasion response and induce tumor regression.
PD-1, a negative costimulatory receptor, is
primarily expressed on the cellular surface of activated T cells
(26,27). PD-L1 is expressed by tumor cells and
tumor-infiltrating immune cells, including macrophages, dendritic
cells and T cells (15). PD-L1 and
PD-L2 mRNAs are expressed in the human heart, placenta, spleen,
lymph nodes and thymus tissues. In addition, PD-L2 messenger RNA
(mRNA), but not PD-L1 mRNA, is expressed in the human lung, liver,
smooth muscle and pancreas tissues (22). In a cohort of 824 NSCLC patients, ≥50%
of tumor cells stained positive for PD-L1 in 23.2% of patients,
1–49% of tumor cells stained positive for PD-L1 in 37.6% of
patients and <1% of tumor cells stained positive for PD-L1 in
39.2% of patients (14). The
objective response rate (ORR) to pembrolizumab treatment is
positively associated with the percentage of tumor cells with
membranous PD-L1 staining, for example: Patients that were <1%
PD-L1+ exhibited an 8.1% ORR; patients that were 1–24% PD-L1+
exhibited a 12.9% ORR; patients that were 25–49% PD-L1+ exhibited a
19.4% ORR; patients that were 50–74% PD-L1+ exhibited a 29.6% ORR;
and patients that were 75–100% PD-L1+ exhibited a 45.4% ORR
(14). In contrast, in a cohort of
272 squamous NSCLC, the ORRs to nivolumab treatment were similar
between PD-L1+ and PD-L1- tumors, namely: Patients that were <1%
PD-L1+ exhibited a 17% ORR; patients that were ≥1% PD-L1+ exhibited
a 17% ORR; patients that were <5% PD-L1+ exhibited a 15% ORR;
patients that were ≥5% PD-L1+ exhibited a 21% ORR; patients that
were <10% PD-L1+ exhibited a 16% ORR; and patients that were
≥10% PD-L1+ exhibited a 19% ORR). This discrepancy may be due to
the differences in sample size or antibodies. However, additional
studies are required to assess expression of PD-1, PD-L1 and PD-L2
in NSCLC. Although Keytruda® and Opdivo® are
not yet approved for use in China, their eventual approval is
possible.
Therefore, the objective of this study was to assess
expression of PD-1, PD-L1, and PD-L2 in 48 cases of NSCLC in China.
We found that PD-L1, but not PD-1 or PD-L2 expression was
associated with stage III NSCLC.
Materials and methods
Human lung cancer tissue samples
The present study was approved by the Institutional
Review Board of The Fourth Hospital of Hebei Medical University
(Shijiazhuang, China). The procedures to obtain human lung cancer
tissue and follow-up information were in accordance with the
Ethical Principles for Medical Research Involving Human Subjects,
as formulated in the World Medical Association Declaration of
Helsinki (revised 2008). All human lung cancer tissue samples were
obtained from the archives of formalin-fixed, paraffin-embedded
tissue blocks in the Department of Thoracic Surgery at The Fourth
Hospital of Hebei Medical University (Shijiazhuang, China). The
specimens were collected from surgeries performed between April
2010 and March 2013. Written informed consent was obtained from all
patients prior to surgery. The patients were followed up until
March 2015, through outpatient visits or correspondences to family
members. In total, 48 patients were included in this retrospective
study. Tumor stage was evaluated according to the Union for
International Cancer Control (UICC) 7th TNM classification system
and histological evaluation was based on the World Health
Organization criteria (28). The
clinicopathological characteristics of the patients are summarized
in Table I.
 | Table I.Clinicopathological characteristics of
patients (n=48). |
Table I.
