Introduction
Primary plasma cell leukemia (pPCL) is an uncommon
form of plasma cell (PC) dyscrasia, and the most aggressive of the
human monoclonal gammopathies (1,2). pPCL is
characterized by the presence of >20% circulating PCs in
peripheral blood and/or an absolute circulating PC count exceeding
2×109 cells/l (1).
Peripheral blood flow cytometry is an important tool to demonstrate
the presence of PCs and to confirm their clonality, as well as to
exclude other lymphoproliferative disorders such as low-grade
B-cell and lymphoplasmacytic lymphoma (2). In regard to cytogenetic findings, the
t(11;14)(q13;q32) rearrangement is the most common alteration in
pPCL (3), promoting cyclin D1
(CCND1) gene overexpression due to its juxtaposition with
the immunoglobulin heavy locus (IGH) chromosome region. The
subsequent deregulation of CCND1 is considered to perturb
the G1-S phase transition of the cell cycle and, therefore, to
contribute to tumor development (4).
However, the IGH/CCND1 rearrangement alone may be
insufficient to cause hematologic malignancies, and may require
other additional genetic aberrations to boost its oncogenic
activity (4). In the present study, a
case of pPCL with t(11;14) characterized by the overexpression of
CCND1 and the myeloma overexpressed (MYEOV)
gene, which maps very close CCND1 on chromosome 11, is
described.
Materials and methods
Clinical history
In July 2014, a previously healthy 65-year-old male
was admitted to the Department of Emergency and Organ
Transplantation, Hematology Section, University of Bari (Bari,
Italy) for anemia, thrombocytopenia and mild leukocytosis
[hemoglobin levels, 11.0 g/dl (normal range, 13.0–16.0 g/dl);
platelets, 49×109 cells/l (normal range,
150–450×109 cells/l); and leukocytes, 13×109
cells/l (normal range, 4–10×109 cells/l)]. Peripheral
blood smear analysis demonstrated the presence of ~40% apparently
undifferentiated cells, a number of which had a large eccentric
nucleus and scattered chromatin, while others had a scanty and
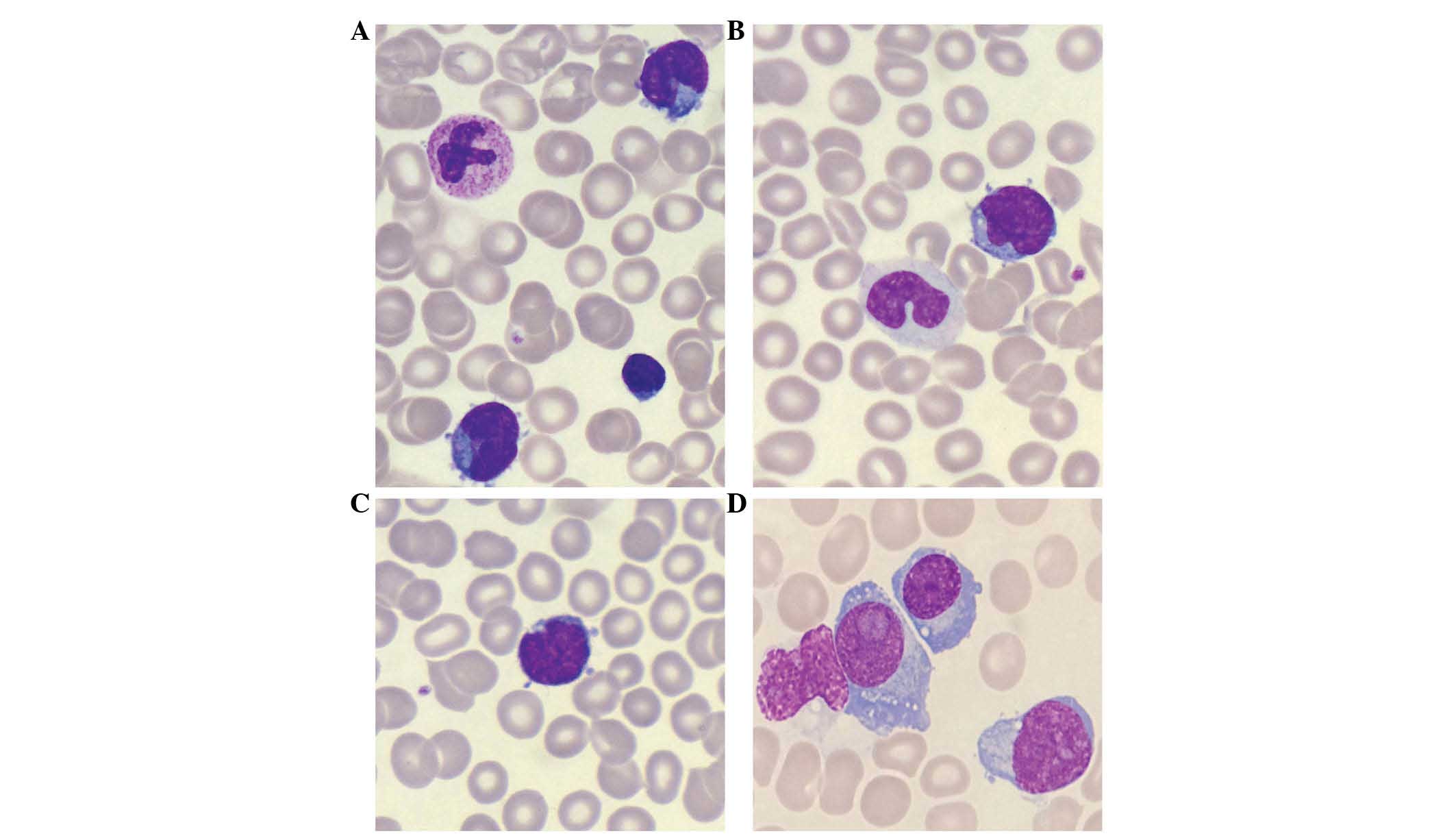
intensely basophilic cytoplasm with protrusions (Fig. 1A-C). Immunophenotypic analysis of bone
