Introduction
Autoimmune pancreatitis (AIP) is a unique form of
pancreatitis that is histopathologically characterized by dense
lymphoplasmacytic infiltration and fibrosis of the pancreas with
obliterative phlebitis (1).
Pancreatic cancer is one of the leading causes of mortality in
Japan and Western countries (2). This
type of tumor is associated with poor prognosis, due to its
aggressive biology and the difficulty in making an early diagnosis.
Patients with AIP share numerous clinical features with pancreatic
cancer patients, including advanced age, painless jaundice, weight
loss, new-onset diabetes mellitus and elevated serum levels of
carbohydrate antigen (CA) 19-9 (3).
Such factors commonly render the differentiation between AIP and
pancreatic cancer rather challenging; however, distinguishing
between the two diseases is crucial, as their treatments and
prognoses are vastly different (4).
An accurate preoperative diagnosis of AIP is required in order to
avoid unnecessary surgery and to achieve clinical remission with
steroid therapy. 18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography (PET)/computed
tomography (CT) has been reported to assist with this
differentiation (2,3,5). The
current study reports the case of a patient who presented with a
new localized 18F-FDG uptake at the pancreatic head and
normal serum immunoglobulin G4 (IgG4) levels during the remission
phase of AIP, and had been strongly suspected of having pancreatic
cancer preoperatively. Written informed consent to publish was
obtained from the patient.
Case report
A 71-year-old male patient was admitted to Shiritsu
Oozu Hospital (Oozu, Japan) after presenting with worsening
diabetes mellitus in April 2009. The patient had a history of two
abdominal surgeries: A choledochectomy and choledochojejunostomy,
due to choledocholithiasis 28 years prior to admission; and a right
lateral hepatic sectoriectomy, due to intrahepatic stones 3 years
prior to admission. AIP was suspected following a workup, which
included examining the serum IgG4 levels and an
18F-FDG-PET/CT, and the patient was referred to the
Ehime University Hospital (Toon, Japan). Abdominal ultrasonography
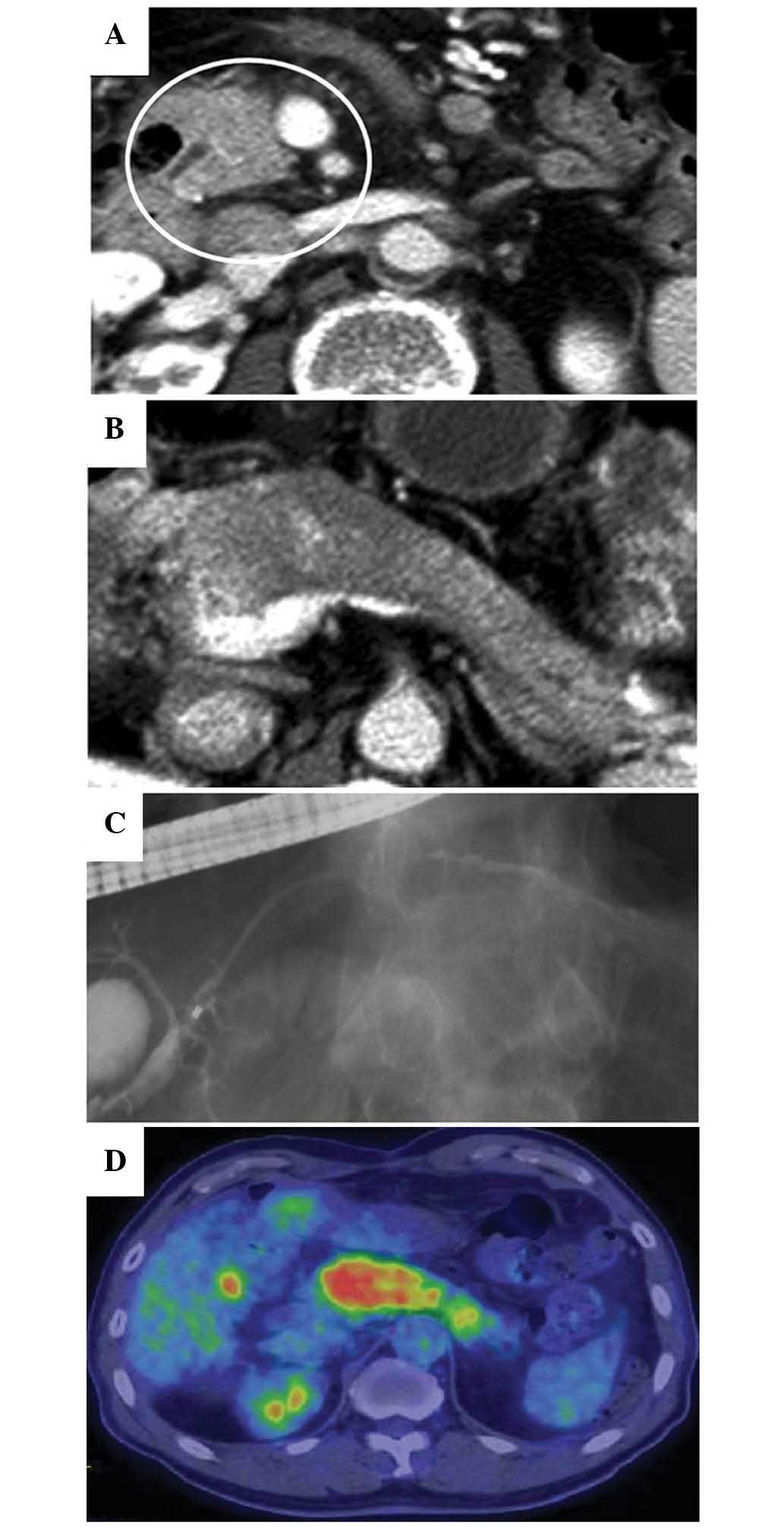
and CT imaging (Brilliance 64; Philips, Tokyo, Japan) revealed
enlargement of the pancreatic head and body (Fig. 1A and B). Endoscopic retrograde
cholangiopancreatography (ERCP; JF-260V; Olympus Corporation,
Tokyo, Japan) showed diffuse narrowing of the main pancreatic duct
(MPD) in the pancreatic head and body (Fig. 1C). 18F-FDG-PET/CT
(Discovery ST Elite; GE Healthcare Life Sciences, Hino, Japan),
