Introduction
Cellular immunotherapy is a novel treatment for
tumors following chemotherapy and hematopoietic stem cell
transplantation (1). It aims to
stimulate the immune system of patients to triggered an anti-tumor
immune response, eventually enhancing the body's natural abilities
to recognize and kill cancer cells (2). Additional research found that cellular
immunotherapy based in different immune cells had various
anti-tumor efficacy (3). Among the
numerous types of immune cells, dendritic cells (DCs) and
cytokine-induced killer cells (CIKs) are extensively used in the
clinic (2,4,5); the
former are highly specialized antigen-presenting cells (APC)
(6) and the latter have a broad
spectrum in the killing of tumor cells (7).
Clinical studies have demonstrated that specifically
designed DC-targeted cancer cell vaccines have different clinical
benefits (8–10). Frank et al demonstrated that
patients who received dendritic cell vaccines generated by the
adherence method demonstrated increased T cell proliferation in
response to the vaccination (11).
Zhu et al noted that DC vaccines and CIK therapy could
induce an immune response against advanced colorectal cancer,
thereby improving quality of life and prolonging overall survival
(12). A large clinical study
demonstrated that the antitumor response of CIKs could be
influenced by DCs in vivo (1,4,12), but the influence of DCs on CIKs
cultured in vitro was unclear.
In this study, data analysis revealed that the
highest amplification fold of CIKs occurred on day 7. Further study
revealed that the DC-CIK cell quantity, partial cell phenotype and
cell cytotoxicity were significantly upregulated compared with
CIKs. The results are likely to be useful for DC-CIK application
and development in antitumor therapies.
Materials and methods
Ethics and consent
Peripheral blood was donated from volunteers after
receiving informed consent, and the study was approved by the
ethics committee of the Second Affiliated Hospital of Nanhua
University, Henyang, China.
CIK culture
Lymphocytes were separated and cultured in
accordance with the studies of Pan et al (13) and Laport et al (14), with certain modifications. Peripheral
blood was mixed 1:1 (V:V) with 0.9% physiological
saline and used for Ficoll density gradient separation (LymphoPrep,
PAA, Cölbe, Germany). Following centrifugation at 1,800 rpm for 20
min, the leukocyte layer was collected in fresh tubes. These cells
were then washed twice with 0.9% physiological saline at 1,500 rpm
for 7 min. Next, the lymphocyte was cultured in GT-T551 medium
(Takara Biotechnology Co., Ltd., Dalian, China) with 1,000 U/ml
γ-interferon (Beijing Biocoen Biotechnology Co., Ltd., Beijing,
China). Ten percent autologous plasma was added on day 0, then 50
µg/ml CD3 monoclonal antibody (Skoda Biotechnology Co., Ltd.,
Beijing, China) and 100 U/ml interleukin 1α (IL-1α; PeproTech,
Suzhou, China) were added on day 1, and 1,000 U/ml rhIL-2
(SL-PHARM, Beijing, China) and 2% autologous plasma was included in
the medium from day 1 onward with the concentration calculated by a
pocH-100i hemtology analyzer (Sysmex, Milton Keynes, UK). The cells
were cultured at 37°C in 5% CO2 until day 13.
DC-CIK culture
The DC cells were cultured in vitro according
to the studies of Miao et al and Pan et al with
certain modifications (15,16). The lymphocyte separated from the
peripheral blood was resuspended with 20 ml GT-T551 medium, and
cultured for 3 h at 37°C in 5% CO2. Finally, the adhered
and suspended cells were separated and cultured as mononuclear
cells and CIK cells, respectively. The mononuclear cells were
cultured with 20 ml AIM-V medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) containing 10% autologous plasma, GM-CSF (0.2
µg/ml, Beijing Biocoen Biotechnology Co., Ltd, Beijing, China) and
IL-4 (1 µg/ml, CELLBO Biotechnology Co., Ltd, Wuxi, China). Half of
the medium was replaced with fresh medium supplemented with
cytokines on day 3, and TNF-α (0.2 µg/ml, Beijing Biocoen
Biotechnology Co., Ltd) was added on day 5 to induce maturation of
the DCs. On day 7, the DCs were collected and co-cultured with CIK
at 37°C in 5% CO2 until day 13.