marrow (BM) demonstrated the specimen to be cluster of
differentiation (CD)38+, CD138+, CD20-, CD23-, CD56+, CD9-, CD117,
human leukocyte antigen-antigen D related- and cytoplasmic
immunoglobulin (CyIg)κ+. Primary antibodies used were as follows:
CD38+ (catalog no. 340926), CD138+ (catalog no. 341097), CD20-
(catalog no. 340954), CD23- (catalog. no. 341008), CD56+ (catalog
no. 340724), CD9- (catalog no. 341639), CD117 (catalog no. 340867),
HLA-DR- (catalog no. 335813), CyIgκ+ (catalog no. 643774) (BD
Biosciences, Franklin Lakes, NJ, USA). BM aspirate and biopsy
revealed the presence of 80% immature plasma cells and
plasmablasts, the majority with considerable atypia (Fig. 1D). Serum protein electrophoresis
identified a monoclonal protein in the gamma region, with a
concentration of 0.55 g/dl. This was classified on immunofixation
electrophoresis as an intact monoclonal immunoglobulin AK. Total
body computed tomography did not reveal the presence of swollen
nodes or lytic bone lesions. Viral serological tests specific for
human immunodeficiency virus, hepatitis B and C viruses, and human
herpes viruses 6 and 8 resulted negative. Conventional cytogenetic
analysis identified the following karyotype: 56, XY, +Y, +del(1p),
+2, +3, +7, +8, +9, t(11;14)(q13;q32), +der(14) t(11;14)(q13;q32), +18, +22[20].
Molecular analysis revealed the presence of the B-Raf V600E
gene mutation. According to these data, a diagnosis of pPCL was
made. The patient refused to start chemotherapy treatment and
succumbed to sepsis 3 months later.
The study was approved by the Ethics Committee of
the Azienda Ospedaliero-Universitaria Consorziale Policlinico di
Bari (Bari, Italy) and written informed consent was obtained from
the patient.
Cytogenetic analysis
Karyotyping was performed at diagnosis on BM cells
according to standard methods (5,6). BM cells
were cultured for 24–48 h, and chromosomes were G-banded with
trypsin-Giemsa staining, according to the recommendations of the
International System for Human Cytogenetic Nomenclature (5). At least 20 metaphases were analyzed.
