Introduction
Non-small lung cancer (NSCLC) has high morbidity and
mortality rates; according to the latest data, 222,520 new cases
and 157,300 mortalities were reported in USA in 2010. NSCLC is the
leading cause of cancer-related mortality in men and women.
According to 2010 Ministry of Health data, lung cancer rose from
the third most common to the most common cause of cancer-related
mortality in 1992, and reached a rate of 30.83/100,000 individuals.
Trends of rising incidence and younger age have become more
obvious, raising greater concern (1,2). There is
a close association between smoking and lung carcinoma; in
addition, atmospheric pollution and automobile exhaust fumes are
also lung cancer risk factors. Furthermore, chronic bronchitis,
pulmonary heart disease, pulmonary fibrosis, pulmonary tuberculosis
and pneumoconiosis are associated with the incidence of lung cancer
(3,4).
MicroRNA (miRNA/miR) is a small single-stranded RNA
(~22 nucleotides) that occurs in eukaryotes. miRNA does not have
the function of the encoded protein; instead, its biological
function is to pair with the 3′-untranslated region of
complementary target mRNAs. If the miRNA is fully complementary it
will cause target mRNA degradation; if the miRNA complementarity is
incomplete, it may prevent the target mRNA translating into protein
(5). Bioinformatic research has shown
that miRNA constitutes 1–5% of the total number of animal genes,
and ~60% encoding genes in animals are regulated by miRNA. Thus,
miRNA is a gene regulation factor that widely exists and has an
important role in living organisms (6). Experimental research has also
demonstrated that miRNAs are key in various biological activities,
such as cell proliferation and differentiation, apoptosis,
generation, migration, invasion and metastasis of tumor cells,
resistance mechanisms and the antivirus effect. Numerous miRNAs can
promote or inhibit metastasis (7).
Thus, miRNAs may become novel targets for cancer treatment.
MET proto-oncogene, which encodes the MET protein,
is a type of tyrosine kinase activity growth factor receptor that
can specifically bind to hepatocyte growth factor (HGF) and
activate its downstream signal transduction pathways, regulating
cell proliferation, energy, migration, angiogenesis, invasion and
morphogenesis (8). The tyrosine
kinase encoded by MET can adjust the invasive growth of tumor
cells, promoting the occurrence and development of lung tumors
(9). Normal MET signaling pathways
regulate embryonic development and tissue damage repair, whereas
abnormal activation promotes tumor metastasis. MET exhibits
abnormal expression in a variety of lung carcinoma tumor tissues
and it is closely associated with tumor progression (10).
Genistein is an antimicrobial toxin precursor that
predominantly exists in the biosynthesis of leguminous plants; it
is the active component and has various physiological functions
(11). It is an effective antioxidant
and protein tyrosine activation enzyme inhibitor, as well as a
plant estrogen. Genistein also has extensive pharmacological
effects in animal cells. A previous study found that genistein has
anticancer effects, including in vitro antitumor effects in
human breast cancer cell lines (12)
and prostate cancer cells (13).
Thus, genistein is a potential cancer chemoprevention agent with a
broad development prospect (14). In
the present study, we hypothesized that the potential anti-cancer
effect of genistein would inhibit A549 human lung cancer cell
proliferation via miR-27a and MET signaling.
Materials and methods
Chemicals
RPMI-1640 medium and fetal bovine serum were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma-Aldrich (St. Louis, MO, USA). Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
assay kit was obtained from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China).
Cell lines and cell growth
The A549 human lung cancer cell line was provided by
Hebei University (Baoding, China), and maintained in RPMI-1640
medium with 10% fetal bovine serum, 100 U/ml penicillin and 100
mg/ml streptomycin (Sangon Biotech Co., Ltd., Shanghai, China) in a
37°C incubator with 5% CO2.
Cell viability
The in vitro effect of genistein on the
viability of A549 cells was determined by MTT assay. Briefly, A549
cells were plated onto a 96-well plate at a density of
1×104 cells/well. Subsequently, A549 cells were exposed
to different concentrations of genistein (0, 10, 25, 50, 100 and
200 µM; Sigma-Aldrich; dissolved in physiological saline) for 1, 2
and 3 days. Subsequently, 20 µl of 0.5% MTT solution with
phosphate-buffered saline (Beyotime Institute of Biotechnology,
Haimen, China) was added to each well and incubated for 4 h at 37°C
with 5% CO2. After the incubation period, the culture
medium was replaced, 200 µl of dimethyl sulfoxide (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) was added to each well
and shaken for 20 min at room temperature. The optical density of
each well was determined with a spectrophotometer at 492 nm
(Varioskan Flash microplate reader; Thermo Fisher Scientific,
Inc.).