Clinicopathological characteristics of
patients (n=48).
| Characteristic | No. of patients |
|---|
| Age,
yearsa | 59.3±7.6 |
| Gender |
|
| Male | 33 |
|
Female | 15 |
| Histology |
|
| SCC | 23 |
| ADC | 25 |
| Differentiation |
|
| Well | 40 |
| Poor | 8 |
| Tumor stage |
|
| I | 17 |
| II | 17 |
| III | 14 |
| Lymph node
metastasis |
|
| No | 30 |
|
Yes | 18 |
Immunohistochemistry
Tissue sections (4-µm thick) were baked at 60°C for
60 min, deparaffinized in xylene and rehydrated through graded
ethanol solutions to water. Antigens were retrieved by heating the
tissue sections in 0.01 M ethylenediaminetetraacetic acid buffer at
95°C for 5 min and then cooling down to room temperature in 20 min.
Endogenous peroxidase activity was blocked by 0.3%
H2O2 for 5 min. Non-specific binding was
blocked with 1.5% normal goat or horse serum (VECTASTAIN Elite ABC
kit; Vector Laboratories, Burlingame, CA, USA). The sections were
incubated with primary antibodies in a humid chamber at 4°C
overnight: Rabbit anti-human PD-L1 polyclonal antibodies (catalog
no., ab58810; dilution, 1:40; Abcam, Cambridge, MA, USA), rabbit
anti-human PD-L2 polyclonal antibodies (catalog no.,
SAB3500395-100UG; dilution, 1:800; Sigma-Aldrich, St. Louis, MO,
USA) and mouse anti-human cluster of differentiation (CD)279 (PD-1)
purified monoclonal antibodies (catalog no., 14-9989-82; dilution,
1:25; eBioscience, Inc., San Diego, CA, USA) were used as the
primary antibodies. Subsequent to being washed 3 times in
phosphate-buffered saline, the sections were incubated with
secondary antibodies from the VECTASTAIN Elite ABC kit for 120 min.
The color was developed using 3,3′-diaminobenzidine (DAB) substrate
kit (Vector Laboratories) following the manufacturer's protocol.
The sections were then counterstained with hematoxylin. Tissue
sections that had previously stained positively were used as a
positive control and tissue sections stained with non-immune serum
rather than primary antibodies served as a negative control.
Positive staining showed brown particles at the cytoplasmic
membrane or in the cytoplasm. Under a microscope, 5 representative
high-power (magnification, ×400) fields, containing tumor islet
cells and stroma, per tissue section were randomly selected and
evaluated by two investigators (Dr Zhiquan Chen from Hebei Medical
University, Shijiazhuang, China, and Dr Jiandong Mei from Sichuan
University, Chengdu, China), who were blinded to the
clinicopathological characteristics. An average of the scores
obtained by the two examiners was used to represent each case. A
two-score system based on a proportion score and an intensity
score, previously described by Allred et al (29), was used. The proportion scores were
assigned based on the percentage of positive staining: 0, none; 1,
<1%; 2, 1–10%; 3, 10–33.3%; 4, 33.3–66.7%; and 5, >66.7%. The
intensity scores were assigned based on the estimated average
staining intensity of positive staining: 0, none; 1, weak; 2,
intermediate; and 3, strong. The overall Allred scores (29) were the sum of the proportion score and
intensity score of each case (range, 0–8).
Statistical analysis
Statistical analysis was performed using the
Statistical Package for the Social Sciences (SPSS) version 16.0 for
Windows (SPSS, Chicago, IL, USA). The results were presented as the
mean ± standard deviation (SD) or median and range for numerical
variables. The comparison of clinicopathological characteristics
between various groups was performed using the χ2 test.
