Introduction
Although pancreatic cancer is not a common human
malignancy, the mortality rate is extremely high at ~100%, which is
the leading cause of cancer-related mortality (1). In recent years, economic development,
improvements in standards of living and aging populations have led
to an increase in the incidence of pancreatic cancer (2).
Matrix metalloproteinase-9 (MMP-9) is a protease
closely associated with tumor invasion and metastasis, as well as
the degradation of the basement membrane during vascular formation
(3). Angiogenesis is closely
associated with tumor growth and metastasis, and microvessel
density is an objective indicator that reflects the angiogenesis of
tumor tissues. Peng et al showed that increased MMP-9
expression induced by pancreatic cancer cells mediates natural
killer cell dysfunction (4). Guo
et al reported that ginsenoside Rg3 inhibited pancreatic
cancer via the downregulation of MMP9/MMP2 expression (5).
Extracellular signal-regulated kinases (ERK) are a
subfamily of the mitogen-activated protein kinase (MAPK) family,
that may be activated by a number of cytokines and growth factors
to mediate cell proliferation, differentiation and signal
transduction (6). ERK1 and ERK2 are
two important family members, and the signal transduction pathways
in which they are involved are closely associated with the
occurrence and development of tumors (7). Furthermore, Tyagi et al (8) indicated that P-21 activated kinase 4
promotes the proliferation and survival of pancreatic cancer cells
via the ERK pathway. In addition, Li et al (9) reported that hyperglycemia regulates the
thioredoxin-interacting protein/thioredoxin/reactive oxygen species
axis of pancreatic cancer via the p38 MAPK and ERK pathways. Zheng
et al (10) reported that
gemcitabine inhibited tumour growth and promoted apoptosis of
pancreatic cancer via upregulation of pERK1/2 levels. Showalter
et al (11) showed that
naturally occurring vitamin K inhibits pancreatic cancer cell
survival via the suppression of ERK phosphorylation.
Paeoniflorin was first isolated from the
Ranunculaceae plant, peony in 1963 (12). Previous studies have shown that
paeoniflorin exhibits antispasmodic, analgesic, antipyretic,
anti-inflammatory, anti-ulcer, anti-oxidation, anti-coagulation and
regulatory effects; however, the mechanism remains unclear, and a
number of receptors and ion channels have been implicated as major
targets of paeoniflorin's pharmacological effects (13–16).
Paeoniflorin inhibited human pancreatic cancer apoptosis, and the
mechanisms are considered to be involved with MMP-9 expression and
ERK signaling pathways. Thus, the aim of the present study was to
investigate the anticancer effects and molecular mechanisms of
paeoniflorin on human pancreatic cancer cell apoptosis.
Materials and methods
Reagents
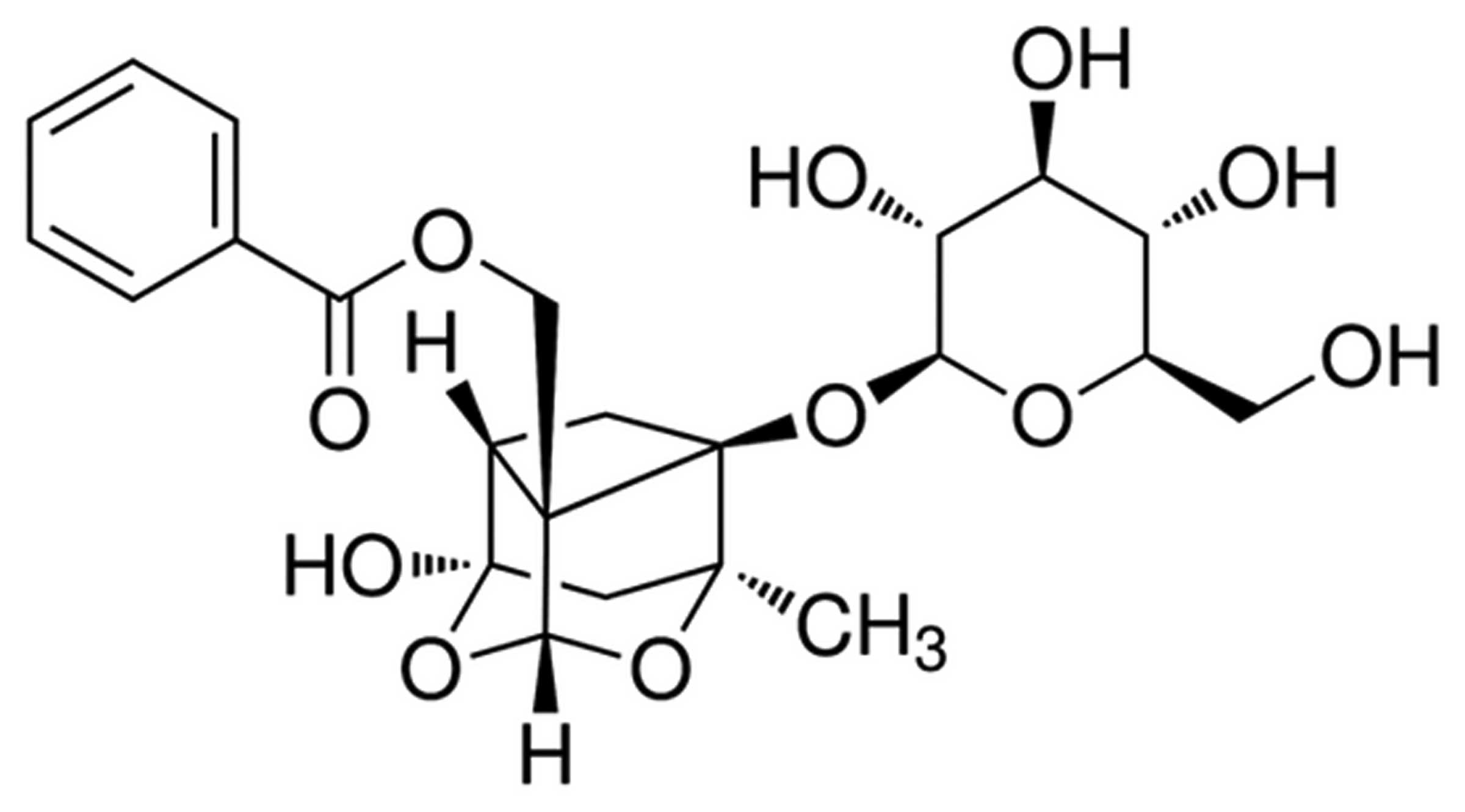
The chemical structure of paeoniflorin (purity ≥98%;
Sigma-Aldrich, St. Louis, MO, USA) is shown in Fig. 1. Gibco Dulbecco's modified eagle
medium (DMEM) and fetal calf serum (FBS) were obtained from Thermo
Fisher Scientific (Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sangon Biotech Co., Ltd., (Shanghai, China). The
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
Apoptosis Detection kit and BCA protein assay kit were obtained
from Sigma-Aldrich. Caspase-3 and caspase-9 Activities Assay Kits
were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
China).