Fluorescence in situ hybridization
(FISH) analysis
FISH analyses were performed on BM samples using
bacterial artificial chromosomes (BACs) and fosmid clones
(Children's Hospital Oakland Research Institute, Oakland, CA, USA),
according to the University of California (Santa Cruz, CA, USA)
database (http://genome.ucsc.edu/; February 2009
release). Chromosome preparations were hybridized in situ
with probes labeled by nick translation (7).
Molecular analyses
Total RNA was extracted from BM cells using the
RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). The RNA
concentration was assessed using a Qubit® 2.0
Fluorometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
total of 1 µg RNA was reverse transcribed into complementary (c)DNA
using the QuantiTect Reverse Transcription kit (Qiagen, Inc.). Gene
expression analysis was conducted by droplet digital polymerase
chain reaction (ddPCR) using the QX200 droplet generator (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The principle of ddPCR
technology is to combine water-oil emulsion droplet technology with
microfluidics and to quantify the absolute target number present in
a sample, thus implementing PCR data with Poisson statistics
(5,6).
ddPCR experiments were performed using primers specific for the
CCND1 and MYEOV genes: CCND1_Forward (F)
TGCCAGAGGCGGAGGAGAACAAAC, CCND1_Reverse (R)
TGGAGGGCGGATTGGAAATGAAC, MYEOV_F GCTGACTGTTGTGACTGTTGAGGC and
MYEOV_R AGGAGGAGAGGAGAAGCACCTGAC. Importin 8 (IPO8) was used
as a control gene to confirm the quality of the cDNA samples, using
the primers IPO8_F TGTGATTGGTTCCCTAGCTGAG and IPO8_R
CATGAAGTACCCAGCAAGATC. A 20-µl reaction mixture containing QX200
ddPCR EvaGreen Supermix (Bio-Rad Laboratories, Inc.), primers at
final concentrations of 100 nM and 50 ng cDNA template was
prepared. Then, each sample was loaded into the Bio-Rad DG8
cartridge with 70 µl droplet generation oil, and next partitioned
into 20,000 droplets by the QX200 droplet generator. The generated
droplets were transferred to a 96-well PCR plate (Eppendorf,
Hamburg, Germany). The plate was sealed with a pierceable foil heat
seal (Bio-Rad Laboratories, Inc.), and the samples were amplified
on the T100 thermal cycler (Bio-Rad Laboratories, Inc.). The
thermal cycling conditions were 95°C for 5 min (1 cycle), 95°C for
30 sec (ramp rate, 2°C/sec; 40 cycles), 60°C for 1 min (ramp rate,
2°C/sec; 40 cycles), 4°C for 5 min (1 cycle), 90°C for 10 min (1
cycle) and 4°C hold. After amplification, the 96-well PCR plate was
loaded onto the QX200 droplet reader (Bio-Rad Laboratories, Inc.),
which counts the fluorescence-positive and -negative droplets to
define the target concentration using QuantaSoft analysis software
version 1.7.4 (Bio-Rad Laboratories, Inc.). The target
concentration in each sample was expressed as number of copies/ng.
Patient's gene expression was compared with that of a healthy BM
control (Human Bone Marrow Total RNA; Clontech Laboratories, Inc.,
Mountainview, CA, USA) and with the myeloma cell lines RPMI-8266
and U266 [American Type Culture Collection, Manassas, VA, USA;
cultured at 1×106 cells/ml in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Euroclone Spa, Pero,
Italy), 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C and
5% CO2]. Molecular analysis of B-Raf V600E was
performed as previously reported (8).
Results
To confirm the presence of the t(11;14)
rearrangement observed in the cytogenetic analysis, FISH
experiments were performed using fosmids and BACs. For chromosome
14q32, the BAC clone RP11-1145H5 and the fosmid G248P83912C7,
specific for the IGH variable (IGHV) region, and the
BACs RP11-815O20 and RP11-676G2, specific for the IGH
constant (IGHC) region, were employed. To study the
breakpoint on chromosome 11, the RP11-156B3 and RP11-378E8 clones
were selected; the former was specific for CCND1, and the
latter mapped upstream, in the region where the breakpoint is
usually localized. The FISH pattern revealed the RP11-156B3 signal
on the normal chromosome 11 and on the two der(14), confirming the CCND1
translocation on der(14).
