Introduction
Anaplastic ependymoma is a high-grade malignant
neoplasm of the central nervous system (CNS), World Health
Organization (WHO) grade III (1),
which is also known as malignant ependymoma. This tumor was listed
as a new type between 1993 and 2007, and was classified as WHO
grade III in 2007 (2). Ependymoma
arises from differentiated ependymal cells lining the ventricles
(1,3),
and the proportion of ependymomas among intracranial glial tumors
is 3–9% (4–7). The majority of these tumors occur in the
ventricle, and while intraparenchymal tumors are rare,
intraparenchymal anaplastic ependymomas are even less commonly
observed (8).
Anaplastic ependymomas are malignant in terms of
their biological behavior, and patients with anaplastic ependymomas
are at high risk of metastasis and relapse, which are responsible
for the poor prognosis (2,5–7,9). A combination of surgery, radiation
therapy and chemotherapy can improve the patient outcome (10). There is a considerable difference in
the treatment and prognosis among the different subtypes of
ependymoma (5), particularly for
anaplastic ependymoma, which requires comprehensive treatment;
therefore, a correct diagnosis is extremely important.
These tumors have various imaging features, some of
which are specific. Few studies have specifically described the
magnetic resonance imaging (MRI) characteristics of these tumors
(7,11), and the majority of these studies have
been case reports. The purpose of the present study was to
retrospectively analyze the characteristics of the tumors in MR
images and pathological findings to improve the accuracy rate of
diagnosis. To the best of our knowledge, the study is the largest
collection thus far of supratentorial extraventricular anaplastic
ependymomas with MRI.
Case report
Patients
The clinical and pathological images of 11 patients
who presented with histologically proven anaplastic ependymoma at
Nanfang Hospital (Southern Medical University, Guangzhou,
Guangdong, China) between September 2004 and March 2015 were
retrospectively reviewed. The histological features of an
anaplastic ependymoma include perivascular pseudorosettes and true
rosettes. MRI scans were obtained in all 11 cases. Computed
tomography scans were obtained in only 3 cases. Tumor recurrence
and patient mortality were recorded, among other data.
Clinical characteristics
The majority of intraparenchymal anaplastic
ependymomas (6/11) appeared in adult patients between the second
and fifth decades of life, while 3 tumors (3/11) occurred during
the first decade. The total male-to-female ratio was 4.5:1