Annexin V-FITC/PI apoptosis assay
The in vitro effect of genistein on A549 cell
viability was determined by performing an Annexin V-FITC/PI assay.
Briefly, A549 cells were plated onto a 6-well plate at a density of
1×106 cells/well. Subsequently, A549 cells were exposed
to different concentrations of genistein (0, 25, 50 and 100 µM) for
2 days. A total of 5 µl Annexin V-FITC (5 µg/ml) and 5 µl PI (1
µg/ml) was added and incubated for 10 min at room temperature in
the dark. Then, cell apoptosis was examined using a flow cytometer
(FC 500; Beckman Coulter, Inc., Brea, CA, USA).
Analysis of caspase-3/9 activity
Following plating and genistein treatment, as
described for the Annexin V-FITC/PI assay, A549 cells were
incubated with cell lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice and then centrifuged at 12,000 × g
for 10 min at 4°C. The protein concentrations of the cell solutions
were determined using a Pierce BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Equal quantities of protein were mixed with 2 mM
Ac-LEHD-pNA for caspase-9 and 2 mM Ac-DEVD-pNA for caspase-3
(Beyotime Institute of Biotechnology). The mixtures were incubated
at 37°C for 2 h in the dark and detected at a wavelength of 405 nm
(Varioskan Flash microplate reader).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following plating and genistein treatment, as
described for the Annexin V-FITC/PI assay, total RNA was extracted
from the A549 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA (1–2 µg) was reverse transcribed to
synthesize complementary DNA using the SuperScript III First-Strand
Synthesis System (Thermo Fisher Scientific, Inc.) miR-27a was
specifically amplified and its expression was quantified by
performing TaqMan miRNA RT-qPCR assays (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The PCR cycling conditions were as follows: 5 min at 94°C, 30 sec
at 94°C, 30 sec at 60°C and 30 sec at 72°C (for a total of 40
cycles), followed by a cycle of 5 min at 72°C. miR-27a levels were
assessed by SYBR Green qPCR (Takara Biotechnology Co., Ltd.,
Dalian, China). The forward (F) and reverse (R) primers were used
were as follows: F, 5′-TCCGTGAGAGCTGGAAAACC-3′ and R,
5′-TGGTTCTAACTAACTCCAGCCG-3′ for miR-27a; F,
5′-CGCTTCGGCACATATACTA-3′ and R, 5′-CGCTTCACGAATTTGCGTGTCA-3′ for
U6. Analysis of mRNA expression of miR 27a was calculated according
to the 2-ΔΔCq method (15).
Western blotting
After plating and genistein treatment, as described
for the Annexin V-FITC/PI assay, A549 cells were incubated with 500
µl RIPA lysis buffer for 30 min on ice and centrifuged at 12,000 ×
g for 10 min at 4°C. The protein concentrations of the cell
solutions were determined using a Pierce BCA protein assay kit
(Thermo Fisher Scientific, Inc.). Equal quantities (80 µg) of
protein was resolved on 12% SDS-PAGE gel and transferred to
nitrocellulose membrane (Santa Cruz Biotechnology, Inc., CA, USA).
Subsequently, the membranes were blocked with non-fat milk in
Tris-buffered saline with Tween 20 and incubated with polyclonal
rabbit MET (1:1,000; catalog no., sc-8307; Santa Cruz
Biotechnology, Inc.) and β-actin (1:500; catalog no., D110007;
Sangon Biotech Co., Ltd.) primary antibodies overnight at 4°C. The
membranes were incubated with horseradish peroxidase-conjugated
mouse anti-rabbit IgG secondary antibodies (1:5,000; catalog no.,
M1003-7; Hangzhou Huaan Biological Technology Co., Ltd., Hangzhou,
China) for 1 h at 37°C. Membranes were visualized using an Enhanced
Chemiluminescence detection kit (Beyotime Institute of
Biotechnoloy). The bands were quantified by densitometric analysis
using ImageJ software (version 1.44p; National Institutes of
Health, Bethseda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
statistical software (version 19.0; IBM SPSS, Chicago, IL, USA) and
results are presented as the mean ± standard deviation of three
replicate experiments. Statistical differences between the control
and treatment samples were determined by Student's t-test.