which had been performed during the previous hospital stay,
revealed a strong and diffuse uptake of 18F-FDG
throughout the entire pancreas (Fig.
1D). The serum IgG4 level was markedly elevated (158 mg/dl;
normal range, 4.8–105.0 mg/dl). A diagnosis of AIP was thereby
established, and steroid therapy was initiated.
The initial oral prednisolone dose administered was
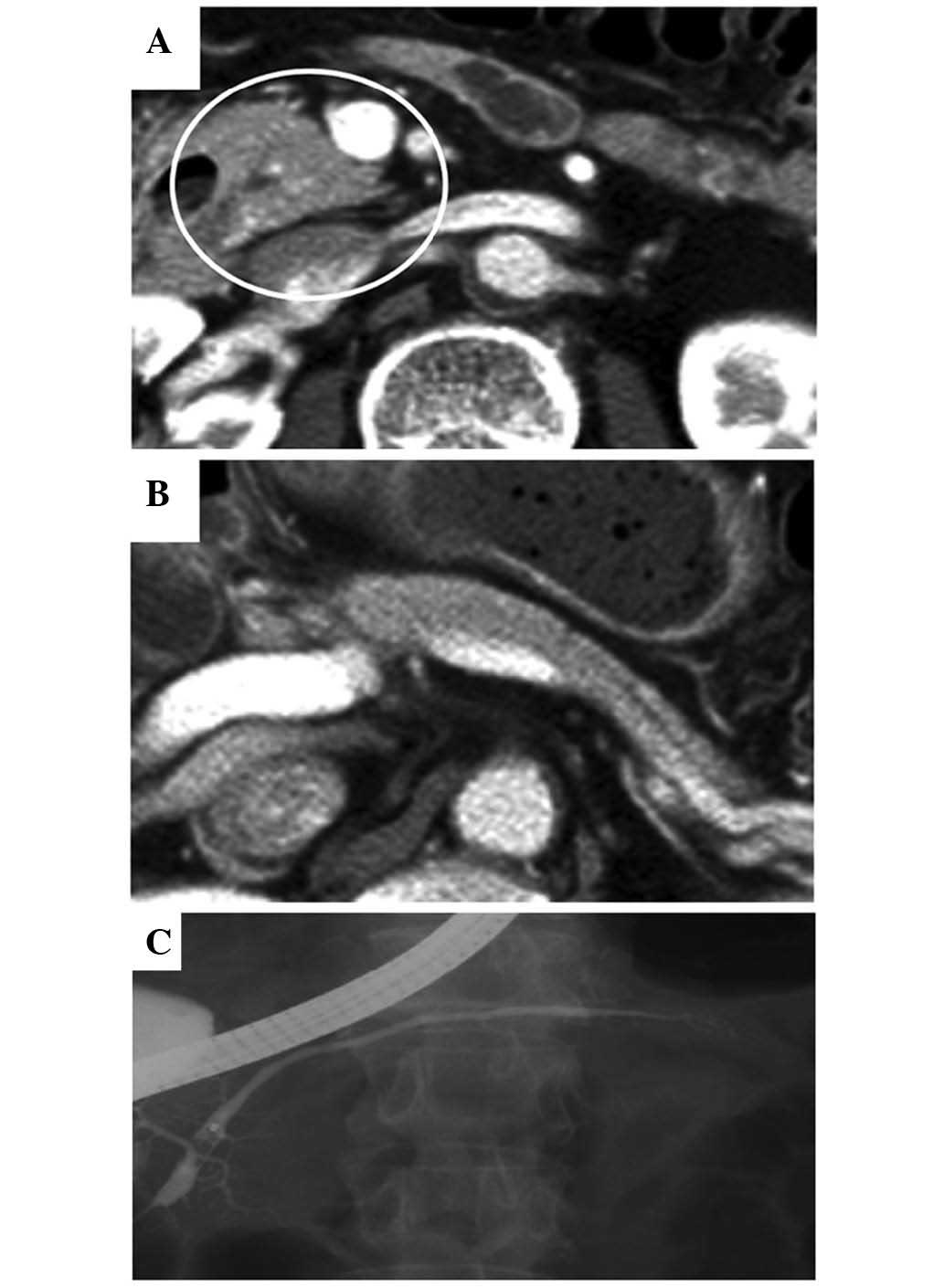
30 mg/day. Following the initiation of the steroid therapy, the
enlargement of the pancreatic head and body markedly improved, the
diffuse narrowing of the MPD fully recovered (Fig. 2A-C), and the IgG4 level dropped to
within normal limits. The oral steroid therapy regimen was as
follows: The initial dose was administered daily for 2 weeks,
followed by gradual tapering of the dose by 5 mg every 2 weeks,
until a daily dose of 5 mg was reached. Subsequently, maintenance
steroid therapy (5 mg/day) was administered, based on the Japanese
consensus guidelines for the management of AIP (6). Follow-up examinations were performed on
an outpatient basis.
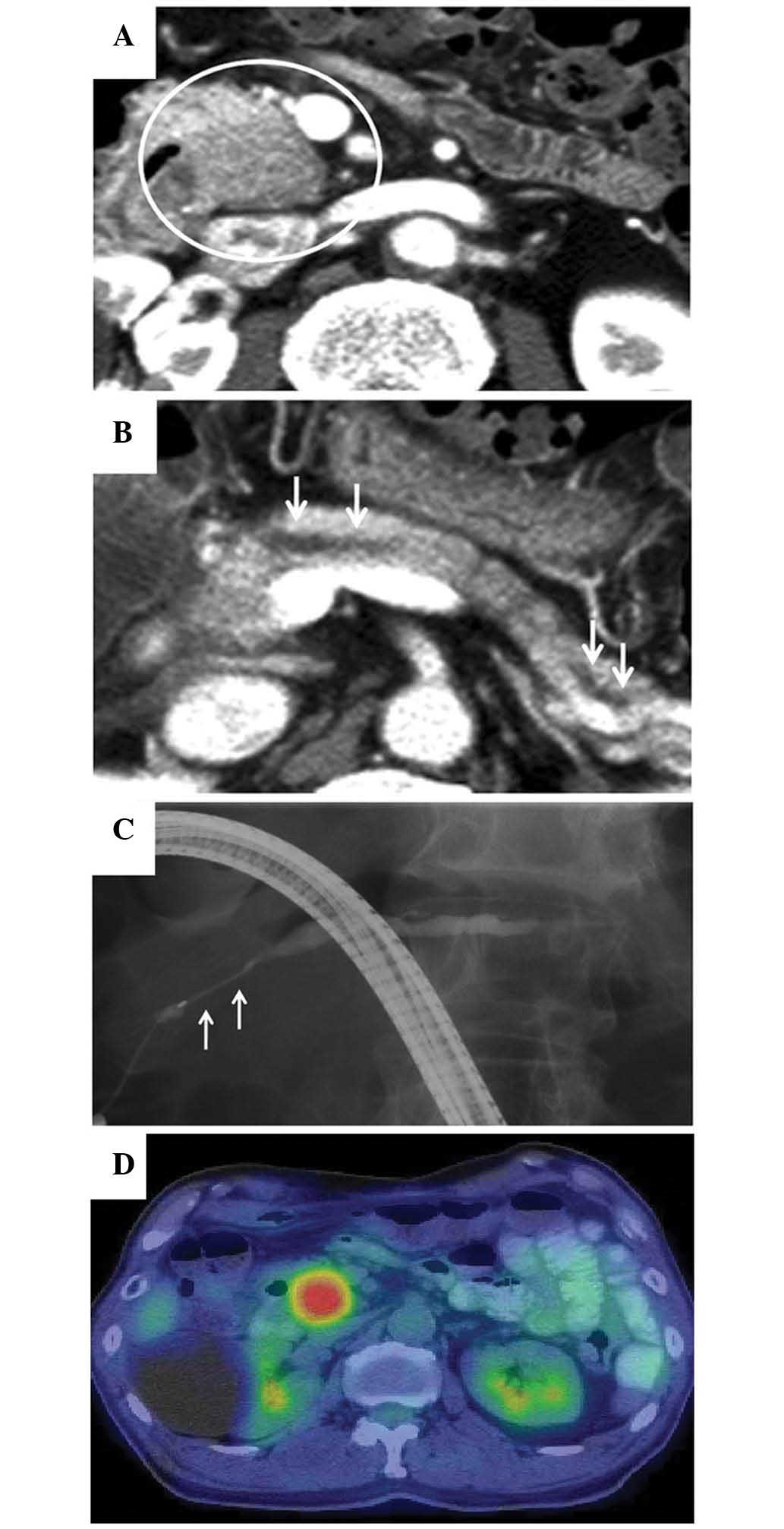
At 10 months after the initiation of the steroid
therapy, elevated serum levels of amylase (255 IU/l; normal range,
37–124 IU/l) and lipase (91 IU/l; normal range, 13–49 IU/l) were