Unexpectedly, the RP11-378E8 signal was not split, but produced
signals on the two der(14) in
addition to the normal chromosome 11 (Fig. 2A). In order to perform a precise
characterization of the breakpoint mapping on chromosome 11,
further FISH experiments using fosmids G248P8216G1, G248P87301E9
and G248P87014D2, specific for the MYEOV gene and which map
upstream to RP11-378E8, demonstrated that they were retained on
der(11) (Fig. 2B). On the basis of these results, the
breakpoint on der(11) was assumed to
be within the RP11-378E8 and G248P87301E9 regions. The CCND1
and MYEOV genes were then translocated on der(14) and retained on der(11), respectively. The breakpoint on the
14q32 locus was identified in the IGHV region, since all the
probes specific for the 14q32 locus induced a signal on the normal
and on the derivative chromosome 14, respectively. Based on the
evidence of a difference from the recurrent breakpoint at the basis
of the t(11;14)(q13;q32) rearrangement in the present patient,
CCND1 and MYEOV gene expression was investigated by
ddPCR. The analysis revealed that, as expected, the CCND1
gene was overexpressed in the patient sample (670 copies/ng) and in
the two myeloma cell lines tested (U266, 294 copies/ng; RPMI-8266,
431 copies/ng), compared with normal BM (47 copies/ng). In
addition, the MYEOV gene was expressed ~70-fold more (54.0
copies/ng) in the patient than in the normal BM control (0.8
copies/ng) and in the myeloma cell lines evaluated, where no
MYEOV gene expression was detected (Fig. 2C).
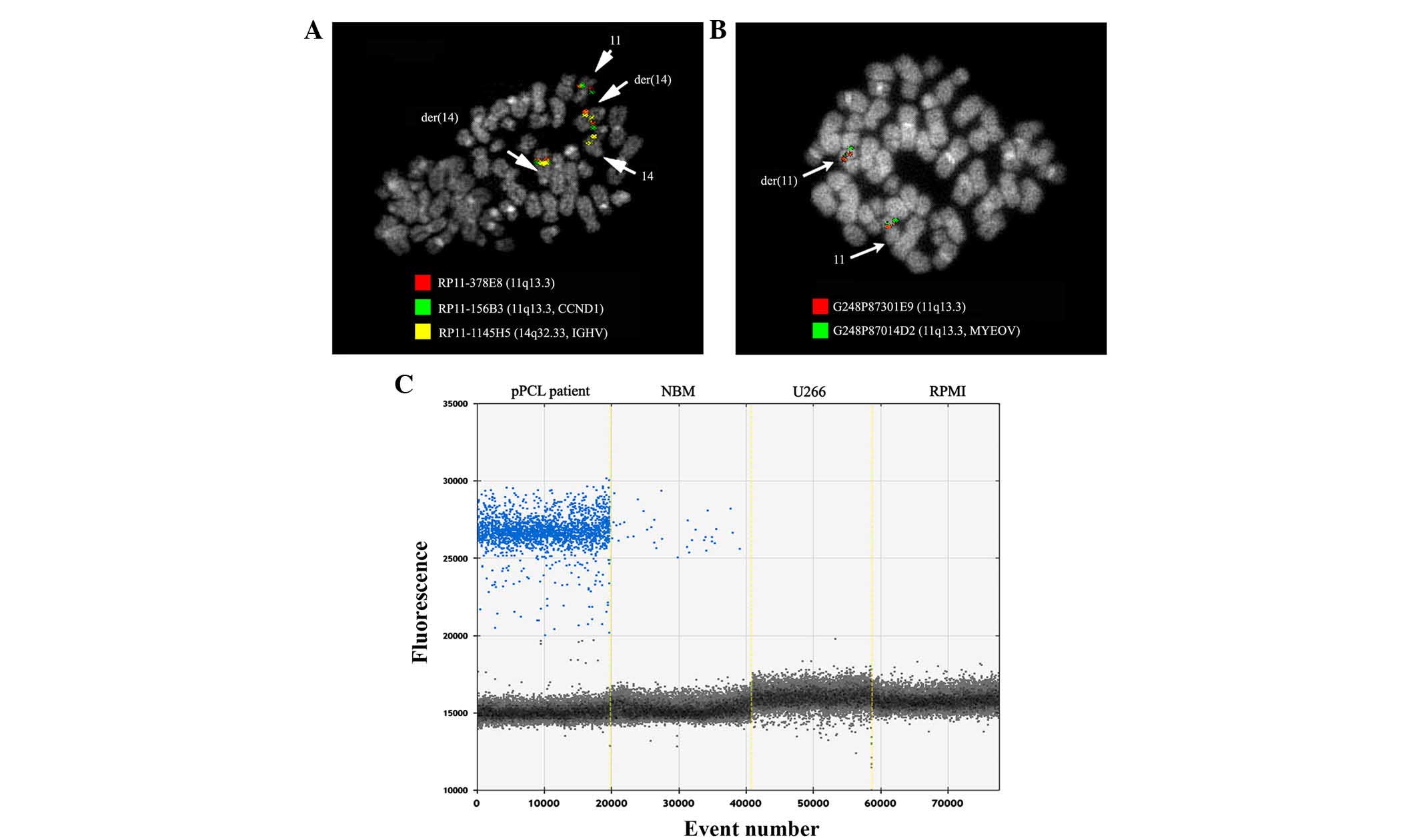 | Figure 2.FISH experiments. FISH
co-hybridization experiments with (A) the bacterial artificial
chromosomes clones RP11-378E8 (red), RP11-156B3 (green)and
RP11-1145H5 (yellow), and (B) with the fosmid clones G248P87301E9
(red) and G248P87014D2 (green), mapping upstream to RP11-378K8,