Spearman's rank correlation coefficient was calculated to reveal
the correlation between PD-1, PD-L1 and PD-L2 scores. The survival
time of various groups was described using Kaplan-Meier curves, and
the statistical significance was analyzed using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PD-1, PD-L1 and PD-L2 are expressed in
NSCLC
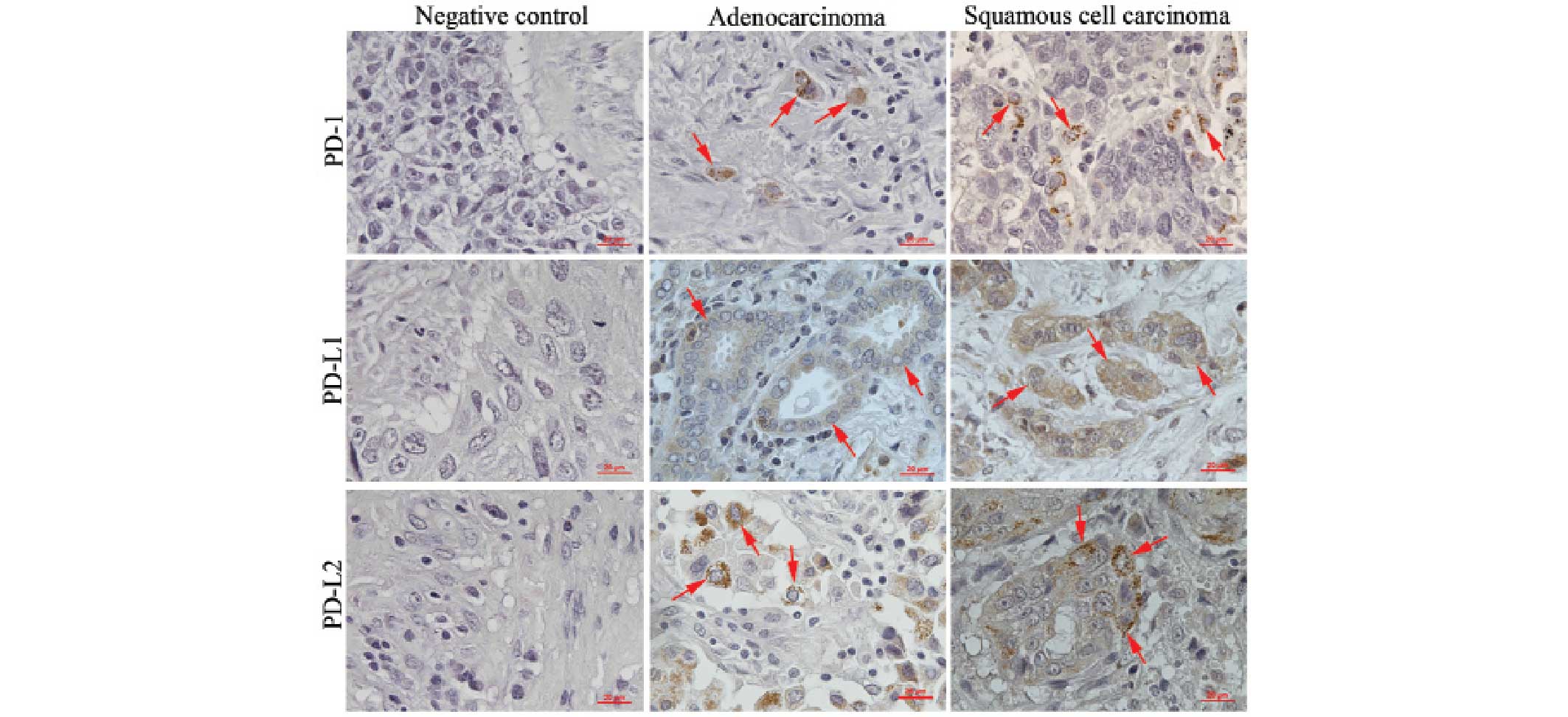
Immunohistochemical staining revealed that PD-1 was
expressed in the immune cells that were located mostly in the
stroma of lung adenocarcinomas and squamous cell carcinomas
(Fig. 1). PD-L1 and PD-L2 were
expressed in the cancer cells of lung adenocarcinomas and squamous
cell carcinomas (Fig. 1).