Flow cytometry analysis
Following culture of CIKs and DC-CIKs for 13 days, 1
ml cell suspension was collected and centrifugated at 1,000 rpm for
10 min, then the precipitate was resuspended in 1 ml 0.9%
physiological saline, centrifugated at 1,000 rpm for 10 min, then
the precipitate was resuspended with 150 µl 0.9% physiological
saline, and divided into two groups. APC mouse IgG1 (5 µl), FITC
mouse IgG2α (5 µl), PE mouse IgG1 (5 µl) and PerCP-CyTM5.5 mouse
IgG1 (1 µl) were added to one group to form the isotype control,
and FITC mouse anti-human CD3 (5 µl), PE mouse anti-human CD4 (5
µl), PerCP-CyTM5.5 mouse anti-human CD8 (1 µl) and APC mouse
anti-human CD56 (5 µl) were added to the second group to form the
experimental group. The two groups were all incubated for 15 min at
room temperature, then resuspended with 1 ml 0.9% physiological
saline, and centrifugated at 1,000 rpm for 10 min. Finally, the
precipitate was resuspended with 0.2 ml 0.9% physiological saline,
and prepared for analysis using a BD Accuri C6 flow cytometer (BD
Biosciences, Shanghai, China).
Cell viability
Following the culture of CIKs and DC-CIKs for 13
days, 1 ml cell suspension was collected and centrifugated at 1,000
rpm for 10 min, then the precipitate was resuspended in 1 ml 0.9%
physiological saline and centrifugated at 1,000 rpm for 10 min.
Next, the precipitate was resuspended and diluted with
physiological saline to 1×105 cells/ml, then the cell
suspension was mixed with 0.4% trypan blue at 9:1
(V:V), and analyzed by Countstar (Inno-Alliance
Biotech, Shanghai, China) within 3 min.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
analysis
Hela cells as target cells were obtained at
logarithmic growth phase and the concentration was adjusted to
1×105 cells/ml. CIKs or DC-CIKs cultured for 13 days