Differences among experimental groups were evaluated by one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant.
Results
In vitro effect of genistein on A549
cell viability
The chemical structure of genistein (Sigma-Aldrich;
with a purity of 98%) is indicated in Fig. 1. In the present study, genistein was
dissolved in physiological saline. The present study investigated
the possible anti-cancer effect of genistein on the viability of
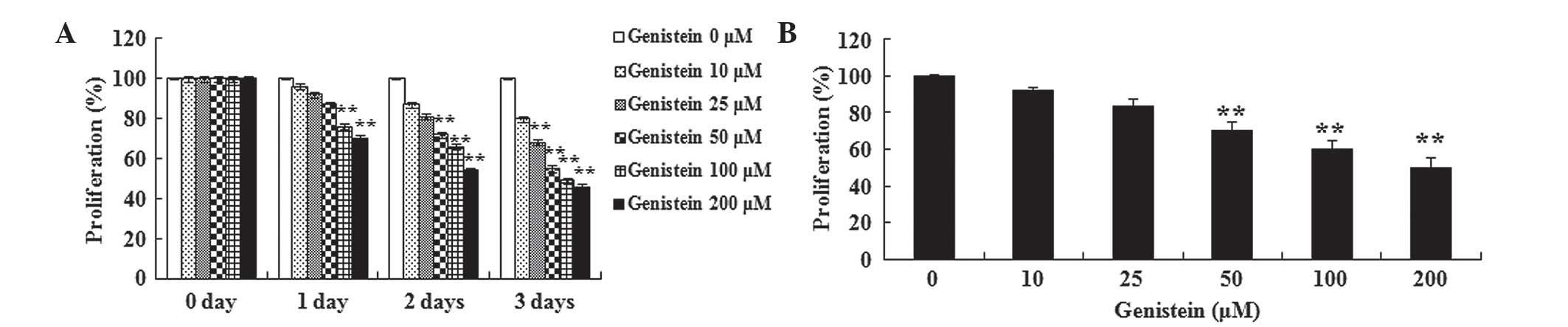
A549 cells by performing an MTT assay. Fig. 2A and B demonstrates that the treatment
with genistein inhibited the viability of A549 cells in a time- and
dose-dependent manner. The results suggest that A549 cell viability
was significantly inhibited by different concentrations of
genistein (25, 50, 100 and 200 µM) for 3 days (P<0.01). When the
treatment was reduced to 2 days, the viability of A549 cells was
significantly inhibited by 50, 100 and 200 µM genistein (P<0.01;
Fig. 2B). Furthermore, A549 cell
viability was significantly suppressed following treatment with 100
and 200 µM genistein for a period of 1 day (P<0.01). These data