Cell line and culture conditions
The BXPC-3 human pancreatic cancer cell line was
obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). BXPC-3 cells were cultured
with DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
U/ml streptomycin in a humidified chamber at 37°C in 5%
CO2. The culture medium was replaced every 2–3 days with
fresh complete medium (DMEM containing 10% FBS with 100 U/ml
penicillin and 100 U/ml streptomycin).
Cell viability assay
BXPC-3 cells (5×104 cells/well) were
seeded in 96-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2 for 24 h. Next, BXPC-3 cells
were cultured with 0, 6.25, 12.5 and 25 µM paeoniflorin for 0, 24,
48 and 72 h, and cell viability was determined by MTT assay. A
total of 20 µl MTT (5 mg/l; Sangon Biotech Co., Ltd.) was added to
each well, and the plates were incubated for 4 h in a humidified
chamber at 37°C in 5% CO2. The medium was discarded and
150 µl dimethyl sulfoxide was added to each well and agitated for
20 min at room temperature. Cell viability was determined at a
wavelength of 490 nm using a multi-well spectrophotometer (XL-818;
Bio-Tek, Winooksi, VT, USA).
Lactate dehydrogenase (LDH) assay
BXPC-3 cells (5×104 cells/well) were
seeded in 96-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were then
treated with 0, 6.25, 12.5 and 25 µM paeoniflorin for 0, 24, 48 and
72 h, and cell cytotoxicity was analyzed by LDH assay. A total of
100 µl LDH solution was added to each well and incubated at room
temperature for 30 min. The absorbance was quantified at a
wavelength of 490 nm using a multi-well spectrophotometer (XL-818;
Bio-Tek).
Flow cytometry
BXPC-3 cells (1×106 cells/well) were
seeded in 6-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were then
treated with 0, 6.25, 12.5 and 25 µM paeoniflorin for 48 h.
Cellular apoptotic rates of the BXPC-3 cells was determined using
an Annexin V-FITC/PI Apoptosis Detection kit (Sigma-Aldrich).
Briefly, BXPC-3 cells were washed twice (5 min each time) with
ice-cold PBS and stained with 10 µl V-FITC at room temperature for
30 min in darkness. Next, 5 µl PI was added to the cells and
incubated at room temperature for 10 min in darkness. Cellular
apoptosis was analyzed by flow cytometry (EPICS® ALTRA™;
Olympus Corporation, Tokyo, Japan).
DAPI staining assay
BXPC-3 cells (1×106 cells/per well) were
seeded in 6-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were treated
with 0, 6.25, 12.5 and 25 µM paeoniflorin for 48 h, and the
apoptotic rate of BXPC-3 cells was determined using a DAPI staining
assay. BXPC-3 cells were washed twice with ice-cold PBS, fixed in
4% paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China), permeabilized with 0.1% Triton X-100 and stained
with 2 µg/ml DAPI (Beyotime Institute of Biotechnology, Haimen,
China) for 10 min. BXPC-3 cells were then observed under a
fluorescence microscope (D810; Nikon Corporation, Tokyo,
Japan).
Caspase-3 and −9 colorimetric protease
assay
BXPC-3 cells (1×106 cells/well) were
seeded in 6-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were treated
with 0, 6.25, 12.5 and 25 µM paeoniflorin for 48 h, and caspase-3
and −9 activity was determined using a colorimetric protease assay.
In accordance with the manufacturer's protocol (Nanjing KeyGen
Biotech Co., Ltd.), BXPC-3 cells lysates were prepared in cell
lysis buffer for 30 min on ice and centrifuged at 12,000 × g for 15
min at 4°C. Supernate was collected and the protein concentrations
were determined using a Pierce BCA Protein Assay Kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Equal amounts of protein (40
ng) were mixed with reaction buffer (Ac-DEVD-pNA for caspase-3,
Ac-LEHD-pNA for caspase-9) and incubated at 37°C for 2 h in the
dark. Caspase-3 and −9 activity was then measured using a
microplate reader (ELX800; Bio-Tek) at an absorbance of 405 nm.