PD-L1, but not PD-1 or PD-L2, is
associated with stage III lung cancer
To assess whether the expression of PD-1, PD-L1 and
PD-L2 is correlated with any clinicopathological characteristics of
the patients, any staining (Allred score ranges 1–8) was defined as
positive (+) and no staining (Allred score=0) was defined as
negative (−). Analysis revealed that neither PD-1 nor PD-L2
expression was associated with the patients' gender, tumor
histological types, tumor differentiation, tumor stage or status of
lymph node metastasis (Tables II and
III). PD-L1 expression was not
associated with the patients' gender, tumor histological types,
tumor differentiation or status of lymph node metastasis (Table IV). However, PD-L1 expression was
associated with the tumor stage (P=0.049). The positive staining
rate was 55.9% (19/34) in the stage 1/II tumors, whereas it was
85.7% (12/14) in the stage III tumors (Table IV).
 | Table II.Association between PD-1 expression
and clincopathological characteristics of patients. |
Table II.
Association between PD-1 expression
and clincopathological characteristics of patients.
|
| PD-1
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | + | − | P-value |
|---|
| No. of
patients | 17 | 31 |
|
| Age,
yearsa | 58.7±8.4 | 59.6±7.3 | 0.667 |
| Gender |
|
|
|
|
Male | 11 | 22 | 0.654 |
|
Female | 6 | 9 |
|
| Histology |
|
|
|
|
SCC | 9 | 14 | 0.606 |
|
ADC | 8 | 17 |
|
|
Differentiation |
|
|
|
|
Well | 16 | 24 | 0.138 |
|
Poor | 1 | 7 |
|
| Tumor stage |
|
|
|
|
I/II | 13 | 21 | 0.525 |
|
III | 4 | 10 |
|
| Lymph node
metastasis |
|
|
|
| No | 12 | 18 | 0.391 |
|
Yes | 5 | 13 |
|
 | Table III.Association between PD-L2 expression
and clincopathological characteristics of patients. |
Table III.
Association between PD-L2 expression
and clincopathological characteristics of patients.
|
| PD-L2
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | + | − | P-value |
|---|
| No. of
patients | 22 | 26 |
|
| Age,
yearsa | 60.6±7.1 | 58.2±8.0 | 0.919 |
| Gender |
|
|
|
|