(9:2).
The average symptom duration was 4 months, with a
range of 1–7 months. The majority of the patients complained of
non-specific symptoms, including headaches (n=9), dizziness (n=4)
and vomiting (n=6). Other presenting symptoms included limb
numbness and weakness with impaired movement (n=4), memory
deterioration (n=1), language disorders (n=2), unconsciousness
(n=1) and an unsteady gait (n=2). All 11 patients underwent
surgical removal of the tumor (total resection), and post-operative
radiation therapy (n=6) or chemotherapy (n=4) was performed in
certain cases. It was found that the tumors were distributed to the
brain parenchyma and the boundaries were not clear in surgery.
Following the resection, the post-operative follow-up period ranged
from 1–48 months (median, 1.5 months; mean, 8 months). Two patients
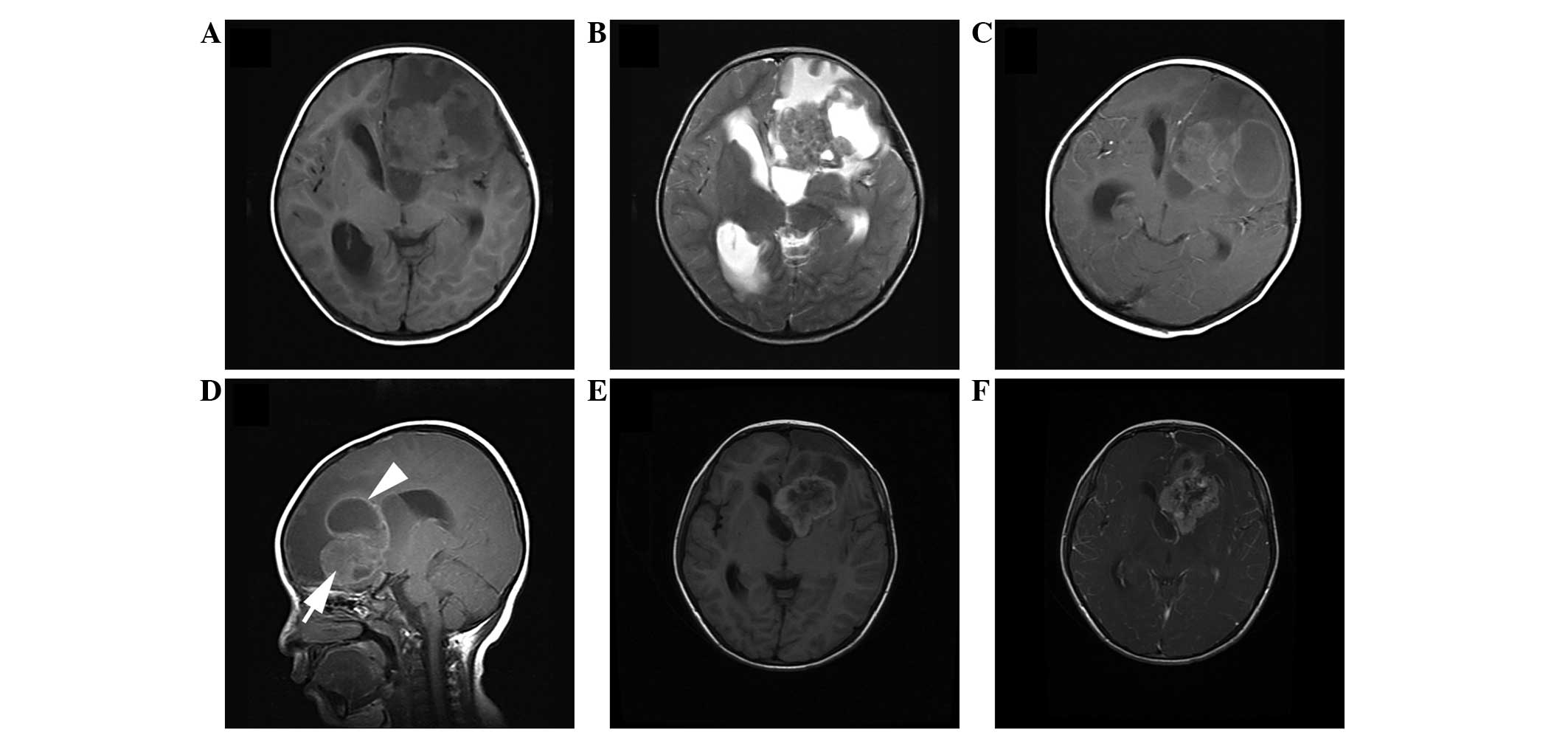
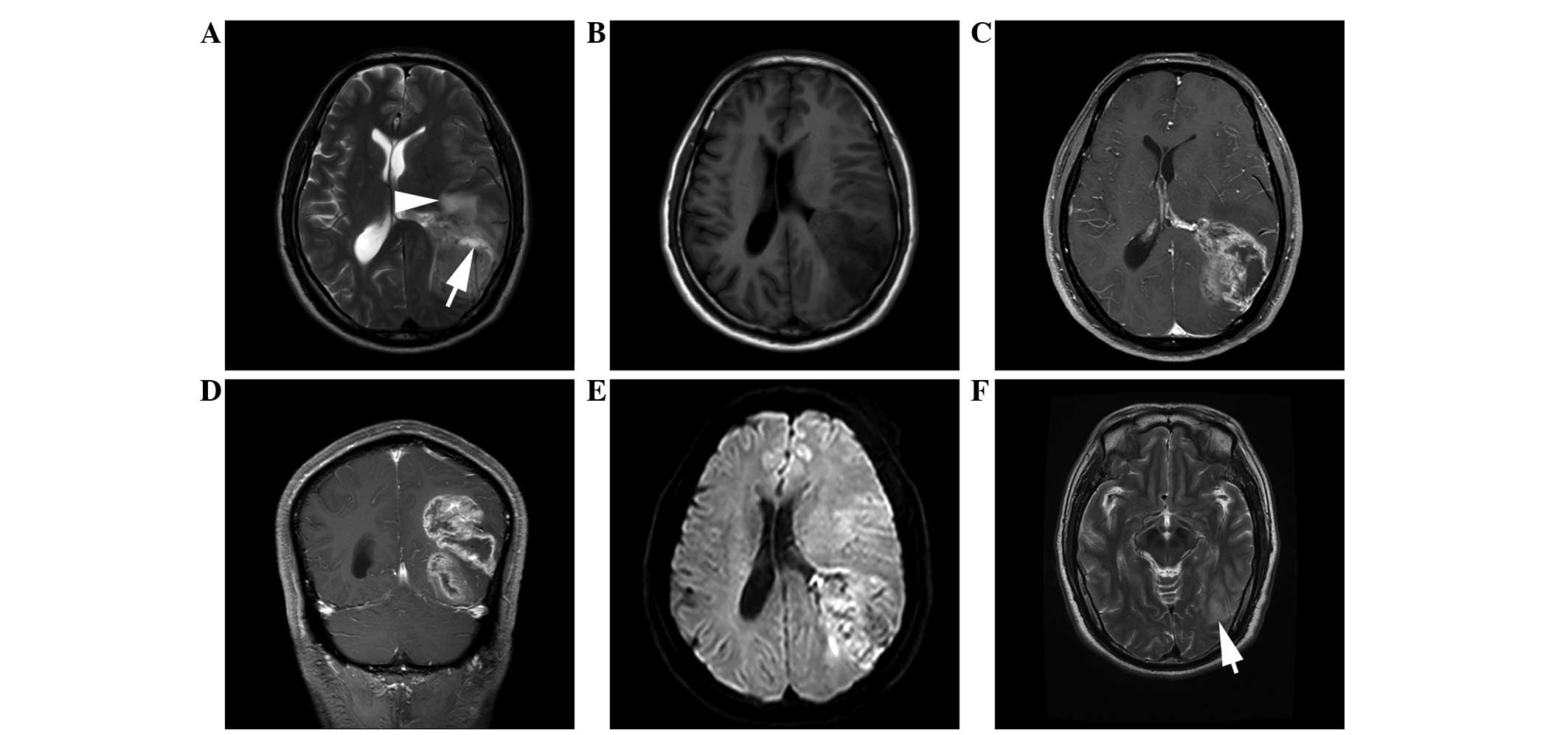
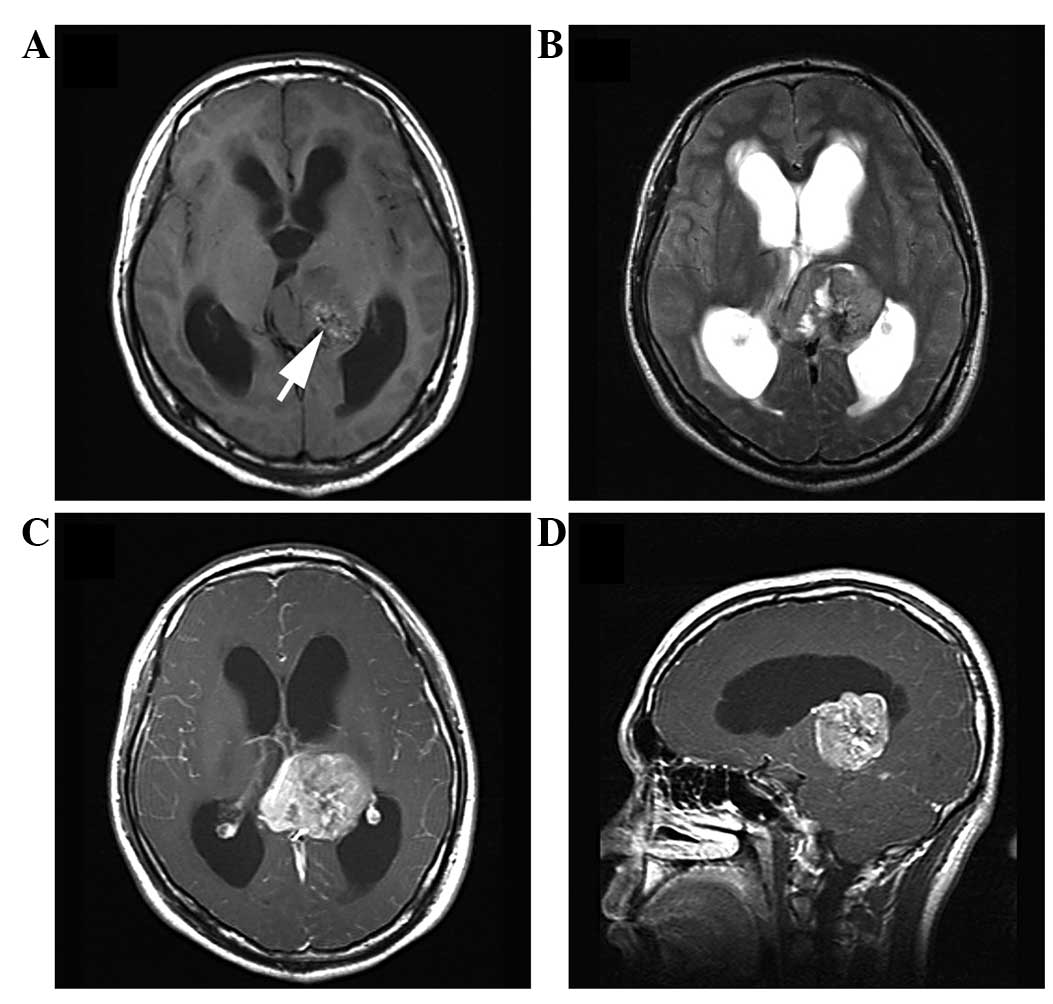
relapsed after follow-up times of 5 months and 4 years (Fig. 1), respectively, and 1 patient
succumbed after a follow-up time of only 1 month. Notably, 1
patient was initially diagnosed with focal cortical dysplasia
(FCD), and the lesion grew rapidly over 1 month, resulting in the
loss of consciousness (Fig. 2).
Imaging findings