Gelatin zymography assays of MMP-9
activity
BXPC-3 cells (1×106 cells/well) were
seeded in 6-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were treated
with 0, 6.25, 12.5 and 25 µM paeoniflorin for 48 h, and the MMP-9
activity of BXPC-3 cells was determined by gelatin zymography
assays. Equal volumes of sample (40 µl) were mixed with sodium
dodecyl sulfate (SDS) sample buffer (Tiandz Inc., Beijing, China).
The miscible liquids were subjected to 10% SDS-PAGE gels
polymerized with 1 mg/ml gelatin (Tiandz Inc.). The gel was then
washed three times for 20 min at room temperature in 2.5% Triton
X-100 to remove SDS following electrophoresis. Next, the gel was
incubated in radioimmunoprecipitation buffer (Nanjing KeyGen
Biotech Co., Ltd.) at 37°C for 12 h. The gel was stained with 0.05%
Coomassie brilliant blue stain R-250 (Beyotime Institute of
Biotechnology) was used to stain followed by destaining with 7%
acetic acid (Sinopharm Chemical Reagent Co., Ltd.).
Western blot analysis of ERK protein
expression
BXPC-3 cells (1×106 cells/well) were
seeded in 6-well plates and cultured with DMEM in a humidified
chamber at 37°C in 5% CO2. BXPC-3 cells were treated
with 0, 6.25, 12.5 and 25 µM paeoniflorin for 48 h, and ERK protein
expression was determined by western blot analysis. Briefly, the
cells were washed with cold PBS and incubated with ice-cold lysis
buffer for 30 min on ice. BXPC-3 cell liquid was collected and
centrifuged at 12,000 × g for 15 min at 4°C. The protein
concentrations were determined using a Pierce BCA Protein Assay Kit
(Thermo Fisher Scientific, Inc.). Equal protein was separated using
12% SDS-polyacrylamide gels with Coomassie Brilliant Blue, and
transferred to a polyvinylidene fluoride membrane (0.22 mm; EMD
Millipore, Bedford, MA, USA). The blotted membranes were then
blocked with Tris-buffered saline containing 5% non-fat milk to
block nonspecific binding sites. The transferred membrane was
incubated with polyclonal rabbit anti-ERK (1:1,500; sc-292838;
Santa Cruz Biotechnology, Dallas, TX, USA) and polyclonal rabbit
anti-β-actin (1:500; sc-130657; Sangon Biotech Co., Ltd.,)