suggest that genistein may significantly inhibit human lung cancer
cell viability.
In vitro effect of genistein on A549
cell apoptosis
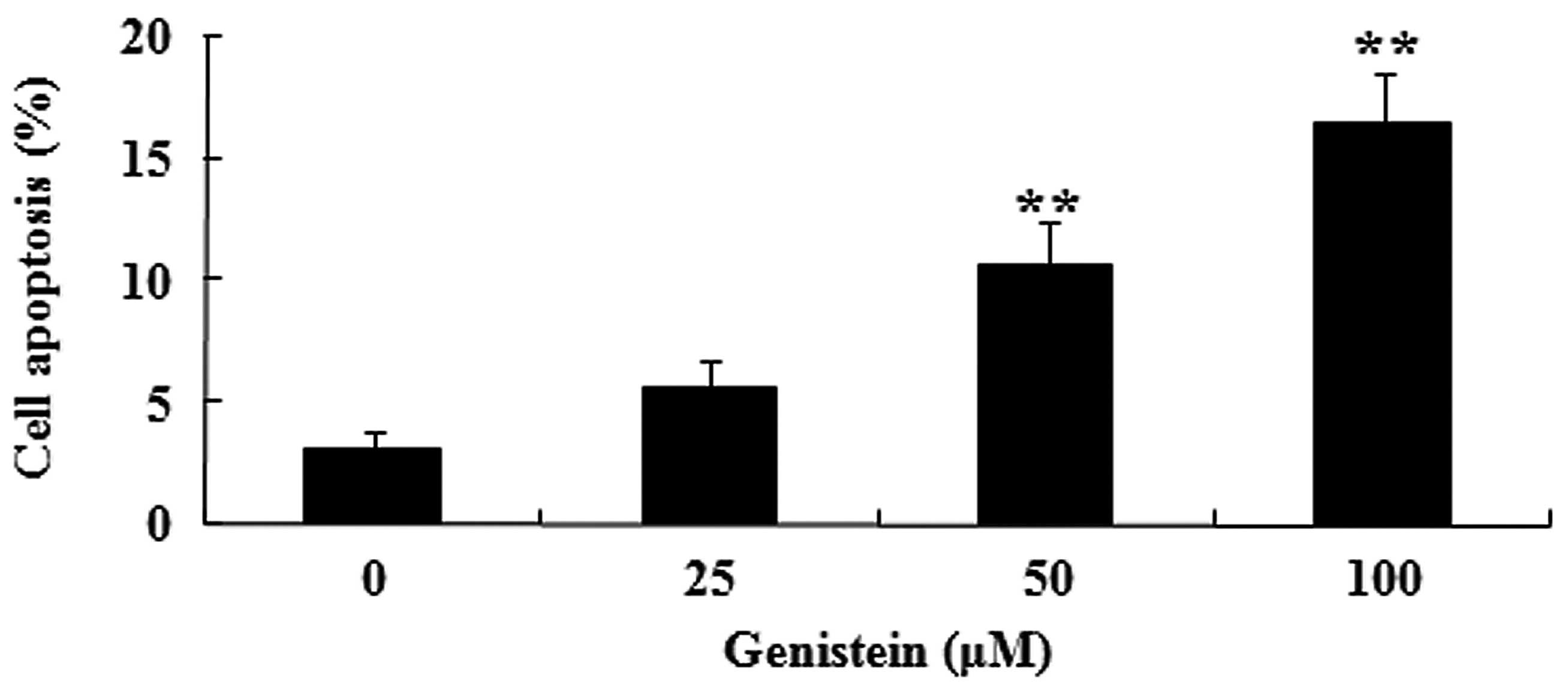
To determine the possible anti-cancer effect of
genistein on A549 cell apoptosis, the present study measured the
apoptosis of A549 cells by performing an Annexin V-FITC/PI
apoptosis assay. Fig 3. indicates
that administration of genistein (50 and 100 µM) significantly
induces the apoptosis of A549 cells in dose-dependent manner
(P<0.01). These data suggest that genistein may induce human
lung cancer cell apoptosis.
In vitro effect of genistein on
caspase-3/9 activation in A549 cells
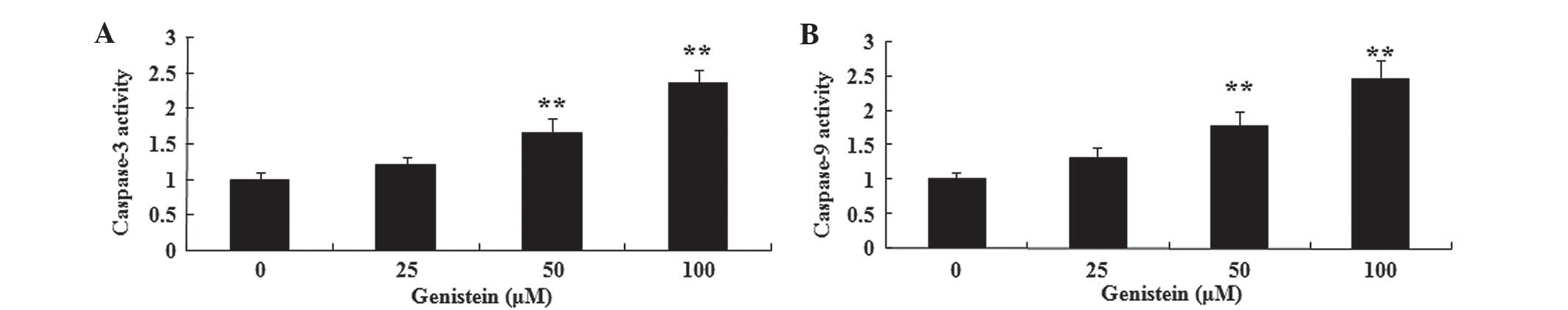
The present study aimed to investigate the possible
anti-cancer effect of genistein on caspase-3/9 activation in A549
cells using commercially available kits. As indicated in Fig. 4A and B, pretreatment with genistein
(50 and 100 µM) for 2 days significantly promoted caspase-3 and −9
activation in A549 cells, respectively (P<0.01). These data
suggest that genistein may promote human lung cancer cell apoptosis
by activation of the caspase-3/9 signaling pathways.