Overall, 8 out of 11 lesions were supratentorial
(Figs. 1–4), while 3 tumors were infratentorial
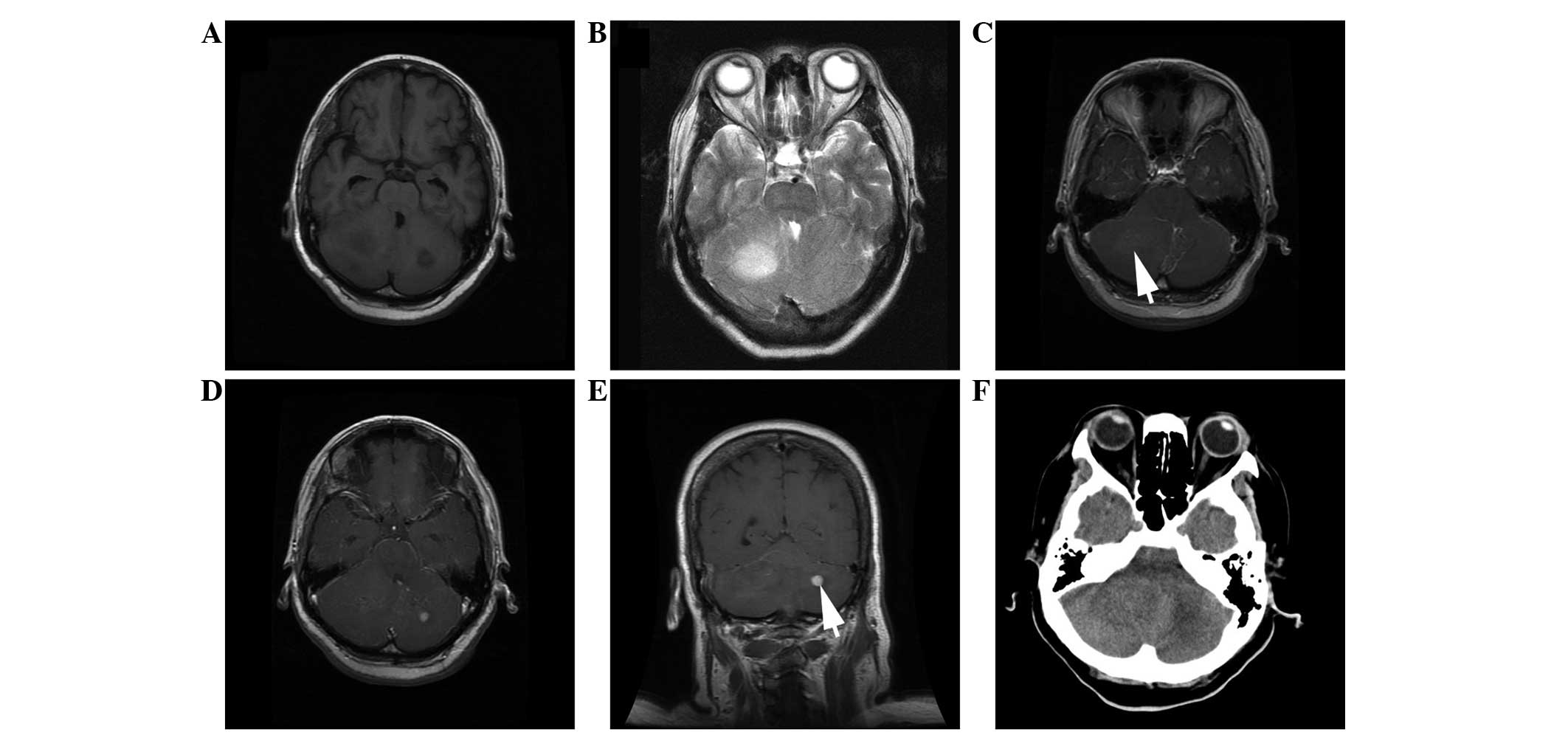
(Fig. 5). Of the former group, 2
tumors were located in the temporoparietal occipital lobe close to
the posterior horn of the lateral ventricle (Fig. 2), 2 were located in the frontal lobe
close to the anterior horn of the lateral ventricle (Fig. 1), 1 was located in the temporal lobe
close to the inferior horn of the lateral ventricle and 3 were
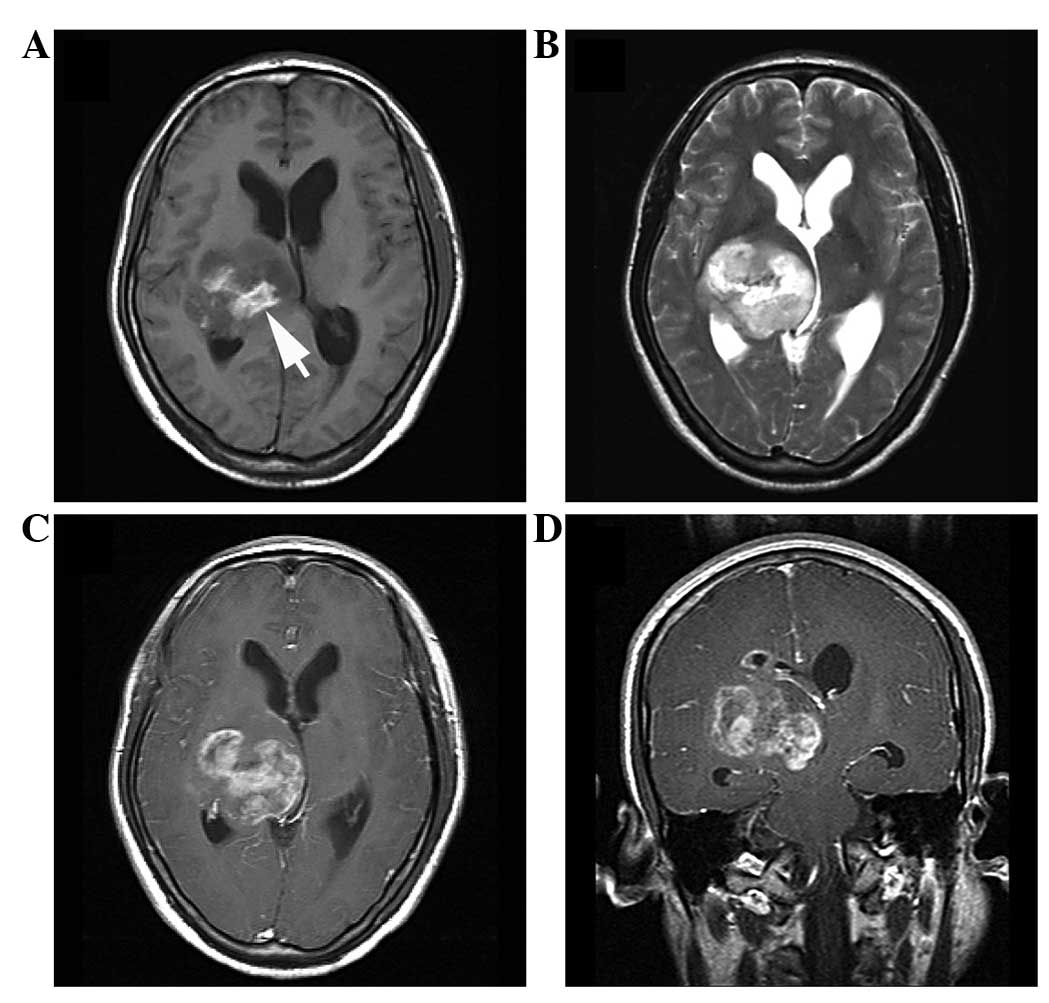
located in the thalamus (Figs. 3 and
4). The infratentorial tumors were
located in the bilateral cerebellar hemispheres or the right
cerebellar hemisphere (Fig. 5).
The tumors ranged in size from 3.0–7.3 cm in the
longest diameter, with a mean longest diameter of 5.1 cm. The
lesions were irregularly lobulated (n=7) (Figs. 1 and 2)
or well-demarcated and quasi-circular (n=4) (Figs. 3–5). In
total, 4 of the tumors presented as mainly solid masses (Figs. 3–5), 5
tumors presented as solid masses with multiple small cysts
(Fig. 2) and 2 lesions were
solid-cystic (Fig. 1).
On T1-weighted imaging (T1WI), all 11 of the tumors
were iso-hypointense relative to the gray matter, and 7 tumors
exhibited additional small (n=3) (Fig.
3A) or medium-sized (n=4) (Fig.