demonstrated that the cyclin D1 and MYEOV genes were
translocated on der(14) and retained
on der(11), respectively. Arrows
indicate chromosomes involved in the rearrangement. (C) Expression
analysis by droplet digital polymerase chain reaction revealed
that, in the present primary plasma cell leukemia patient, the
MYEOV gene was expressed ~70-fold more (54.0 copies/ng) than
in normal bone marrow samples (0.8 copies/ng) and myeloma cell
lines (0.0 copies/ng). CCND1, cyclin D1;
MYEOV, myeloma overexpressed; IGHV,
immunoglobulin heavy locus variable; pPCL, primary plasma
cell leukemia; NBM, normal bone marrow; FISH, fluorescence in
situ hybridization. |
Discussion
The present study reports a pPCL case characterized
by a t(11;14) chromosomal rearrangement associated with
overexpression of MYEOV, a gene mapped in close proximity to
the CCND1 locus (9). In fact,
MYEOV is located 390 kb centromeric of CCND1, and its
activation [which is concurrent to that of CCND1 through
juxtaposition of MYEOV to either the 5′ intronic Em
gene located in the intron between the IGH joining and
switch sequences, or the 3′ regulatory region (RR) IGH
enhancers located downstream of the constant region genes] was
first described in a subset of multiple myeloma (MM) cell lines
with t(11;14) (9). Since then,
MYEOV overexpression has rarely been reported in MM patients
with t(11;14) (10). Since the
enhancers are joined to both CCND1 and MYEOV with a
breakpoint in switch sites, and MYEOV expression is lost in
the majority of these cases, the authors concluded that MM does not
favor MYEOV expression (10).
Furthermore, gene expression profile experiments revealed that the
MYEOV gene was expressed in malignant PCs in 79% of newly
diagnosed patients with MM, and that MYEOV is a prognostic
factor, partly through a role of MYEOV in the control of
neoplastic cell proliferation (11).