detected, and a CT scan revealed a 2-cm low-attenuation mass at the
pancreatic head and dilation of the MPD (Fig. 3A and B). The patient was readmitted to
the hospital due to a suspected relapse of AIP. Magnetic resonance
imaging (MRI) revealed tumor-like enlargement at the pancreatic
head, and obstruction of the MPD with dilatation of the upstream
MPD. ERCP showed a ~2-cm long stricture of the MPD at the
pancreatic head and a dilatation of the body and tail portion of
MPD that measured 5 mm in diameter (Fig.
3C). Based on these radiographic findings, it was difficult to
decide between recurrence of AIP and pancreatic cancer. The serum
levels of CA19-9, duke pancreatic monoclonal antigen type 2, and
Span-1 were normal. The serum level of carcinoembryonic antigen was
slightly elevated (7.1 ng/ml; normal range, <5.0 ng/ml). The
serum level of IgG4 was 106 mg/dl, which was below the cutoff value
(≥135 mg/dl) of the Japanese clinical diagnostic criteria for AIP
(1). 18F-FDG-PET/CT
(Aquiduo PCA-7000B; Toshiba Medical Systems, Ootawara, Japan)
showed a well-circumscribed, solitary, nodular and homogenous
18F-FDG uptake, with a maximum standardized uptake value
of 7.82 at the location where the pancreatic head mass was
identified by CT scan (Fig. 3D). No
abnormal extrapancreatic uptake of 18F-FDG was
observed.