4A) foci of hyperintensity. By contrast, all these lesions
presented as heterogeneous (with areas of hypointense and
hyperintense signal intensity) or slightly hypointense relative to
the gray matter on T2WI. Contrast-enhanced MRI presented areas of
marked (n=9) or mild (n=2) enhancement; among the former were
wreath-like or ring-like appearances with intratumoral nodule
enhancement (n=3) (Figs. 1 and
2) or flake-like heterogeneous
enhancement (n=6) (Figs. 3 and
4). Certain patchy areas of
hypointensity on T2WI showed no contrast enhancement (Figs. 1, 3 and
4). Cystic or necrotic areas were
frequently observed in 8 of the lesions, and multiple cysts of
different sizes coexisted, with regions of them merged together;
indeed, in 2 lesions, the cystic region occupied more than half of
the tumor (Fig. 1). Small point-like
enhancements were found in the frontal and occipital lobes of
infratentorial lesions. The tumors presented with moderate (n=4)
(Figs. 1 and 2), mild (n=3) (Fig. 6) or absent (n=4) (Figs. 3 and 4)
peritumoral edema.
On computed tomography (CT) scans, the tumors of 3
of the patients presented as low density or equidensity lesions. In
the diffusion-weighted sequences, moderate restriction was observed
in 3 patients. MR spectroscopy for 1 patient demonstrated reduced
N-acetylaspartate and elevated choline levels. In the
susceptibility-weighted angiography (SWAN) sequence of 1 patient,
the tumor presented with multiple patchy hypointense areas in the
center.
Pathology findings
All of the tumors were surgically resected and
displayed a gray-red or gray-white fish flesh-like appearance,
mixed with cysts, necrosis, hemorrhage and vascular proliferation.
Surgery confirmed that the tumors were derived from the brain
parenchyma. Microscopic examination revealed that the resected
tumor cells in all patients were plentiful and distributed
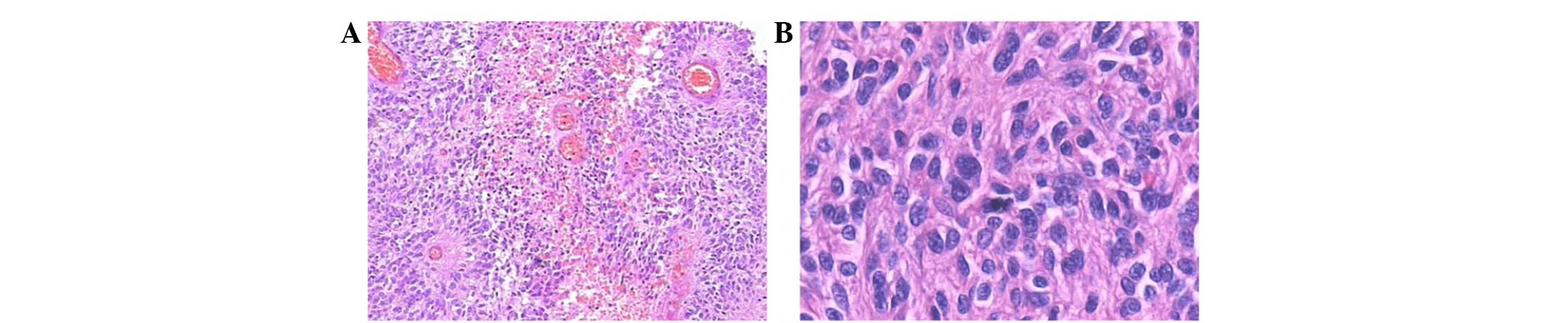
diffusely. Perivascular pseudorosettes were frequently observed
(Fig. 6A). The space around the
vessels without cells was wide. The tumor cells were small, round
or fusiform, lacking cytoplasm, and the cell nuclei appeared large
and polymorphic (Fig. 6B). Nuclear
fission was active. In all 11 cases, immunohistochemical staining
demonstrated that the tumors were positive for glial fibrillary
acidic protein (GFAP) and p53 protein, negative or positive for
vimentin and S-100 protein, and negative or weakly positive for
epithelial membrane antigen (EMA).
Discussion
Ependymoma is a neoplasm consisting of cellular
elements derived from the differentiated ependymal cells lining the
ventricles of the brain or the central canal of the spinal cord
(3). Intracranial ependymomas mostly
occur in the fourth ventricle, while other ependymomas occur in the
lateral ventricle, brain parenchyma, spinal cord or cauda equina.
The lesions vary in biological behavior (2,12). Of all
intracranial ependymomas, ~60% are infratentorial and 40% are
supratentorial (13). According to a
previous study, supratentorial ependymomas have a higher occurrence
rate among high-grade tumors compared with infratentorial tumors,
and they principally occur in the brain parenchyma, while
infratentorial extraventricular lesions are more often located in
the cerebellar hemispheres (14). In
the present series, 8 supratentorial lesions were derived from the
parenchyma, while 1 infratentorial case was rooted in the bilateral
cerebellar hemispheres and 2 infratentorial tumors were derived
from the right cerebellar hemisphere. Ependymomas are rare
neoplasms of the CNS, accounting for 3–9% of intracranial glial
neoplasms (4–7), while anaplastic ependymomas are even
less commonly observed (8),
accounting for ~25% of ependymomas (15). This lesion is the most malignant of
all ependymomas (1,5,7) and can
also develop from low-grade ependymoma.
Histologically, ependymomas are moderately cellular
tumors characterized by perivascular pseudorosettes (2,5). The
cellular atypia and karyokinesis, cell proliferation and necrosis
of anaplastic tumors are more evident than those of low-grade
ependymomas. A positive Ki-67 labeling index is more common in
anaplastic ependymoma than in low-grade ependymoma (15). Shuangshoti et al (3) reported that an increased Ki-67 labeling
index was strongly correlated with increased mitotic activity and
histological malignancy. Ritter et al (16) found an increased Ki-67 labeling index
was linked to a poor prognosis. Immunohistochemical staining has
demonstrated that the tumors are positive for GFAP, vimentin and
S-100 protein, and negative for EMA (5,15).