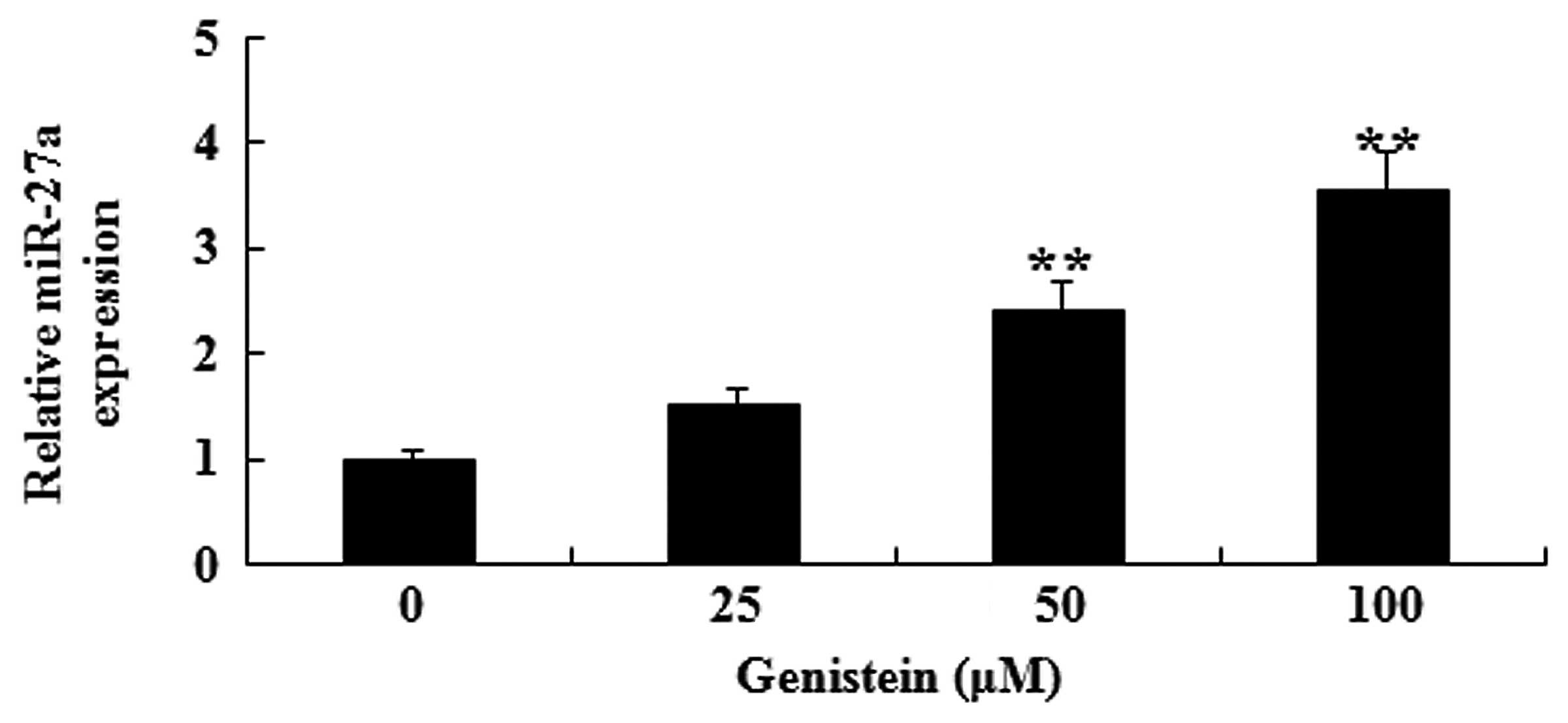
In vitro effect of genistein on
miR-27a expression in A549 cells
To explain the possible anti-cancer effect of
genistein on miR-27a expression in human lung cancer, miR-27a
expression of A549 cell was analyzed by RT-qPCR. As presented in
Fig. 5, after 2 days of treatment
with genistein (50 and 100 µM), miR-27a expression in A549 cells
was significantly increased (P<0.01). These data indicate that
genistein may activate miR-27a expression levels in human lung
cancer.