The patient was referred to the Department of
Hepatobiliary-Pancreatic and Breast Surgery, Ehime University
Hospital with a suspected diagnosis of concomitant pancreatic
cancer with AIP, and pancreatoduodenectomy was performed. The
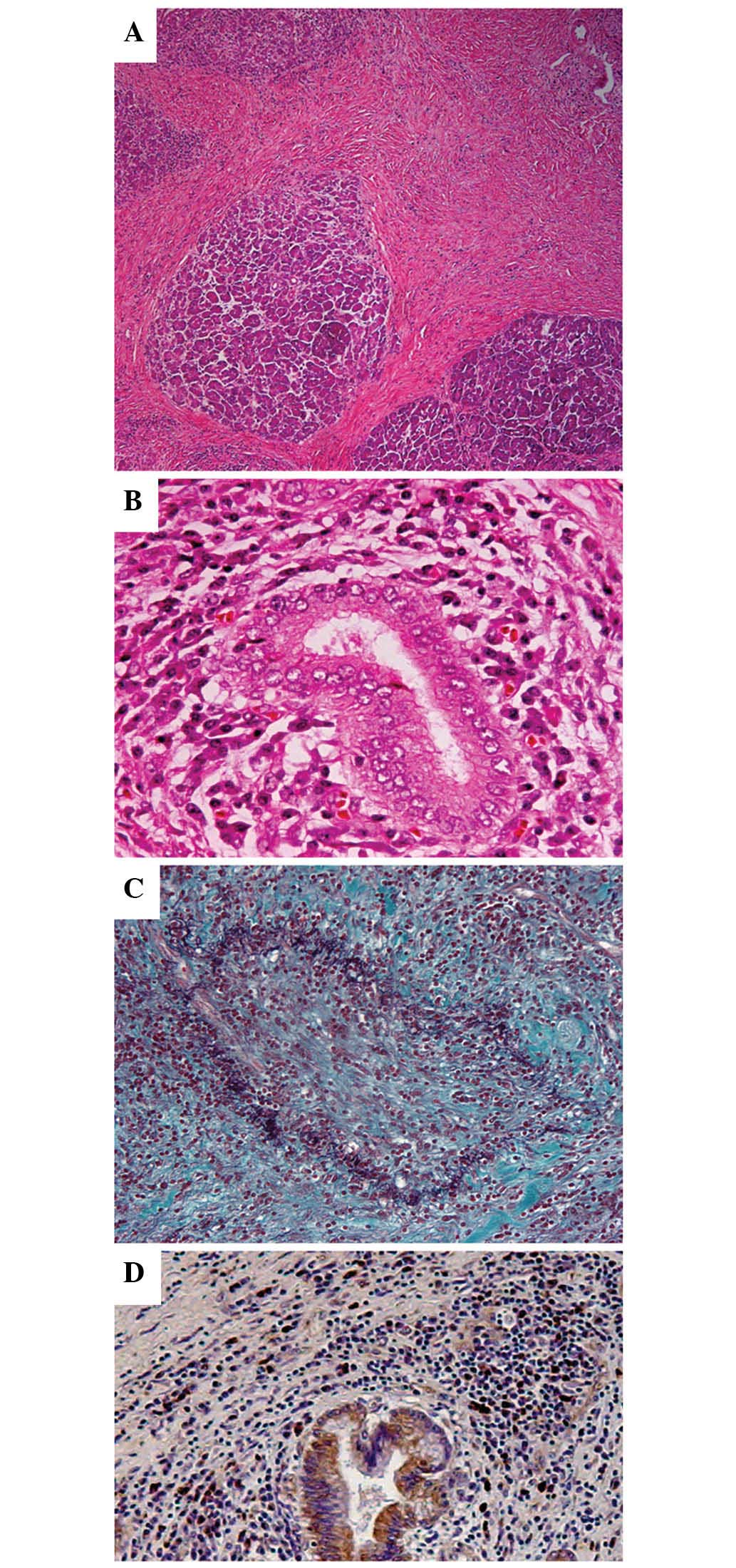
formalin-fixed paraffin-embedded 4-µm sections were used for
hematoxylin and eosin staining, Elastica-Masson staining and IgG4
immunostaining (mouse anti-human IgG4 monoclonal antibody;
dilution, 1:400; catalog no., GTX75819; GeneTex, Irvine, CA, USA).