Male | 15 | 18 | 0.938 |
|
Female | 7 | 8 |
|
| Histology |
|
|
|
|
SCC | 10 | 13 | 0.753 |
|
ADC | 12 | 13 |
|
|
Differentiation |
|
|
|
|
Well | 19 | 21 | 0.897 |
|
Poor | 3 | 5 |
|
| Tumor stage |
|
|
|
|
I/II | 16 | 18 | 0.791 |
|
III | 8 | 6 |
|
| Lymph node
metastasis |
|
|
|
| No | 15 | 15 | 0.454 |
|
Yes | 7 | 11 |
|
 | Table IV.Association between PD-L1 expression
and clincopathological characteristics of patients. |
Table IV.
Association between PD-L1 expression
and clincopathological characteristics of patients.
|
| PD-L1
expression |
|
|---|
|
|
|
|
|---|
| Characteristic | + | − | P-value |
|---|
| No. of
patients | 31 | 17 |
|
| Age,
yearsa | 60.5±6.7 | 57.2±8.8 | 0.164 |
| Gender |
|
|
|
|
Male | 23 | 10 | 0.272 |
|
Female | 8 | 7 |
|
| Histology |
|
|
|
|
SCC | 14 | 9 | 0.606 |
|
ADC | 17 | 8 |
|
|
Differentiation |
|
|
|
|
Well | 24 | 16 | 0.138 |
|
Poor | 7 | 1 |
|
| Tumor stage |
|
|
|
|
I/II | 19 | 15 | 0.049 |
|
III | 12 | 2 |
|
| Lymph node
metastasis |
|
|
|
| No | 18 | 12 | 0.107 |
|
Yes | 13 | 5 |
|
PD-1, PD-L1 and PD-L2 expression is
independent of each other in lung cancer
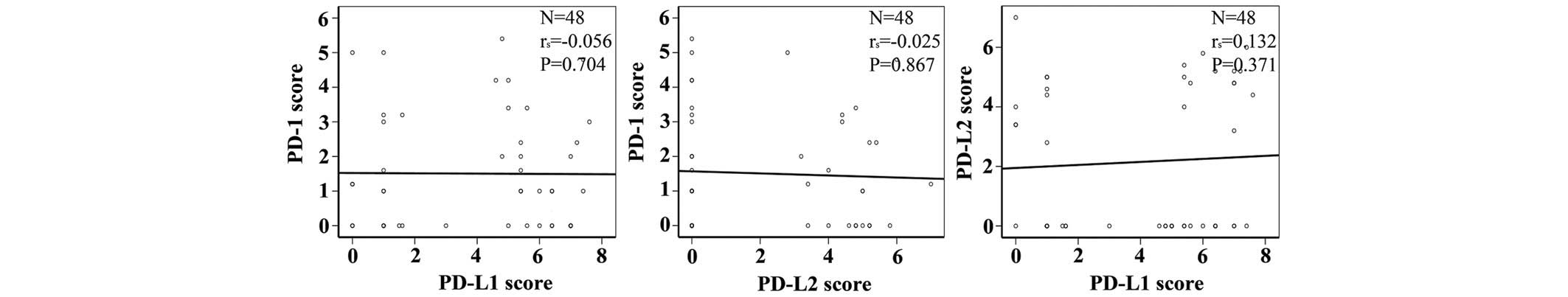
Correlation analysis found that the expressions of
PD-1, PD-L1 and PD-L2 were independent of each other. No
correlation was identified between PD-1 and PD-L1 expression, PD-1
and PD-L2 expression or PD-L1 and PD-L2 expression (Fig. 2; Table
V).
 | Table V.Correlation between PD-1 and PD-L1 or
PD-L2 expression. |
Table V.
Correlation between PD-1 and PD-L1 or
PD-L2 expression.
|
| PD-1
expression |
|
|---|
|
|
|
|
|---|
| Protein | + | − | P-value |
|---|
| PD-L1 |
|
|
|
| + | 12 | 19 | 0.519 |
| − | 5 | 12 |
|
| PD-L2 |
|
|
|
| + | 8 | 14 | 0.900 |
| − | 9 | 17 |
|
PD-1, PD-L1 and PD-L2 expression is
not associated with the survival time in lung cancer patients
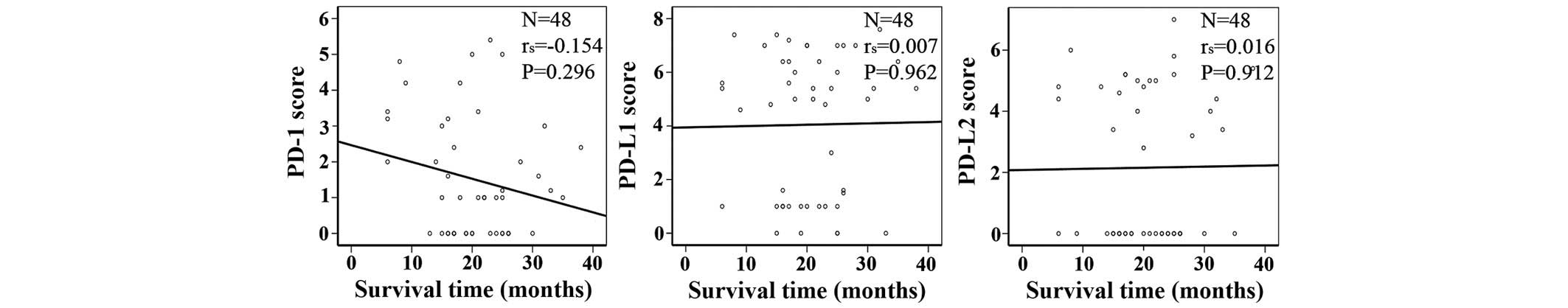
Kaplan-Meier analysis showed that PD-1, PD-L1 and
PD-L2 expression was not associated with the survival time of
patients with lung cancer (Fig. 3).