antibodies overnight at 4°C. The membrane was washed with TBST and
incubated with rabbit anti-mouse immunoglobulin G horseradish
peroxidase-conjugated antibody (1:5,000; A1949, Sigma-Aldrich) for
1 h. Then the proteins were detected using enhanced
chemiluminescence using an ECL Advanced Western Blot detection Kit
(Tiangen Biotech Co., Ltd., Beijing, China). and then the proteins
were detected using enhanced chemiluminescence using an ECL
Advanced Western Blot detection Kit (Tiangen Biotech Co., Ltd.,
Beijing, China).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 statistical software (SPSS Inc., Chicago, IL, USA) and
representative of at least three independent experiments. Analysis
of variance was performed to compare multiple data. Data are
expressed as the mean ± standard deviation of at least three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of paeoniflorin on cell
viability
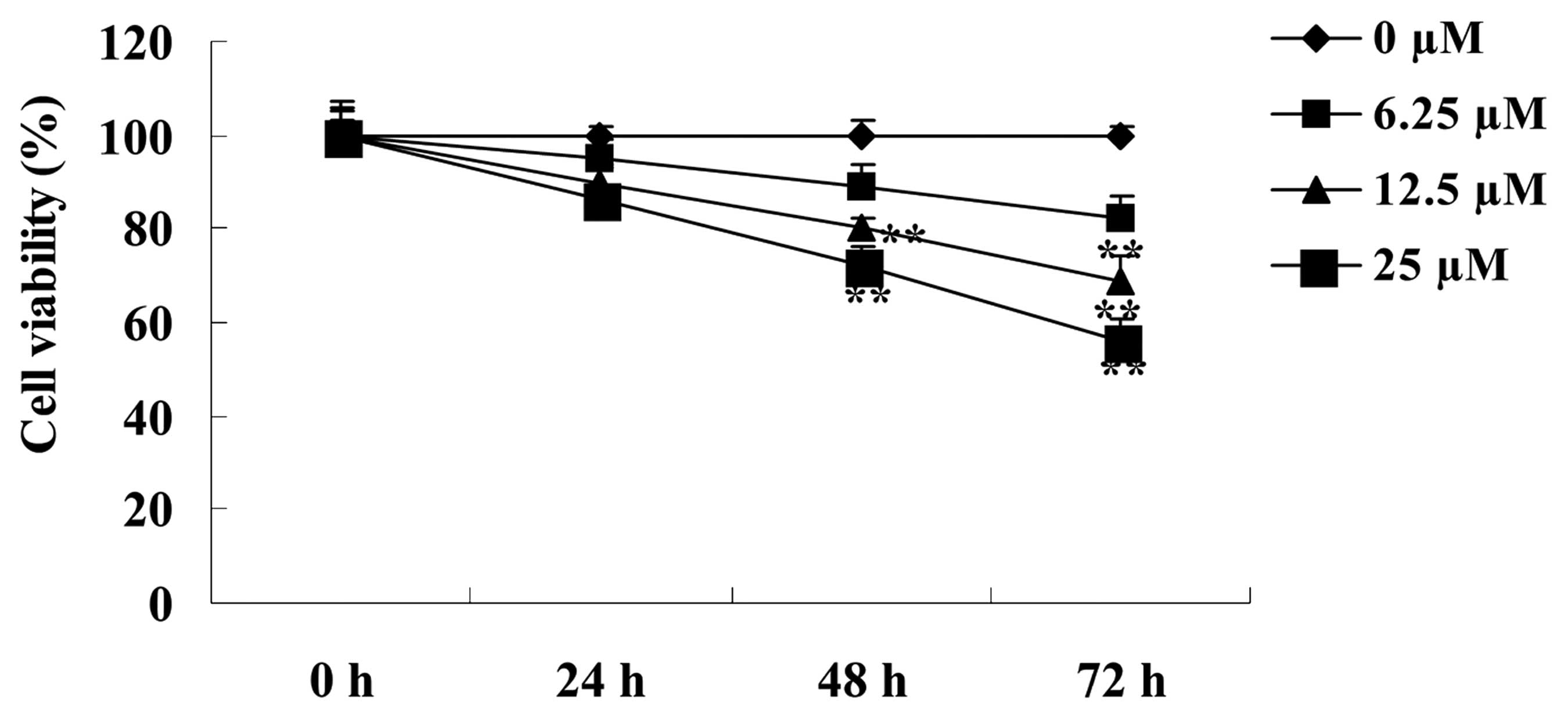
The effect of paeoniflorin on the cell viability of
BXPC-3 cells was determined using MTT assays. As shown in Fig. 2, treatment with 6.25, 12.5 and 25 µM
paeoniflorin decreased cell viability in a dose- and time-dependent
manner. Following treatment with 6.25 µM paeoniflorin for 72 h, and
treatment with 12.5 and 25 µM paeoniflorin for 48 and 72 h, the
cell viability of BXPC-3 cells was significantly reduced when
compared with the 0 µM paeoniflorin-treatment group (P<0.01;
Fig. 2). Based on these results, a
dose of 12.5 µM paeoniflorin and a treatment duration of 48 h were
selected for further study.
Effect of paeoniflorin on cell
cytotoxicity
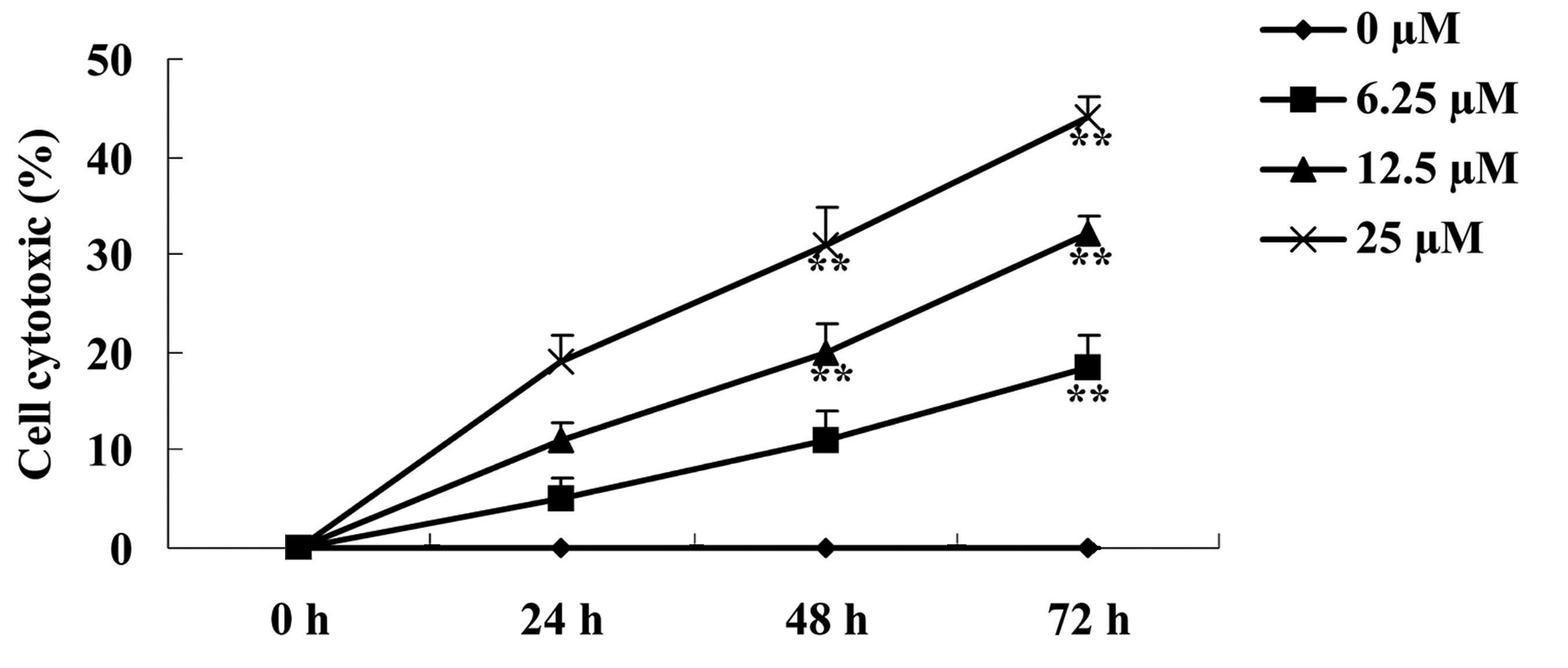
The effect of paeoniflorin on BXPC-3 cell
cytotoxicity was investigated using the LDH release assay. As shown
in Fig. 3, cell cytotoxicity of
BXPC-3 cells increased following treatment with 6.25, 12.5 and 25
µM paeoniflorin, in a dose- and time-dependent manner. Notably, in
cells treated with 6.25 µM paeoniflorin for 72 h, and 12.5 and 25
µM paeoniflorin for 48 and 72 h, cell cytotoxicity of BXPC-3 cells
was significantly increased when compared with the 0 µM
paeoniflorin treatment group (P<0.01; Fig. 3).