Pathological examination revealed diffuse lymphoplasmacytic
infiltrate with fibrosis, periductal lymphoplasmacytic infiltrate
and obliterative phlebitis (Fig.
4A-C). The lymphoplasmacytic infiltrate included an abundance
of IgG4-positive cells [>10 cells/high power field (HPF)]
(Fig. 4D), which forms one of the
histological criteria for AIP proposed by the Mayo Clinic (7). No malignant cells were found. Recurrent
AIP was therefore diagnosed. Maintenance steroid therapy was
resumed following surgery, and, at the time of writing the present
study, no recurrent AIP in the pancreatic remnant has been
identified for 4 years after surgery.
Discussion
AIP is a distinct form of pancreatitis characterized
by the involvement of autoimmune mechanisms, such as
hypergammaglobulinemia, increased serum levels of IgG, increased
serum levels of IgG4 or the presence of autoantibodies (1). AIP has been associated with an effective
response to steroid therapy (1). The
pathological features of this disorder are characterized by
periductal lymphoplasmacytic infiltrate and lymphoplasmacytic
infiltrate showing abundant (>10 cells/HPF) IgG4-positive cells
(7). This lymphoplasmacytic
infiltration is often accompanied by stroriform fibrosis and
obliterative phlebitis (7). These
characteristic features can distinguish AIP from normal chronic
pancreatitis (7). The clinical
spectrum of AIP includes sclerosing cholangitis, retroperitoneal
fibrosis, hilar lymphadenopathy, salivary gland swelling and
interstitional pneumonia (8). Some of
these extrapancreatic lesions show pathological findings similar to
those of pancreatic lesions (8).
AIP and pancreatic cancer share several
characteristics; however, the therapeutic methods for each of these
diseases are vastly different. Pancreatic cancer requires surgery,
while steroid therapy is effective for AIP without the need for
surgical intervention (4). It is
therefore crucial to distinguish AIP from pancreatic cancer;
however, in certain cases, differential diagnosis is challenging,
despite the use of numerous different diagnostic modalities, such
as CT and MRI scans, and ERCP. Nakazawa et al (9) reported that 7/37 (18.9%) patients with
AIP underwent surgical intervention due to having been misdiagnosed
with pancreatic or bile duct cancer. Kamisawa et al
(4) also reported that 6/17 (35.3%)
patients with focal mass-forming AIP were surgically treated due to
the suspicion of pancreatic cancer.
Other studies have reported the utility of
18F-FDG-PET/CT for the differentiation of AIP from
pancreatic cancer (2,3,5).
18F-FDG-PET/CT is a sensitive modality used for the
diagnosis of malignancies. Since 18F-FDG uptake is
caused by increased glucose utilization of tumor cells and is also
observed at inflammatory sites, 18F-FDG uptake is a
shared finding between AIP and pancreatic cancer. Kamisawa et
al (5) concluded that
18F-FDG-PET/CT can assist in the differentiation between
the two diseases by assessing 18F-FDG uptake patterns in
the pancreas and extrapancreatic lesions. Lee et al
(3) indicated that, in severe cases,
using PET/CT can detect the presence of diffuse 18F-FDG
uptake by the pancreas, or concomitant extrapancreatic uptake by
the salivary glands, which can aid in differentiation. Ozaki et
al (2) also reported that the
typical 18F-FDG-PET findings for AIP are an irregular
contour, longitudinal shape, heterogeneous accumulation and
multiple localizations, whereas those for pancreatic cancer are a
smooth contour, nodular shape, homogenous accumulation and solitary
localization. Shigekawa et al (8) indicated that the accumulation patterns
of 18F-FDG were nodular and solitary in the majority of
cases of pancreatic cancer that they examined, and that the
possibility of AIP was increased if the 18F-FDG
accumulation in the pancreas had a longitudinal shape; however,
nodular and solitary 18F-FDG accumulations were also
observed in AIP, corresponding with focal changes in the pancreas
on CT or ERCP. It was also reported that 18F-FDG uptake
in extrapancreatic lesions, including the extra-abdominal lymph
nodes, salivary glands, eyes and biliary duct, may be helpful in
differentiating between pancreatic cancer and AIP.