Increased levels of PD-1 expression appeared to be inversely
associated with the survival time; however, this result was not
statistically significant (Fig. 3).
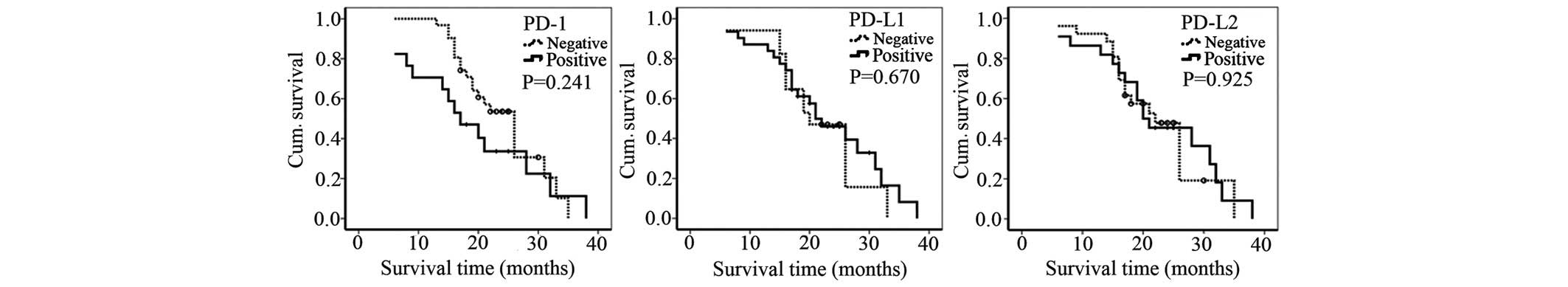
In addition, the survival time of patients with tumors that were
positively stained for PD-1, PD-L1 and PD-L2 expression was not
significantly different from the survival time of patients with
negatively stained tumors (Fig.
4).
Discussion
In the present study of a cohort of 48 patients with
NSCLC, 35.4% (17/48) of patients were positive for PD-1 expression,
64.6% (31/48) of patients were positive for PD-L1 expression and
45.8% (22/48) of patients were positive for PD-L2 expression.
Neither PD-1 nor PD-L2 expression was associated with gender,
histology, differentiation status, tumor stage or lymph node
metastasis. PD-L1 expression was not associated with gender,
histology, differentiation status or lymph node metastasis.
However, PD-L1 expression was significantly increased in stage III
NSCLC (85.7% PD-L1+) compared with stage I/II NSCLC (55.9% PD-L1+)
(P=0.049). The lack of statistically significant associations with
the majority of the clinicopathological characteristics may be due
to the small sample size used in the present study. In a cohort of
331 patients with squamous NSCLC in a previous study, neither PD-L1
nor PD-L2 expression was associated with gender, age, smoking
history, tumor size, tumor stage or lymph node metastasis (30). However, PD-L1 expression was
marginally associated with tumor stage (P=0.059) (30). The present study also found that the
expressions of PD-1, PD-L1 and PD-L2 were independent of each
other, which is consistent with the previous study (30). This independence may suggest that any
component of the PD-1-PD-L1/L2 pathway may be upregulated to
suppress immune responses in the tumor microenvironment. In
addition, the present study indicated that the expression of PD-1,
PD-L1 and PD-L2 was not associated with the survival of the
patient. In a meta-analysis of 9 studies that included 1,550 NSCLC
patients, PD-L1 expression was associated with differentiation
status, but not with gender, smoking status, histology, tumor stage
or lymph node status (31). These
findings suggest that PD-L1 may have limited use for predicting
prognosis.
The present study provides essential information
regarding the expression of PD-1, PD-L1 and PD-L2 in patients with
NSCLC, which may be useful for guiding future treatment with
Keytruda® and Opdivo®. Given the
unsatisfactory clinical outcomes with current therapies, the
adoption of immunotherapy may help to improve the survival rate of
our patients.