Effect of paeoniflorin on cellular
apoptosis
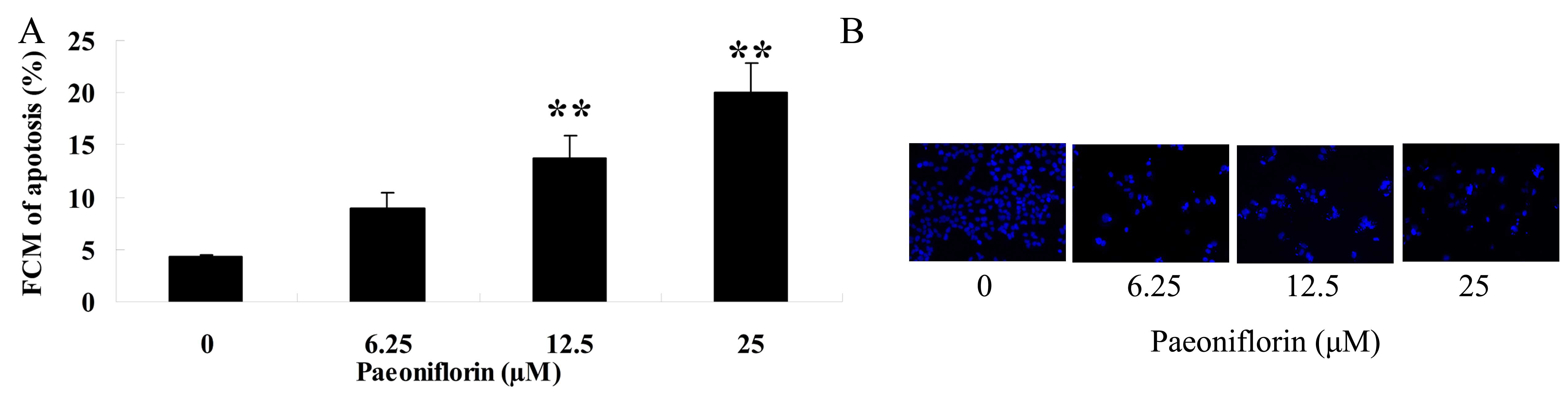
To determine the effect of paeoniflorin on cellular
apoptosis of BXPC-3 cells, the levels of cellular apoptosis were
analyzed by Annexin V-FITC/PI apoptosis and DAPI staining assays.
Treatment with 6.25, 12,5 and 25 µM paeoniflorin for 48 h induced a
concentration-dependent increase in cellular apoptosis of BXPC-3
cells (Fig. 4A-B). As shown in
Fig. 4A, treatment with 12.5 and 25
µM paeoniflorin resulted in a significant increase in cellular
apoptosis of BXPC-3 cells at 48 h when compared with that of the 0
µM paeoniflorin-treatment group (P<0.01; Fig. 4A). In addition, DAPI staining revealed
that cellular apoptosis of BXPC-3 cells was increased in the 6.25,
12.5 and 25 µM paeoniflorin treatment groups at 48 h when compared
with the 0 µM paeoniflorin treatment group (Fig. 4B).
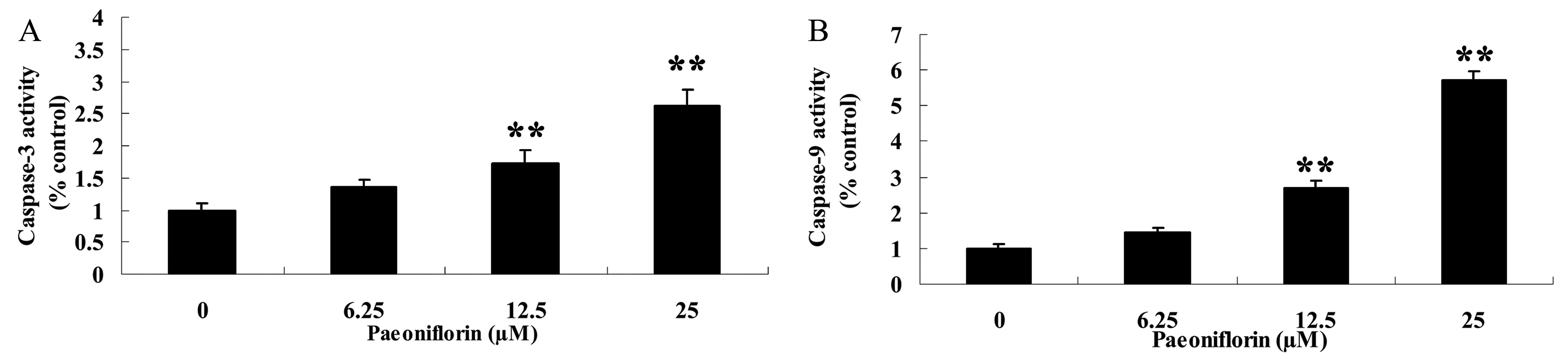
Effect of paeoniflorin on caspase-3
and −9 activity
The effect of paeoniflorin treatment on caspase-3
and caspase-9 activity of BXPC-3 cells was also assessed. Caspase-3
and caspase-9 activity of BXPC-3 cells was significantly increased
following 48 h treatment with 12.5 and 25 µM paeoniflorin when
compared with that of 0 µM paeoniflorin (P<0.01; Fig. 5A and B). These results indicate that
paeoniflorin may promote cellular apoptosis of BXPC-3 cells.