Kamisawa et al (4) proposed an algorithm for the clinical
management of a mass-like lesion on the pancreatic head, with
particular emphasis on the differentiation between AIP and
pancreatic cancer. They identified 6 imaging characteristics, a
combination of CT and ERCP findings, that were highly suggestive of
AIP. The findings were as follows: i) Delayed enhancement of the
enlarged pancreas on CT scan; ii) a capsule-like rim on CT scan,
iii) the presence of extrapancreatic lesions, such as salivary
gland swelling, retroperitoneal mass or stenosis of the upper or
intrahepatic bile duct on CT scan or ERCP; iv) ≥3 cm-long narrowed
portion of the MPD on ERCP; v) skipped lesions of the MPD on ERCP,
and vi) a maximal diameter of <5 mm of the upstream MPD on ERCP.
In the present case, none of these imaging characteristics were
observed. According to this algorithm, in cases with no positive
imaging factors for AIP, surgery should be considered under the
provisional diagnosis of pancreatic cancer.
Several studies have reported cases of pancreatic
cancer complicated with AIP simultaneously (10–13) or
during follow-up (14–18). Loos et al (15) reported a case of a patient who
developed a metastatic adenocarcinoma of the pancreatobiliary
system within a year after the histologically confirmed diagnosis
of AIP. In addition, among the reports, 3 patients were diagnosed
with pancreatic cancer during maintenance steroid therapy (16–18). In
general, the risk of pancreatic cancer is markedly increased in
patients with chronic pancreatitis (14); however, the association between AIP
and pancreatic cancer remains unknown. Based on earlier reports of
cancer development during the course of maintenance steroid therapy
for AIP, three key findings of the present study, and the algorithm
proposed by Kamisawa et al (4), the patient was diagnosed with pancreatic
cancer during the course of maintenance steroid therapy for AIP,
and pancreatoduodenectomy was performed. The aforementioned key
findings of the present study were the following: i) Detection by
PET/CT scan of a well-circumscribed, solitary, nodular and
homogenous 18F-FDG uptake at the same area where a
pancreatic head mass was identified; ii) no extrapancreatic uptake;
and iii) normal serum IgG4 levels. Pathological examination
revealed diffuse lymphoplasmacytic infiltrate with fibrosis,
periductal infiltrate, obliterative phlebitis, IgG4-positive cells
and absence of malignant cells; therefore, post-treatment relapse
of AIP was eventually diagnosed.
At the time of writing the present study, no
recurrent AIP in the pancreatic remnant of the patient had been
identified for 4 years after surgery. Of note, authors from the
Mayo Clinic recently reported that the relapse rate of AIP patients
who underwent pancreatoduodenectomy as the initial treatment was
markedly lower than that of patients who had not undergone
pancreatoduodenectomy (the corticosteroid-treated group) (19). While the underlying mechanisms are
unclear, this is a noteworthy observation that requires further
study.
In summary, the current study reported the case of a
patient who presented with a new mass at the pancreatic head and an
upstream dilatation of the MPD while receiving a maintenance dosage
of steroids in the remission phase of AIP. The present study
highlights the challenges faced by clinicians in the diagnosis and
management of AIP in remission. In certain cases, the
differentiation between pancreatic cancer and AIP remains
difficult, despite the use of the latest diagnostic modalities.
References
|
1
|
Okazaki K, Kawa S, Kamisawa T, Naruse S,
Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, et al:
Clinical diagnostic criteria of autoimmune pancreatitis: Revised
proposal. J Gastroenterol. 41:626–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozaki Y, Oguchi K, Hamano H, Arakura N,
Muraki T, Kiyosawa K, Momose M, Kadoya M, Miyata K, Aizawa T and
Kawa S: Differentiation of autoimmune pancreatitis from suspected
pancreatic cancer by fluorine-18 fluorodeoxyglucose positron
emission tomography. J Gastroenterol. 43:144–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee TY, Kim MH, do Park H, Seo DW, Lee SK,
Kim JS and Lee KT: Utility of 18F-FDG PET/CT for differentiation of
autoimmune pancreatitis with atypical pancreatic imaging findings
from pancreatic cancer. AJR Am J Roentgenol. 193:343–348. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamisawa T, Imai M, Yui Chen P, Tu Y,
Egawa N, Tsuruta K, Okamoto A, Suzuki M and Kamata N: Strategy for
differentiating autoimmune pancreatitis from pancreatic cancer.