The MYEOV gene was reported to be co-amplified and
co-overexpressed with CCND1 in a subset of esophageal
squamous cell carcinomas, breast cancers, gastric cancers,
neuroblastomas and colorectal cancers (12,13). The
oncogenic role of MYEOV has also been investigated in
functional studies, where in vitro small interfering
RNA-mediated knockdown of MYEOV resulted in an inhibition of
the proliferation, invasion and migration of colorectal cancer cell
lines (13). To the best of our
knowledge, MYEOV gene overexpression has never been
described in pPCL. In MM, IGH translocations were shown to
be definite, non-random chromosomal fusions of IGHC with the
loci of the fibroblast growth factor receptor 3 (4p16.3),
CCND1 (11q13.3), CCND3 (6p21.1), v-Maf avian
musculoaponeurotic fibrosarcoma oncogene homolog (MAF)
(16.q23.2) and MAFB (20q12) genes, and of IGHV with
the locus of the multiple myeloma SET domain (4p16.3) gene
(14). On the contrary, in the
present pPCL case, the breakpoint on the 14q32 region was in the
IGHV region, resulting in the juxtaposition of the
MYEOV and CCND1 genes near to Em, which could
promote their transcription. Therefore, in the present pPCL case,
it was the MYEOV proximity to Em that could favor its
expression rather than the RR enhancer. On this basis, as this
rearrangement recurred with a high frequency, cytogenetic molecular
characterization of the t(11;14) rearrangement in pPCL cases could
highlight the role and the mechanism regulating MYEOV
overexpression. The current pPCL case was also characterized by the
presence of the B-Raf V600E gene mutation. B-Raf
mutations were observed in 4% of MM cases (15) and were found to be significantly
associated with relapsed myeloma, extensive extramedullary disease
and a decreased overall survival (16). Molecular analyses of 15 pPCL cases
identified the presence of B-Raf V600E gene mutation in 1
case (6.7%), together with t(14;16) (3). Notably, an MM patient was described to
exhibit the B-Raf V600E gene mutation together with a
plasmablastic differentiation during the relapse (17). The current case presented a marked
plasmablastic differentiation, a phenotype difficult to link with
the molecular characteristics reported above. There are few reports
in the literature describing the morphological features of
circulating PCs in pPCL (2,18). However, certain cases were described
with an irregular nuclear contour or containing multiple immature
cells with a high nuclear-cytoplasmic ratio, finely dispersed
chromatin, prominent nucleolus and limited or absent Golgi zone,
which corresponded to blasts or plasmablasts (18). In patients with sepsis, viral
infections, autoimmune conditions and less common peripheral T-cell
lymphomas such as angioimmunoblastic T-cell lymphoma, polytypic
plasmacytosis in the peripheral blood may mimic pPCL, particularly
when PCs display atypical features (19). Furthermore, in MM, the plasmablastic
morphology is highly associated with adverse clinical risk features
and a high proliferation rate, but not with prognostically adverse
IGH rearrangements (20).
In conclusion, the present study reports the first
pPCL case with t(11;14) in which the breakpoint located in the
IGHV region was associated with a concomitant MYEOV
and CCND1 gene overexpression. The pathogenic role of the
MYEOV gene in pPCL remains to be elucidated.
Acknowledgements
The authors would like to thank Ms. M.V.C. Pragnell
for the language revision of the present manuscript. The present
study was supported by the ‘Il sorriso di Antonio’ association
(Corato, Italy), and the Italian Association Against Leukemia
(Bari, Italy).
References
|
1
|
Chng WJ, Dispenzieri A, Chim CS, Fonseca
R, Goldschmidt H, Lentzsch S, Munshi N, Palumbo A, Miguel JS,
Sonneveld P, et al: IMWG consensus on risk stratification in
multiple myeloma. Leukemia. 28:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelinek T, Kryukov F, Rihova L and Hajek
R: Plasma cell leukemia: From biology to treatment. Eur J Haematol.
95:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mosca L, Musto P, Todoerti K, Barbieri M,
Agnelli L, Fabris S, Tuana G, Lionetti M, Bonaparte E, Sirchia SM,
et al: Genome-wide analysis of primary plasma cell leukemia
identifies recurrent imbalances associated with changes in
transcriptional profiles. Am J Hematol. 88:16–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lovec H, Grzeschiczek A, Kowalski MB and
Möröy T: Cyclin D1/bcl-1 cooperates with myc genes in the
generation of B-cell lymphoma in transgenic mice. EMBO J.
13:3487–3495. 1994.PubMed/NCBI
|
|
5
|
Shaffer LG, Slovak ML and Campbell LJ: An
International System for Human Cytogenetic Nomenclature (2009).