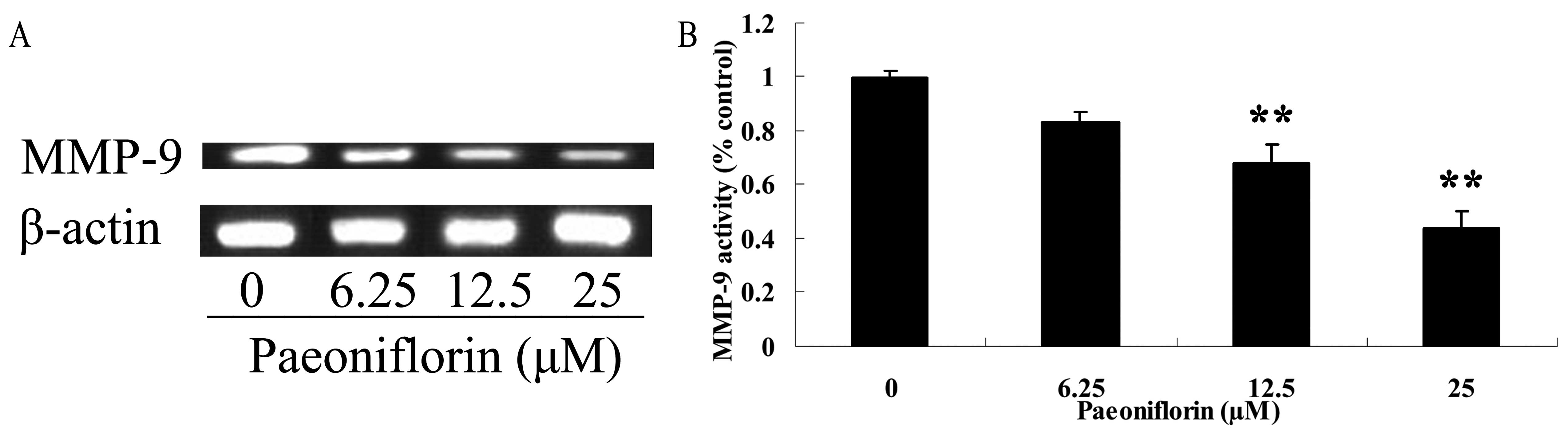
Effect of paeoniflorin on MMP-9
activity
To investigate the effect of paeoniflorin on BXPC-3
cells, MMP-9 activity of BXPC-3 cells was evaluated by gelatin
zymography assays. The MMP-9 activity of BXPC-3 cells was
significantly reduced by treatment with 12.5 and 25 µM paeoniflorin
for 48 h when compared with that of the 0 µM paeoniflorin treatment
group (P<0.01; Fig. 6A and B).
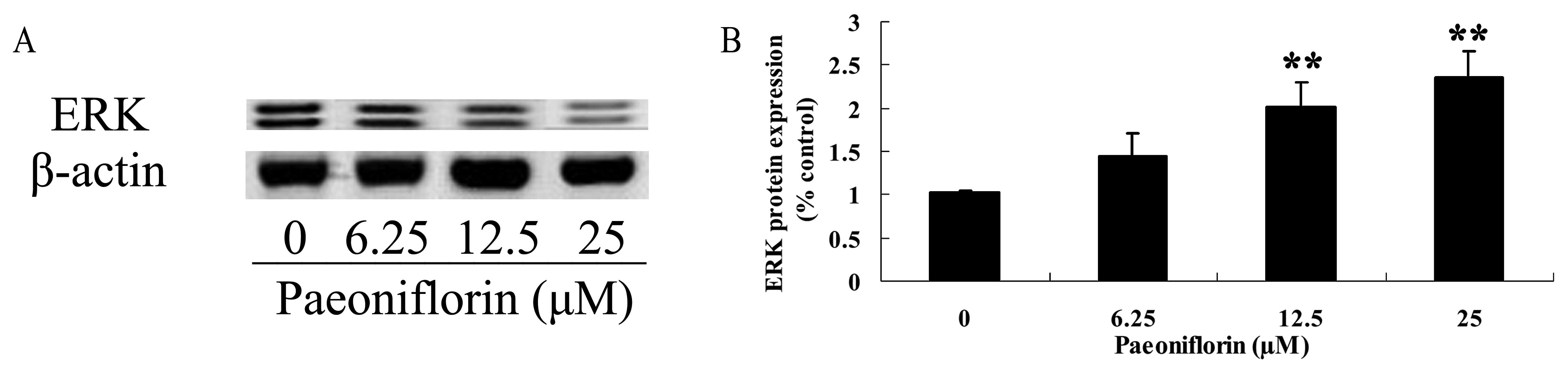
Effect of paeoniflorin on ERK protein
expression
ERK protein expression of BXPC-3 cells was analyzed
using western blot analysis. The ERK protein expression of BXPC-3
cells was significantly increased following 12.5 and 25 µM
paeoniflorin treatment for 48 h, when compared with that of the 0
µM paeoniflorin treatment group (P<0.01; Fig. 7A and B).