Pancreas. 37:e62–e67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamisawa T, Takum K, Anjiki H, Egawa N,
Kurata M, Honda G and Tsuruta K: FDG-PET/CT findings of autoimmune
pancreatitis. Hepatogastroenterology. 57:447–450. 2010.PubMed/NCBI
|
|
6
|
Kamisawa T, Okazaki K, Kawa S, Shimosegawa
T and Tanaka M: Research Committee for Intractable Pancreatic
Disease and Japan Pancreas Society: Japanese consensus guidelines
for management of autoimmune pancreatitis: III. Treatment and
prognosis of AIP. J Gastroenterol. 45:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chari ST, Smyrk TC, Levy MJ, Topazian MD,
Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS
and Farnell MB: Diagnosis of autoimmune pancreatitis: The Mayo
Clinic experience. Clin Gastroenterol Hepatol. 4:1010–1016. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shigekawa M, Yamao K, Sawaki A, Hara K,
Takagi T, Bhatia V, Nishio M, Tamaki T, El-Amin H, Sayed Zel-A and
Mizuno N: Is (18)F-fluorodeoxyglucose positron emission tomography
meaningful for estimating the efficacy of corticosteroid therapy in
patients with autoimmune pancreatitis? J Hepatobiliary Pancreat
Sci. 17:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakazawa T, Ohara H, Sano H, Ando T, Imai
H, Takada H, Hayashi K, Kitajima Y and Joh T: Difficulty in
diagnosing autoimmune pancreatitis by imaging findings.
Gastrointest Endosc. 65:99–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue H, Miyatani H, Sawada Y and Yoshida
Y: A case of pancreas cancer with autoimmune pancreatitis.
Pancreas. 33:208–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witkiewicz AK, Kennedy EP, Kennyon L, Yeo
CJ and Hruban RH: Synchronous autoimmune pancreatitis and
infiltrating pancreatic ductal adenocarcinoma: Case report and
review of the literature. Hum Pathol. 39:1548–1551. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motosugi U, Ichikawa T, Yamaguchi H,
Nakazawa T, Katoh R, Itakura J, Fujii H, Sato T, Araki T and
Shimizu M: Small invasive ductal adenocarcinoma of the pancreas
associated with lymphoplasmacytic sclerosing pancreatitis. Pathol
Int. 59:744–747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandrasegaram MD, Chiam SC, Nguyen NQ,
Ruszkiewicz A, Chung A, Neo EL, Chen JW, Worthley CS and
Brooke-Smith ME: A case of pancreatic cancer in the setting of
autoimmune pancreatitis with nondiagnostic serum markers. Case Rep
Surg. 2013:8090232013.PubMed/NCBI
|
|
14
|
Ghazale A and Chari S: Is autoimmune
pancreatitis a risk factor for pancreatic cancer? Pancreas.
35:3762007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loos M, Esposito I, Hedderich DM, Ludwig
L, Fingerle A, Friess H, Klöppel G and Büchler P: Autoimmune
pancreatitis complicated by carcinoma of the pancreatobiliary
system: A case report and review of the literature. Pancreas.
40:151–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukui T, Mitsuyama T, Takaoka M, Uchida K,
Matsushita M and Okazaki K: Pancreatic cancer associated with
autoimmune pancreatitis in remission. Intern Med. 47:151–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kubota K, Iida H, Fujisawa T, Yoneda M,
Inamori M, Abe Y, Kirikoshi H, Saito S, Ohshiro H, Kakuta Y and
Nakajima A: Clinical factors predictive of spontaneous remission or
relapse in cases of autoimmune pancreatitis. Gastrointest Endosc.
66:1142–1151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta R, Khosroshahi A, Shinagare S,
Fernandez C, Ferrone C, Lauwers GY, Stone JH and Deshpande V: Does
autoimmune pancreatitis increase the risk of pancreatic carcinoma?
A retrospective analysis of pancreatic resections. Pancreas.
42:506–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sah RP, Chari ST, Pannala R, Sugumar A,
Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD,
et al: Differences in clinical profile and relapse rate of type 1
versus type 2 autoimmune pancreatitis. Gastroenterology.
139:140–148; quiz e12–e13. 2010. View Article : Google Scholar : PubMed/NCBI
|