Shuangshoti et al (3)
demonstrated positive rates for GFAP (87%), S-100 protein (77%) and
EMA (17%). The present study findings confirmed these observations.
All of the anaplastic ependymomas in the present series expressed
p53 protein, which is consistent with the results of the study by
Shuangshoti et al (3). This
study found 91% of anaplastic ependymomas expressed p53 protein.
Zamecnik et al (17) found
that p53 immunopositivity was one of the strongest indicators of
aggressive tumor behavior and a poor prognosis.
Clinically, ependymomas usually occur in individuals
within the range of 3–6 years old, with approximately one-third
diagnosed prior to 3 years old (18).
The second peak age of onset is in the third decade of life
(19). Supratentorial ependymomas
frequently occur in adults (12,20). In
the present series, the majority of the patients presented with
tumors during the second to fifth decades, likely indicating that
anaplastic ependymoma has an older age of onset than low-grade
ependymoma. There is no apparent gender predilection (3), but the present series had a male
predominance, with a male-to-female ratio of 4.5:1 (9:2), perhaps
due to the small sample size.
In the present study, the clinical manifestations
were non-specific and dependent on the lesion location (5). It has reported that the symptoms of
anaplastic ependymomas can develop earlier than those of low-grade
ependymomas (2). Intracranial
hypertension is the most common specific symptom, particularly in
children, and the present findings were in accordance with this
observation (5,11). The tumors also frequently present with
other neurological deficits, such as dyskinesia and seizures
(11).
Various sites of extraventricular ependymoma have
been reported, with these tumors appearing mainly at the angled
margins of the ventricles (3). It is
believed that the extraventricular location of a tumor depends on
whether it originates from extraventricular ependymal cells
(21). Shuangshoti et al
(3) and Molina et al (22) reported that supratentorial
extraventricular ependymomas frequently occurred in the left
hemisphere, particularly the frontal region. Of the 8
supratentorial tumors in the present series, 4 tumors were located
in the left hemisphere, while 4 lesions were derived from the right
hemisphere; these results did not indicate a left predominance,
perhaps due to the small sample size. It has been reported that
this tumor type has higher occurrence rates in the frontal and
occipital lobes (12). In the present
study, the tumors were located in the frontal lobe (2/11), the
temporoparietal occipital lobe (2/11), the temporal lobe (1/11),
the cerebellar hemispheres (3/11) and the thalamus (3/11), similar
to the distributions of tumor location observed in a previous study
(12). In the present study, 6
supratentorial tumors had a close association with the lateral
ventricle, indicating that the tumor cells may have originated from
the ependymal cells lining the ventricles of the brain or perhaps
indicating the direct evolution of ectopic original ependymal cells
around the lateral ventricle.
Anaplastic ependymomas are malignant and easily
metastasize or relapse, and the prognosis is poor (2,5–7); these ependymomas, particularly
infratentorial tumors, often metastasize into the CNS, displaying
multifocal nodules in the subependymal region. In the present
series, 3 patients relapsed after resection; 1 patient suffered a
relapse 5 months after tumor resection, displaying multiple nodular
enhancements in the left occipital lobe and centrum semiovale, in
line with the previous literature (2,5–7). Occasionally, the tissue around the
tumors displays nodules or patchy enhancement, perhaps revealing
CSF dissemination; in the infratentorial case in the present study,
point-like enhancements were found in the frontal and occipital
lobes, likely indicating that the tumor invaded the surrounding
parenchyma. Therefore, when abnormal enhancement is present in the
surrounding parenchyma, it may be indicative of anaplastic
ependymoma. It is worth noting that 1 tumor in the present study
demonstrated rapid deterioration; it was diagnosed as FCD with a
patchy high signal on T2WI imaging in the initial diagnosis. The
tumor had developed markedly 5 months later. The short duration and
quick deterioration of anaplastic ependymoma can aid in
distinguishing anaplastic ependymomas from low-grade ependymomas,
perhaps suggesting that anaplastic ependymoma, with its rapid
growth, poor clinical course and biological behavior, should be
defined as malignant.
Imaging features facilitate the pre-treatment
diagnosis when an anaplastic ependymoma is clinically suspected.
However, only a few studies have specifically described the MRI
characteristics of supratentorial extraventricular anaplastic
ependymomas (7,11), and the majority of these studies were
case reports and non-specific. According to the previous
literature, ependymomas appear as well-circumscribed lesions with
varying degrees of contrast enhancement (5), while supratentorial ependymomas are
commonly cystic (11,23). Supratentorial ependymomas also cause
calcification and intra-tumoral hemorrhages, with certain studies
reporting that supratentorial ependymomas can occasionally cause
peripheral calcification with a cystic center (23). Peritumoral edema and brain
infiltration have been observed occasionally (5). The present study confirms these
findings.
MRI provides important information on these lesions,
including their location, shape, boundaries and internal
architecture. Anaplastic ependymomas often grow rapidly with a
great volume; the diameters of 90% of reported ependymomas have
been >4 cm (13,23). The majority of the tumors in the
present study were large (mean longest diameter, 5.1 cm), in
keeping with the previous literature. In the present series, the
majority of the tumors were irregularly-lobulated, which was likely
due to the fact that the tumors grew actively or the cell
proliferation was not uniform. The tumors were hypointense to
isointense relative to the gray matter on non-enhanced T1WI, and
they were hyperintense or slightly hypointense on T2WI, similar to
the appearances of other intracranial tumors (24). The architecture of the tumors in the
present study was commonly heterogeneous, correlating with the MRI
results and the microscopic findings; heterogeneous intensity can
indicate various components of the lesion, such as cysts, necrosis,
intratumoral hemorrhage, calcification, fibrosis or vascular
proliferation (25). Cysts and
necrosis are characteristic appearances of anaplastic ependymomas
(21), particularly supratentorial
tumors. Furie and Provenzale reported that these tumors were
typically large cystic masses (11).