Acknowledgements
Dr Zongbing You was supported partially by National
Institutes of Health (Bethesda, MD, USA; grant nos. P20GM103518 and
R01CA174714), Department of Defense (Fort Detrick, MD, USA; grant
nos. W81XWH-14-1-0050, W81XWH-14-1-0149, W81XWH-14-1-0458 and
W81XWH- 15-1-0444), the Developmental Fund of Tulane Cancer Center,
Louisiana Cancer Research Consortium Fund and Tulane's Institute of
Integrated Engineering for Health and Medicine (New Orleans, LA,
USA; grant no. TI2EHM). Dr Lunxu Liu was partially supported by
National Natural Science Foundation of China (Beijing, China; grant
nos. NSFC 81,172,236, ‘The mechanism of TAMs activation in lung
cancer and a novel immunotherapy’ and NSFC 81,372,505, ‘The role of
IL-17 in formation and progression of primary lung cancer and the
underlying molecular mechanisms’) and the Key Science and
Technology Program of Sichuan Province (Chengdu, China; grant no.,
2013SZ0005). Dr Jiandong Mei was a visiting scholar at Tulane
University School of Medicine sponsored by the China Scholarship
Council (Beijing, China; grant no., 201,406,240,145).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zhang S and Zou X: Evaluation on
the incidence, mortality and tendency of lung cancer in China.
Thoracic Cancer. 1:35–40. 2010. View Article : Google Scholar
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledford H: Cancer treatment: The killer
within. Nature. 508:24–26. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harvey RD Immunologic and clinical effects
of targeting of pd-1 in lung cancer. Clin Pharmacol Ther. 2014.
|
|
8
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-pd-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-pd-l1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipson EJ, Sharfman WH, Drake CG, Wollner
I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, et al: Durable
cancer regression off-treatment and effective reinduction therapy
with an anti-pd-1 antibody. Clin Cancer Res. 19:462–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-pd-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-pd-l1
antibody mpdl3280a in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha E, Wallin J and Kowanetz M: Pd-l1
inhibition with mpdl3280a for solid tumors. Semin Oncol.
42:484–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of pd-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI
|
|
18
|
Nishimura H, Minato N, Nakano T and Honjo
T: Immunological studies on pd-1 deficient mice: Implication of
pd-1 as a negative regulator for b cell responses. Int Immunol.
10:1563–1572. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the pd-1 gene encoding an itim motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the pd-1 immunoinhibitory receptor by a
novel b7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong H, Zhu G, Tamada K and Chen L: B7-h1,
a third member of the b7 family, co-stimulates t-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
Pd-l2 is a second ligand for pd-1 and inhibits t cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng SY, Otsuji M, Gorski K, Huang X,
Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM and Tsuchiya H:
B7-dc, a new dendritic cell molecule with potent costimulatory
properties for t cells. J Exp Med. 193:839–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okazaki T and Honjo T: Pd-1 and pd-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated b7-h1 promotes t-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: Pd-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharpe AH and Freeman GJ: The b7-cd28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology and Genetics of Tumours of the Lung, Pleura,
Thymus and Heart. Lyon, IARC Press. pp9–11. 2004.
|
|
29
|
Allred DC, Clark GM, Elledge R, Fuqua SA,
Brown RW, Chamness GC, Osborne CK and McGuire WL: Association of
p53 protein expression with tumor cell proliferation rate and
clinical outcome in node-negative breast cancer. J Natl Cancer
Inst. 85:200–206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim MY, Koh J, Kim S, Go H, Jeon YK and
Chung DH: Clinicopathological analysis of pd-l1 and pd-l2
expression in pulmonary squamous cell carcinoma: Comparison with
tumor-infiltrating t cells and the status of oncogenic drivers.
Lung Cancer. 88:24–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan ZK, Ye F, Wu X, An HX and Wu JX:
Clinicopathological and prognostic significance of programmed cell
death ligand1 (pd-l1) expression in patients with non-small cell
lung cancer: A meta-analysis. J Thorac Dis. 7:462–470.
2015.PubMed/NCBI
|