Karger. Basel: 2009.
|
|
6
|
Sykes PJ, Neoh SH, Brisco MJ, Hughes E,
Condon J and Morley AA: Quantitation of targets for PCR by use of
limiting dilution. Biotechniques. 13:444–449. 1992.PubMed/NCBI
|
|
7
|
Lichter P, Tang Chang CJ, Call K,
Hermanson G, Evans GA, Housman D and Ward DC: High-resolution
mapping of human chromosome 11 by in situ hybridization with cosmid
clones. Science. 247:64–69. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arcaini L, Zibellini S, Boveri E, Riboni
R, Rattotti S, Varettoni M, Guerrera ML, Lucioni M, Tenore A, Merli
M, et al: The BRAF V600E mutation in hairy cell leukemia and other
mature B-cell neoplasms. Blood. 119:188–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janssen JW, Vaandrager JW, Heuser T, Jauch
A, Kluin PM, Geelen E, Bergsagel PL, Kuehl WM, Drexler HG, Otsuki
T, et al: Concurrent activation of a novel putative transforming
gene, myeov, and cyclin D1 in a subset of multiple myeloma cell
lines with t(11;14)(q13;q32). Blood. 95:2691–2698. 2000.PubMed/NCBI
|
|
10
|
Specht K, Haralambieva E, Bink K, Kremer
M, Mandl-Weber S, Koch I, Tomer R, Hofler H, Schuuring E, Kluin PM,
et al: Different mechanisms of cyclin D1 overexpression in multiple
myeloma revealed by fluorescence in situ hybridization and
quantitative analysis of mRNA levels. Blood. 104:1120–1126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moreaux J, Hose D, Bonnefond A, Reme T,
Robert N, Goldschmidt H and Klein B: MYEOV is a prognostic factor
in multiple myeloma. Exp Hematol. 38:1189.e3–1198.e3. 2010.
View Article : Google Scholar
|
|
12
|
Carneiro A, Isinger A, Karlsson A,
Johansson J, Jönsson G, Bendahl PO, Falkenback D, Halvarsson B and
Nilbert M: Prognostic impact of array-based genomic profiles in
esophageal squamous cell cancer. BMC Cancer. 8:982008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lawlor G, Doran PP, MacMathuna P and
Murray DW: MYEOV (myeloma overexpressed gene) drives colon cancer
cell migration and is regulated by PGE2. J Exp Clin Cancer Res.
29:812010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian E, Sawyer JR, Heuck CJ, Zhang Q, van
Rhee F, Barlogie B and Epstein J: In multiple myeloma, 14q32
translocations are nonrandom chromosomal fusions driving high
expression levels of the respective partner genes. Genes
Chromosomes Cancer. 53:549–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chapman MA, Lawrence MS, Keats JJ,
Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann
GJ, Adli M, et al: Initial genome sequencing and analysis of
multiple myeloma. Nature. 471:467–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andrulis M, Lehners N, Capper D, Penzel R,
Heining C, Huellein J, Zenz T, von Deimling A, Schirmacher P, Ho
AD, et al: Targeting the BRAF V600E mutation in multiple myeloma.
Cancer Discov. 3:862–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bohn OL, Hsu K, Hyman DM, Pignataro DS,
Giralt S and Teruya-Feldstein J: BRAF V600E mutation and clonal
evolution in a patient with relapsed refractory myeloma with
plasmablastic differentiation. Clin Lymphoma Myeloma Leuk.
14:e65–e68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson MR, Del Carpio-Jayo D, Lin P,
Giralt S, Anderlini P, Champlin RE, Khouri IF, Vadhan-Raj S,
Medeiros LJ and Bueso-Ramos CE: Primary plasma cell leukemia:
Morphologic, immunophenotypic, and cytogenetic features of 4 cases
treated with chemotherapy and stem cell transplantation. Ann Diagn
Pathol. 10:263–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song JY and Popplewell L: Circulating
reactive plasma cells in the setting of peripheral T-cell lymphoma
mimicking plasma cell leukemia. Blood. 126:11502015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Møller HE, Preiss BS, Pedersen P,
Kristensen IB, Hansen CT, Frederiksen M, Abildgaard N and Møller
MB: Clinicopathological features of plasmablastic multiple myeloma:
A population-based cohort. APMIS. 123:652–658. 2015. View Article : Google Scholar : PubMed/NCBI
|