In the present series, 8 of the lesions exhibited cystic
components; multiple cysts coexisted of different sizes, with
regions of them merged together, and the cystic regions occupied
more than half of the tumors in 2 patients. Of the 2 solid-cystic
lesions, the ratios of the diameter of the cystic regions to the
tumor were 0.54 and 0.66. Thus, supratentorial lesions frequently
presented as solid tumors with large or small cystic components in
the present series. Notably, patients between 28 and 63 years old
tended to present with solid tumors or solid tumors with small
cysts, while patients between 3 and 24 years old were inclined to
present with solid-cystic lesions with large cysts. The cystic
components demonstrated a low signal on T1WI imaging, but a high
signal on T2WI imaging, like a water signal. Necrosis may be an
important indicator of malignant tumors, as malignant tumors grow
rapidly and cause vascular invasion, which leads to ischemic
necrosis of tumor cells.
Hemorrhage occurs occasionally in these cases, and
the literature has suggested an occurrence rate of 0–13% (26). Hemorrhage can be caused by the
fragility of the vessels perfusing these tumors, and by the
invasion and erosion of the vessel walls. In the present series, 7
tumors caused hemorrhage, which presented as a high signal on T1WI
and as a low signal on T2WI. The SWAN sequence presented with
hyperintensity in the center of the tumor, which is representative
of hemorrhage. The rate of hemorrhage was higher than that reported
in the literature, most likely as anaplastic ependymomas can more
easily cause hemorrhage than benign ependymomas. Calcification,
ranging from small punctate foci to large masses, is common
(2,5).
Hypointensity on T1WI plus T2WI imaging can indicate calcification,
but also hemorrhage. CT is superior to MRI in the detection of
calcification, but only 3 patients underwent CT scans in the
present series, and they did not present with calcification; if
more patients had undergone CT scans, the proportion may have been
different. This was a shortcoming of the present study. Certain
lesions in the present series appeared hypointense at the
peripheral rim of the tumor, perhaps due to a fiber envelope.
MR spectroscopy in 1 patient showed reduced
N-acetylaspartate and elevated choline levels, similar to the
findings of a previous report (2).
The appearance of these tumors is similar to that of high-grade
glioma (5), suggesting that the
tumors are malignant. The diffusion-WI showed moderate restriction
in the present study, which is consistent with the literature
(2). The tumors mostly presented with
moderate or mild peritumoral edema. As the large mass oppresses the
surrounding parenchyma, it can result in ischemia or the
obstruction of venous return, and 7 of the tumors displayed
moderate or mild peritumoral edema in the present study.
The majority of the anaplastic ependymomas in the
present series showed contrast enhancement on gadolinium-enhanced
T1WI, with a variety of heterogeneous appearances, including
ring-like, wreath-like with a thick wall, and intratumoral nodular
enhancement. The varying enhancement patterns of anaplastic
ependymoma have been described previously (7). Heterogeneous enhancement likely occurs
due to the arrangement of the tumor cells, which occurs due to
vascular proliferation. Cystic, calcified and hemorrhagic
components also contribute to their heterogeneous nature. The
ring-like and wreath-like enhancement could indicate plentiful
blood vessels on the peripheral rim of the neoplasms.
According to previous studies and the present
results, certain imaging features can contribute to differentiating
WHO grade III ependymomas from WHO grade II ependymomas, although
there is considerable overlap in the imaging features of these two
tumors (2–4,8,11): i) Regarding location, WHO grade II
ependymomas often derive from the brain surface close to the
cerebral falx or cistern, while WHO grade III ependymomas usually
occur in the deep parenchyma and close to the ventricle. ii)
Regarding gross morphology, WHO grade II ependymomas are usually
regularly quasi-circular with clear boundaries, and the ratio of
the cystic region to the tumor is less than that of anaplastic
ependymomas, while anaplastic ependymomas frequently appear
irregularly-lobulated, with obscure boundaries and a solid-cystic
nature, or as large cysts with solid nodules. iii) Regarding the
enhancement pattern, WHO grade III ependymomas show more marked
heterogeneous enhancement than low-grade tumors. iv) Regarding
tumoral hemorrhage and peritumoral edema, tumoral hemorrhage is
rarely observed in WHO grade II ependymomas; however, it is more
frequently observed in WHO grade III ependymomas. There is always
no or mild edema around WHO grade II ependymomas, while anaplastic
ependymomas often present with mild or moderate peritumoral
edema.
The differential diagnosis for ependymoma includes
pilocytic astrocytoma, oligodendroglioma, hemangioblastoma and
glioblastoma multiform. Pilocytic astrocytoma often occurs in
childhood, appearing as a large cystic component, with small
enhancing mural nodules, without peritumoral edema (27). Oligodendroglioma infrequently presents
as cystic, and it usually displays mild enhancement.
Hemangioblastoma appears as a solid-cystic mass, accompanied by
multiple voids. The intensity of glioblastoma multiform is more
heterogeneous, and the peritumoral edema is more marked; these
tumors often grow contralaterally across the midline and involve
the bilateral frontal lobes.
Anaplastic ependymomas are rare tumors in adults.
The standard treatment consists of maximal safe resection and
radiation therapy (10,28). The role of surgery is clearly
extremely important. Radiotherapy is applied to manage
disseminating, residual or recurrent anaplastic ependymomas
(10). By contrast, chemotherapy and
genetic alterations can be used and could develop into promising
therapeutic strategies. Stem-like cells in ependymomas are
vulnerable to certain components of the Notch pathway. It has been
suggested that inhibitors of these enzyme may become a treatment in
the future (10).