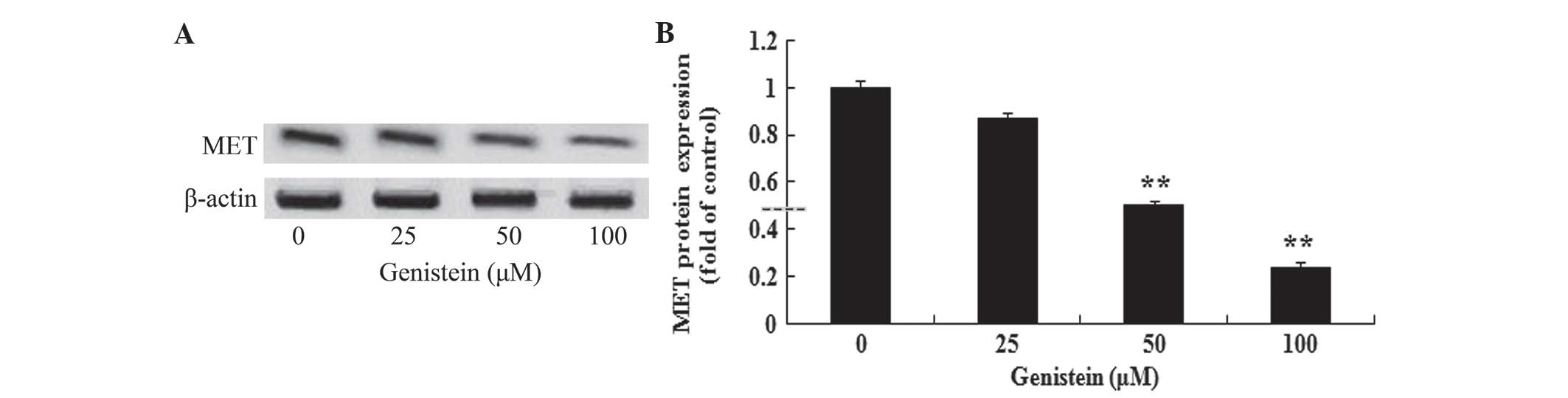
In vitro effect of genistein on MET
protein expression in A549 cells
To investigate the possible anti-cancer effect of
genistein on MET protein expression in human lung cancer, the
present study determined MET protein expression levels in A549
cells by performing western blotting. As shown in Fig. 6A and B, the effect of incubation with
genistein (50 and 100 µM) for 2 days significantly reduced MET
protein expression in A549 cells (P<0.01). These data suggest
that genistein may decrease MET protein expression levels in human
lung cancer.
Discussion
NSCLC has the highest morbidity and mortality rates
of all malignant tumors in the majority of the world's countries
(16). Approximately two-thirds of
patients are diagnosed with locally advanced disease or distant
metastasis at the time of diagnosis, thus, the opportunity for
surgery is not possible. Instead, the primary treatment of advanced
NSCLC is systemic chemotherapy treatment, however, the associated
analysis indicates that the curative effect of chemotherapy on
advanced NSCLC has entered a plateau period. The use of radiation
therapy and biological treatment are also poor at improving its
curative effect (17). Therefore, the
identification of effective treatments is currently the top
priority in lung cancer research. To the best of our knowledge, the
present study is the first to demonstrate that genistein inhibits
viability, induces apoptosis and promotes caspase-3/9 activation of
A549 cells in a dose-dependent manner. Similarly, Xiao et al
(18) demonstrated that genistein
inhibits human colorectal cancer metastasis. Suzuki et al
(19) also reported that genistein
induces the apoptosis of human pancreatic cancer cells. These data
suggest that genistein may be a potential targeting drug for human
lung cancer.
The occurrence and development of lung cancer is a
complex, multiple links process, including the oncogene activation
and mutations or deficiency of tumor suppressor genes, in the
process of the occurrence and metastasis of lung carcinoma, genetic
variation is the extensive research focus for many years (20). miRNA widely exists in human
chromosomes, and it have very strong organization specificity.
Based regulation on proliferation, differentiation, and apoptosis
process of cells, miRNA can regulate tumor biological behavior,
affect the occurrence and development of lung carcinoma. Therefore,
targeted therapy to the miRNA has the vital significance in the
gene therapy of lung carcinoma. Acunzo et al (21) reported that miR-27a can regulate MET
and EGFR in NSCLC. In the current study, genistein activated
miR-27a expression of NSCLC. Xia et al (22) demonstrated that genistein inhibits
cell growth of pancreatic cancer cells through regulation of
miR-27a and human uveal melanoma cells (23).