Discussion
The early symptoms of high-grade malignant
pancreatic tumors are not clear, and thus the majority of tumors
are identified at an advanced stage, yielding a low surgical
resection rate (17). As a result of
recent changes in human behavior and diet, the worldwide incidence
of pancreatic cancer has been increasing annually (18). Pancreatic cancer results from the
interaction between genetic and environmental factors, which may be
associated with genetic susceptibility as a result of genetic
mutation, genetic polymorphism and epigenetic factors. Recently,
certain risk factors which are associated with pancreatic cancer,
including smoking, obesity, alcohol consumption, chronic
pancreatitis and diabetes, have increasingly gained attention
(19). In the current study,
paeoniflorin was found to decrease cell viability and increase cell
cytotoxicity of BXPC-3 cells in a dose- and time-dependent manner.
In addition, levels of BXPC-3 cellular apoptosis were increased
following paeoniflorin treatment. Notably, the activity of
caspase-3 and −9 in BXPC-3 cells was increased by paeoniflorin
treatment. In a previous study, Lu et al reported that
paeoniflorin inhibited the tumor invasion and metastasis of HepG2
and Bel-7402 human hepatocellular carcinoma cells via the
suppression of MMP-9 and ERK (20).
Wang et al showed that paeoniflorin inhibited the growth of
human colorectal cancer cells via regulation of p53/14-3-3ζ
(21).
MMP-9 degrades IV and V collagen and gelatin in the
extracellular matrix, which facilitates the growth of tumor blood
vessels to mesenchyme and subsequently, the microvessel density of
tumor blood vessels increases, leading to continual tumor growth
and distant metastasis (22). A
previous study showed that the positive expression rate of MMP-9 is
50% in pancreatic cancer patients, and in cases accompanied by
liver metastasis, the positive rate of MMP-9 expression is 66.7%,
which indicates MMP-9 may be associated with pancreatic cancer
liver metastases (23). The present
study demonstrated that MMP-9 activity of human pancreatic cancer
BXPC-3 cells was significantly reduced by paeoniflorin treatment,
which is in agreement with the results of Lu et al (20) which indicated paeoniflorin inhibited
the tumor invasion and metastasis of human hepatocellular cancer
cells via suppression of MMP-9 and ERK. Ji et al (24) reported that paeoniflorin suppressed
type I collagen synthesis via the inhibition of MMP-1 mRNA
expression.
Activated ERK kinase causes cell proliferation and
differentiation (25). Jun protein
Jen encoded by c-jun belongs to the members of activated protein
(AP-1) family, and is a downstream target gene of the MAPK family.
Many tumor genes mediate transformed tumorigenic effect by AP-1
family signal transduction pathway, and activated AP-1 family
promotes cell transformation and cancerization, participating in
all the links including extracellular matrix degradation,
abnormality and angiogenesis of metastatic tumor newborn vessels by
downstream target gene expression (26). The results of the present study showed
that paeoniflorin significantly reduced ERK protein expression
levels in human pancreatic cancer BXPC-3 cells. Chen et al
showed that paeoniflorin attenuated cerebral infarction via
downregulation of mitogen-activated protein kinase kinase (MEK),
phosphorylated-MEK and ERK (27). In
addition, paeoniflorin inhibited the tumor invasion and metastasis
of hepatocellular carcinoma cells via downregulation of MMP-9 and
ERK expression (20).
In conclusion, the results of this study suggest
that paeoniflorin decreases cell viability and promote cellular
apoptosis of human pancreatic cancer BXPC-3 cells. Furthermore,
these results indicate that MMP-9 and ERK may significantly
contribute to the anticancer effects of paeoniflorin. However,
further studies are required to identify additional signaling
pathways that are affected by paeoniflorin, which may elucidate to
the mechanism of its anticancer effects in pancreatic cancer
cells.
References
|
1
|
Nakamura M, Oida Y, Abe Y, Yamazaki H,
Mukai M, Matsuyama M, Chijiwa T, Matsumoto H and Ueyama Y:
Thrombospondin-2 inhibits tumor cell invasion through the
modulation of MMP-9 and uPA in pancreatic cancer cells. Mol Med
Rep. 1:423–427. 2008.PubMed/NCBI
|
|
2
|
Bakshi H, Sam S, Rozati R, Sultan P, Islam
T, Rathore B, Lone Z, Sharma M, Triphati J and Saxena RC: DNA
fragmentation and cell cycle arrest: A hallmark of apoptosis
induced by crocin from kashmiri saffron in a human pancreatic
cancer cell line. Asian Pac J Cancer Prev. 11:675–679.
2010.PubMed/NCBI
|
|
3
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrix metalloproteinase
(MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng YP, Zhang JJ, Liang WB, Tu M, Lu ZP,
Wei JS, Jiang KR, Gao WT, Wu JL, Xu ZK, et al: Elevation of MMP-9
and IDO induced by pancreatic cancer cells mediates natural killer
cell dysfunction. BMC Cancer. 14:7382014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo JQ, Zheng QH, Chen H, Chen L, Xu JB,
Chen MY, Lu D, Wang ZH, Tong HF and Lin S: Ginsenoside Rg3
inhibition of vasculogenic mimicry in pancreatic cancer through
downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J
Oncol. 45:1065–1072. 2014.PubMed/NCBI
|
|
6
|
Xie G, Yang S, Chen A, et al:
Electroacupuncture at quchi and zusanli treats cerebral
ischemia-reperfusion injury through activation of ERK signaling.