In summary, extraventricular anaplastic ependymomas
are rare, malignant and rapidly growing tumors that usually occur
in the supratentorial deep brain white matter. A correct diagnosis
is important for guiding clinical therapy and estimating the
prognosis. The present series of 11 patients revealed that these
tumors have specific MRI features, such as an irregularly-lobulated
and solid or solid-cystic mass, with different-sized cysts and
necrosis; they frequently cause hemorrhage and occasionally
calcification, with moderate or mild peritumoral edema and markedly
heterogeneous enhancement on post-contrast MRI. If a middle-aged or
elderly patient presents with a short duration of clinical symptoms
associated with a lesion that has a close association with the
lateral ventricle, and if the imaging features are similar to those
aforementioned, the presence of an anaplastic ependymoma should be
considered.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martínez León MI, Denis Vidal M and Lara
Weil B: Magnetic resonance imaging of infratentorial anaplastic
ependymoma in children. Radiologia. 54:59–64. 2012.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shuangshoti S, Rushing EJ, Mena H, Olsen C
and Sandberg GD: Supratentorial extraventricular ependymal
neoplasms: A clinicopathologic study of 32 patients. Cancer.
103:2598–2605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Metellus P, Figarella-Branger D, Guyotat
J, Barrie M, Giorgi R, Jouvet A and Chinot O: Club de
Neuro-Oncologie de la Société Française de Neurochirurgie and the
Association des Neuro-Oncologues d'Expression Française:
Supratentorial ependymomas: Prognostic factors and outcome analysis
in a retrospective series of 46 adult patients. Cancer.
113:175–185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reni M, Gatta G, Mazza E and Vecht C:
Ependymoma. Crit Rev Oncol Hematol. 63:81–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D
and Epstein FJ: Disseminated ependymomas of the central nervous
system. J Neurosurg. 85:618–624. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuh EL, Barkovich AJ and Gupta N: Imaging
of ependymomas: MRI and CT. Childs Nerv Syst. 25:1203–1213. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niazi TN, Jensen EM and Jensen RL: WHO
Grade II and III supratentorial hemispheric ependymomas in adults:
Case series and review of treatment options. J Neurooncol.
91:323–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar R, Singhal N, Jaiswal SK and
Mahapatra AK: Recurrence in supratentorial anaplastic ependymoma.
Pediatr Neurosurg. 43:364–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shim KW, Kim DS and Choi JU: The history
of ependymoma management. Childs Nerv Syst. 25:1167–1183. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furie DM and Provenzale JM: Supratentorial
ependymomas and subependymomas: CT and MR appearance. J Comput
Assist Tomogr. 19:518–526. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGuire CS, Sainani KL and Fisher PG:
Incidence patterns for ependymoma: A surveillance, epidemiology,
and end results study. J Neurosurg. 110:725–729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mermuys K, Jeuris W, Vanhoenacker PK, Van
Hoe L and D'Haenens P: Best cases from the AFIP: Supratentorial
ependymoma. Radiographics. 25:486–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Armington WG, Osborn AG, Cubberley DA,
Harnsberger HR, Boyer R, Naidich TP and Sherry RG: Supratentorial
ependymoma: CT appearance. Radiology. 157:367–372. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koeller KK and Sandberg GD: Armed Forces
Institute of Pathology: From the archives of the AFIP. Cerebral
intraventricular neoplasms: Radiologic-pathologic correlation.
Radiographics. 22:1473–1505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ritter AM, Hess KR, McLendon RE and
Langford LA: Ependymomas: MIB-1 proliferation index and survival. J
Neurooncol. 40:51–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamecnik J, Snuderl M, Eckschlager T,
Chanova M, Hladikova M, Tichy M and Kodet R: Pediatric intracranial
ependymomas: Prognostic relevance of histological,
immunohistochemical, and flow cytometric factors. Mod Pathol.
16:980–991. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perilongo G, Massimino M, Sotti G,
Belfontali T, Masiero L, Rigobello L, Garrè L, Carli M, Lombardi F,
Solero C, et al: Analyses of prognostic factors in a retrospective
review of 92 children with ependymoma: Italian pediatric
neuro-oncology group. Med Pediatr Oncol. 29:79–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fokes EC Jr and Earle KM: Ependymomas:
Clinical and pathological aspects. J Neurosurg. 30:585–594. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guyotat J and Metellus P: Intracranial
ependymomas in adult patients. Prognostic factors, place of surgery
and complementary treatment. Neurochirurgie. 53:85–94. 2007.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swartz JD, Zimmerman RA and Bilaniuk LT:
Computed tomography of intracranial ependymomas. Radiology.
143:97–101. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molina OM, Colina JL, Luzardo GD, Mendez
OE, Cardozo D, Velasquez HS and Cardozo JJ: Extraventricular
cerebral anaplastic ependymomas. Surg Neurol. 51:630–635. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Tassel P, Lee YY and Bruner JM:
Supratentorial ependymomas: Computed tomographic and pathologic
correlations. J Comput Tomogr. 10:157–165. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yurt A, Selçuki M, Ertürk AR and
Küpelioglu A: Large supratentorial cortical ependymoma in a child.
Clin Med Res. 8:25–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maksoud YA, Hahn YS and Engelhard HH:
Intracranial ependymoma. Neurosurg Focus. 13:e42002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fine MJ, Kricheff II, Freed D and Epstein
FJ: Spinal cord ependymomas: MR imaging features. Radiology.
197:655–658. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasalkar DD, Chu WC, Paunipagar BK, Cheng
FW and Li CK: Paediatric intra-axial posterior fossa tumours:
Pictorial review. Postgrad Med J. 89:39–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lombardi G, Pambuku A, Bellu L, Della
Puppa A, Rumanò L, Gardiman MP, Pomerri F and Zagonel V: Cisplatin
and temozolomide combination in the treatment of supratentorial
anaplastic ependymoma. Chemotherapy. 59:176–180. 2013. View Article : Google Scholar : PubMed/NCBI
|