MET protein is the receptor of hepatocyte growth
factor/scatter factor (HGF/SF). MET as the proto-oncogene, the
product has tyrosine kinase activity after it is activated, HGF/SF
specificity combine with it, activate a series of trans-membrane
signal transduction pathways, thus stimulate epithelial cell
morphogenesis, proliferation and migration (24). Research has demonstrated that the MET
gene has excessive expression in various types of malignant tumor,
such as lung and breast carcinoma. Under normal circumstances, MET
signaling pathways involve in embryogenesis, while abnormal MET
signaling pathways are closely associated with tumor occurrence and
development; in particular, MET has an important role in promoting
tumor cell invasion and metastasis (25). Increased MET expression appears to be
a prognostic factor in patients with NSCLC following curative
surgery (26). In the present study,
genistein was observed to decrease MET protein expression levels in
human lung cancer. Similarly, Singletary and Ellington (27) reported that genistein suppresses the
proliferation of human breast epithelial cells through
downregulation of the proto-oncogene MET.
In conclusion, the present study demonstrates for
the first time that genistein inhibits A549 human lung cancer cell
proliferation via miR-27a and MET signaling. Additional studies are
required to clarify the mechanism underlying the interaction
between the miRNAs and the potential anti-cancer effect of
genistein; in particular, it is important to determine the
molecular mechanisms of genistein in cancer.
Acknowledgements
The present study was supported by Hebei University
Special Funds for Medical Science Construction Project (grant no.,
2015B1001).
References
|
1
|
Chen Q, Cheng P, Yin T, He H, Yang L, Wei
Y and Chen X: Therapeutic potential of bone marrow-derived
mesenchymal stem cells producing pigment epithelium-derived factor
in lung carcinoma. Int J Mol Med. 30:527–534. 2012.PubMed/NCBI
|
|
2
|
Chen X, Ni J, Meng H, Li D, Wei Y, Luo Y
and Wu Y: Interleukin15: A potent adjuvant enhancing the efficacy
of an autologous whole-cell tumor vaccine against Lewis lung
carcinoma. Mol Med Rep. 10:1828–1834. 2014.PubMed/NCBI
|
|
3
|
Tatematsu T, Sasaki H, Shimizu S, Okuda K,
Shitara M, Hikosaka Y, Moriyama S, Yano M, Brown J and Fujii Y:
Investigation of neurotrophic tyrosine kinase receptor 1 fusions
and neurotrophic tyrosine kinase receptor family expression in
non-small-cell lung cancer and sensitivity to AZD7451 in vitro. Mol
Clin Oncol. 2:725–730. 2014.PubMed/NCBI
|
|
4
|
Lo HM, Shieh JM, Chen CL, Tsou CJ and Wu
WB: Vascular endothelial growth factor induces CXCL1 chemokine
release via JNK and PI-3K-dependent pathways in human lung
carcinoma epithelial cells. Int J Mol Sci. 14:10090–10106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macchiaroli N, Cucher M, Zarowiecki M,
Maldonado L, Kamenetzky L and Rosenzvit M: microRNA profiling in
the zoonotic parasite Echinococcus canadensis using a
high-throughput approach. Parasit Vectors. 8:832015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Zhong J, Ji Y, Li J, Jian Y, Zhang
J and Yang W: The expression and clinical significance of microRNAs
in colorectal cancer detecting. Tumour Biol. 36:2675–2684. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lerebours F, Cizeron-Clairac G, Susini A,
Vacher S, Mouret-Fourme E, Belichard C, Brain E, Alberini JL,
Spyratos F, Lidereau R and Bieche I: miRNA expression profiling of
inflammatory breast cancer identifies a 5-miRNA signature
predictive of breast tumor aggressiveness. Int J Cancer.
133:1614–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang L, An SJ, Chen ZH, Su J, Yan HH and
Wu YL: MET expression plays differing roles in non-small-cell lung
cancer patients with or without EGFR mutation. J Thorac Oncol.