were used as effector cells, and then mixed with target cells at a
proportion of 50:1 (effector cells to target cells). CIK or DC-CIK
culture medium (10 ml) was collected, and following centrifugation
at 1,000 rpm for 10 min, the precipitate was resuspended with
GT-T551 containing 2% autologous plasma and diluted to
5×106 cells/ml. The cells were divided into three
groups: the effector-target group comprised 100 µl effector cells
and target cells, respectively; the effector cells group comprised
100 µl effector cells and GT-T551 culture medium; and the target
cells group comprised 100 µl target cells and GT-T551 culture
medium. All groups were cultured at 37°C in 5% CO2 for
24 h. There were five parallel tubes in every group. Ten
microliters MTT (5 mg/ml) was added and cultured at 37°C in 5%
CO2 for 4 h. Following centrifugation at 2,000 rpm for 5
min, the precipitate was dissolved in 100 µl dimethyl sulfoxide,
agitated for 15 min, and analyzed at an optical density (OD) of 490
nm. The killing rate was calculated as follows:
Rate = [1 - (ODeffector-target cell well
- ODeffector cell well) / ODtarget cell well]
× 100%.
Results
Effect of DCs on CIK cell
quantity
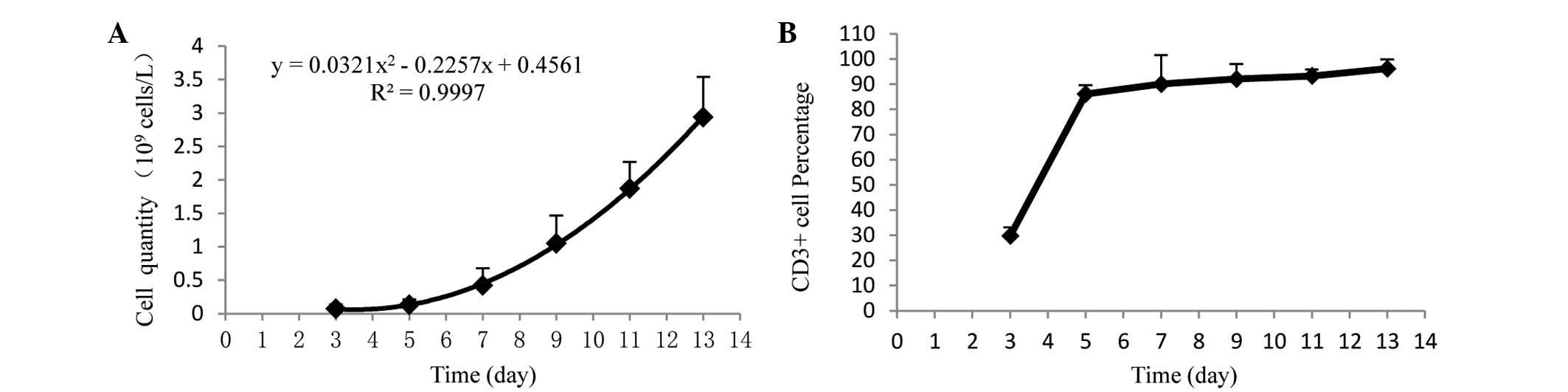
To determine the appropriate co-culture time of DCs
and CIKs, the cell proliferation of CIKs was analyzed. From day 7,
the CIKs were in a period of rapid proliferation (Fig. 1A), and the percentage of
CD3+ T cells was over 90% (Fig. 1B). Of these, DCs were co-cultured with
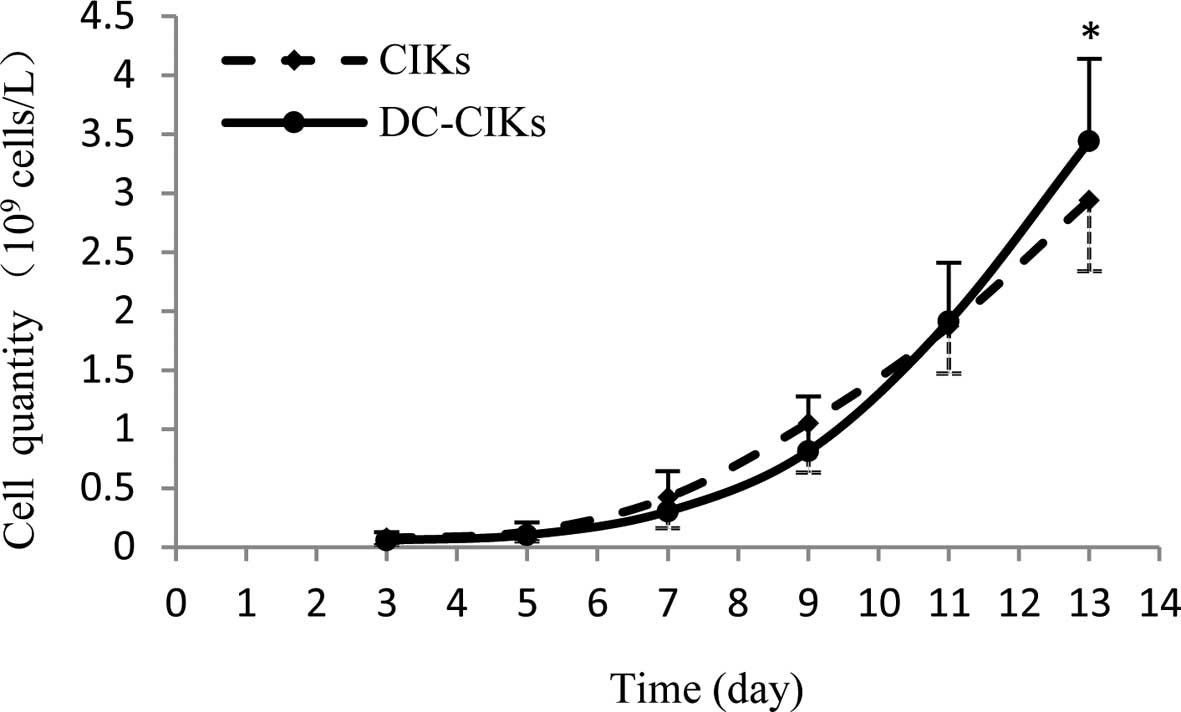
CIKs for 7 days. As shown in Fig. 2,
the DC-CIKs were in rapid proliferation on day 7, but the cell
quantity was lower than that of CIKs until day 11 and the DC-CIK
quantity was significantly (1.17-fold) greater than that of CIKs on
day 13.