Exp Ther Med. 5:1593–1597. 2013.PubMed/NCBI
|
|
7
|
Amsterdam A, Shezen E, Raanan C, Schreiber
L, Prus D, Slilat Y, Ben-Arie A and Seger R: Nuclear localization
of phosphorylated ERK1 and ERK2 as markers for the progression of
ovarian cancer. Int J Oncol. 39:649–656. 2011.PubMed/NCBI
|
|
8
|
Tyagi N, Bhardwaj A, Singh AP, McClellan
S, Carter JE and Singh S: p-21 activated kinase 4 promotes
proliferation and survival of pancreatic cancer cells through AKT-
and ERK-dependent activation of NF-kB pathway. Oncotarget.
5:8778–8789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Wu Z, Ma Q, et al: Hyperglycemia
regulates TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in
pancreatic cancer. Curr Cancer Drug Targets. 14:348–356. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng C, Jiao X, Jiang Y and Sun S: ERK1/2
activity contributes to gemcitabine resistance in pancreatic cancer
cells. J Int Med Res. 41:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Showalter SL, Wang Z, Costantino CL, et
al: Naturally occurring K vitamins inhibit pancreatic cancer cell
survival through a caspase-dependent pathway. J Gastroenterol
Hepatol. 25:738–744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu F, Zhong X, Mao Q and Huang Z: The
antidepressant-like effects of paeoniflorin in mouse models. Exp
Ther Med. 5:1113–1116. 2013.PubMed/NCBI
|
|
13
|
Li J, Ji X, Zhang J, Shi G, Zhu X and Wang
K: Paeoniflorin attenuates Aβ25-35-induced neurotoxicity in PC12
cells by preventing mitochondrial dysfunction. Folia Neuropathol.
52:285–290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Wang CM, Liu JD and Wang YT:
Influence of paeoniflorin on intracellular calcium ion
concentration in the sphincter of Oddi of hypercholesterolemic
rabbits. Genet Mol Res. 13:5001–5010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuboi H, Hossain K, Akhand AA, Takeda K,
Du J, Rifa'i M, Dai Y, Hayakawa A, Suzuki H and Nakashima I:
Paeoniflorin induces apoptosis of lymphocytes through a
redox-linked mechanism. J Cell Biochem. 93:162–172. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Yuan J, Wu HX, Chang Y, Wang QT,
Wu YJ, Liu LH and Wei W: Paeoniflorin inhibits inflammatory
responses in mice with allergic contact dermatitis by regulating
the balance between inflammatory and anti-inflammatory cytokines.
Inflamm Res. 62:1035–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori-Iwamoto S, Kuramitsu Y, Ryozawa S,
Taba K, Fujimoto M, Okita K, Nakamura K and Sakaida I: A proteomic
profiling of gemcitabine resistance in pancreatic cancer cell
lines. Mol Med Rep. 1:429–434. 2008.PubMed/NCBI
|
|
18
|
Taghavi A, Fazeli Z, Vahedi M, Baghestani
AR, Zali MR and Pourhoseingholi MA: Pancreatic cancer mortality and
misclassification-bayesian analysis. Asian Pac J Cancer Prev.
12:2271–2274. 2011.PubMed/NCBI
|
|
19
|
Li S, Sun J, Yang J, Zhang L, Wang L, Wang
X and Guo Z: XIAP expression is associated with pancreatic
carcinoma outcome. Mol Clin Oncol. 1:305–308. 2013.PubMed/NCBI
|
|
20
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
21
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Wu JZ, Zhang JY, Xue J, Ma R, Cao
HX and Feng J: Detection of circulating vascular endothelial growth
factor and matrix metalloproteinase-9 in non-small cell lung cancer
using Luminex multiplex technology. Oncol Lett. 7:499–506.
2014.PubMed/NCBI
|
|
23
|
Yuan J, Wu Y and Lu G: α-Mangostin
suppresses lipopolysaccharide-induced invasion by inhibiting matrix
metalloproteinase-2/9 and increasing E-cadherin expression through
extracellular signal-regulated kinase signaling in pancreatic
cancer cells. Oncol Lett. 5:1958–1964. 2013.PubMed/NCBI
|
|
24
|
Ji Y, Wang T, Wei ZF, Lu GX, Jiang SD, Xia
YF and Dai Y: Paeoniflorin, the main active constituent of Paeonia
lactiflora roots, attenuates bleomycin-induced pulmonary fibrosis
in mice by suppressing the synthesis of type I collagen. J
Ethnopharmacol. 149:825–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Wadei HA, Al-Wadei MH, Ullah MF and
Schuller HM: Celecoxib and GABA cooperatively prevent the
progression of pancreatic cancer in vitro and in xenograft models
of stress-free and stress-exposed mice. PLoS One. 7:e433762012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen YJ, Zhu XX, Yang X, Jin B, Lu JJ,
Ding B, Ding ZS and Chen SH: Cardamonin inhibits angiotensin
II-induced vascular smooth muscle cell proliferation and migration
by downregulating p38 MAPK, Akt, and ERK phosphorylation. J Nat
Med. 68:623–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen YF, Wu KJ and Wood WG: Paeonia
lactiflora extract attenuating cerebral ischemia and arterial
intimal hyperplasia is mediated by paeoniflorin via modulation of
VSMC migration and Ras/MEK/ERK signaling pathway. Evid Based
Complement Alternat Med. 2013:4824282013.PubMed/NCBI
|