9:725–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuda M, Davis IJ, Argani P, Shukla N,
McGill GG, Nagai M, Saito T, Laé M, Fisher DE and Ladanyi M: TFE3
fusions activate MET signaling by transcriptional up-regulation,
defining another class of tumors as candidates for therapeutic MET
inhibition. Cancer Res. 67:919–929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han S, Wu H, Li W and Gao P: Protective
effects of genistein in homocysteine-induced endothelial cell
inflammatory injury. Mol Cell Biochem. 403:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pons DG, Nadal-Serrano M,
Blanquer-Rossello MM, Sastre-Serra J, Oliver J and Roca P:
Genistein modulates proliferation and mitochondrial functionality
in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell
Biochem. 115:949–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pavese JM, Krishna SN and Bergan RC:
Genistein inhibits human prostate cancer cell detachment, invasion
and metastasis. Am J Clin Nutr. 100(Suppl 1): S431–S436. 2014.
View Article : Google Scholar
|
|
14
|
Spoerlein C, Mahal K, Schmidt H and
Schobert R: Effects of chrysin, apigenin, genistein and their
homoleptic copper (II) complexes on the growth and metastatic
potential of cancer cells. J Inorg Biochem. 127:107–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishii H, Azuma K, Yamada K, Kinoshita T,
Imamura Y and Hoshino T: Predictive factors in patients with EGFR
mutation-negative non-small cell lung cancer treated with
erlotinib. Oncol Lett. 8:2699–2704. 2014.PubMed/NCBI
|
|
17
|
Jiang L, Liang X, Liu M, Wang W, Ma J, Guo
Q, Han L, Yang C and Nan K: Reduced expression of liver kinase B1
and Beclin1 is associated with the poor survival of patients with
non-small cell lung cancer. Oncol Rep. 32:1931–1938.
2014.PubMed/NCBI
|
|
18
|
Xiao X, Liu Z, Wang R, Wang J, Zhang S,
Cai X, Wu K, Bergan RC, Xu L and Fan D: Genistein suppresses FLT4
and inhibits human colorectal cancer metastasis. Oncotarget.
6:3225–3239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki R, Kang Y, Li X, Roife D, Zhang R
and Fleming JB: Genistein potentiates the antitumor effect of
5-Fluorouracil by inducing apoptosis and autophagy in human
pancreatic cancer cells. Anticancer Res. 34:4685–4692.
2014.PubMed/NCBI
|
|
20
|
Capodanno A, Boldrini L, Proietti A, Alì
G, Pelliccioni S, Niccoli C, D'Incecco A, Cappuzzo F, Chella A,
Lucchi M, et al: Let-7 g and miR-21 expression in non-small cell
lung cancer: Correlation with clinicopathological and molecular
features. Int J Oncol. 43:765–774. 2013.PubMed/NCBI
|
|
21
|
Acunzo M, Romano G, Palmieri D, Laganá A,
Garofalo M, Balatti V, Drusco A, Chiariello M, Nana-Sinkam P and
Croce CM: Cross-talk between MET and EGFR in non-small cell lung
cancer involves miR-27a and Sprouty2. Proc Natl Acad Sci USA.
110:8573–8578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F
and Wang Z and Wang Z: Genistein inhibits cell growth and invasion
through regulation of miR-27a in pancreatic cancer cells. Curr
Pharm Des. 20:5348–5353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Q, Cong R, Yan H, Gu H, Zeng Y, Liu N,
Chen J and Wang B: Genistein inhibits growth of human uveal
melanoma cells and affects microRNA-27a and target gene expression.
Oncol Rep. 22:563–567. 2009.PubMed/NCBI
|
|
24
|
Freudlsperger C, Alexander D, Reinert S
and Hoffmann J: Prognostic value of c-Met expression in oral
squamous cell carcinoma. Exp Ther Med. 1:69–72. 2010.PubMed/NCBI
|
|
25
|
Hanna JA, Bordeaux J, Rimm DL and Agarwal
S: The function, proteolytic processing and histopathology of Met
in cancer. Adv Cancer Res. 103:1–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kowalczuk O, Kozlowski M, Niklinska W,
Kisluk J, Niklinska BJ and Niklinski J: Increased MET gene copy
number but not mRNA level predicts postoperative recurrence in
patients with non-small cell lung cancer. Transl Oncol. 7:605–612.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singletary K and Ellington A: Genistein
suppresses proliferation and MET oncogene expression and induces
EGR-1 tumor suppressor expression in immortalized human breast
epithelial cells. Anticancer Res. 26:1039–1048. 2006.PubMed/NCBI
|