Difference in cell phenotype between
CIKs and DC-CIKs
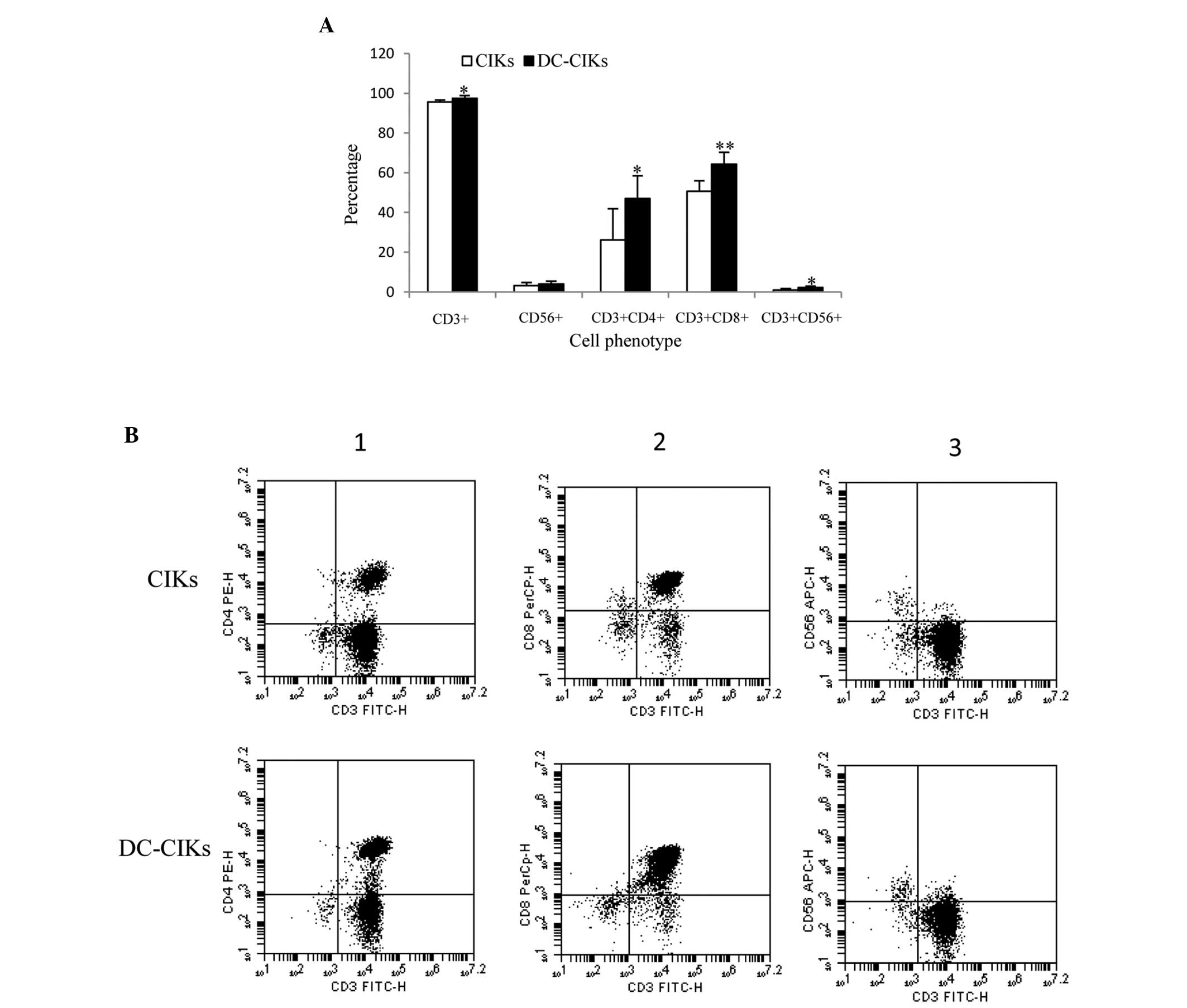
Next, cell phenotype was analyzed by flow cytometry.
As Fig. 3A reveals, the expression of
CD3+, CD56+, CD3+CD4+,
CD3+CD8+ and CD3+CD56+
T cells in DC-CIKs was higher than that in CIKs, and the expression
of CD3+, CD3+CD4+
CD3+CD8+ and CD3+CD56+
T cells was significantly upregulated 1.02, 1.79, 1.26 and
2.44-fold, respectively.
Cell viability and cell
cytotoxicity
For further analysis of the influence of DCs on CIKs
in vitro, cell viability analysis and MTT were used. The CIK
and DC-CIK cell viability was 96 and 98%, respectively (Fig. 4), and there was no significant
difference between CIKs and DC-CIKs. In addition, MTT results
revealed that the CIK and DC-CIK cell cytotoxicity in Hela cells
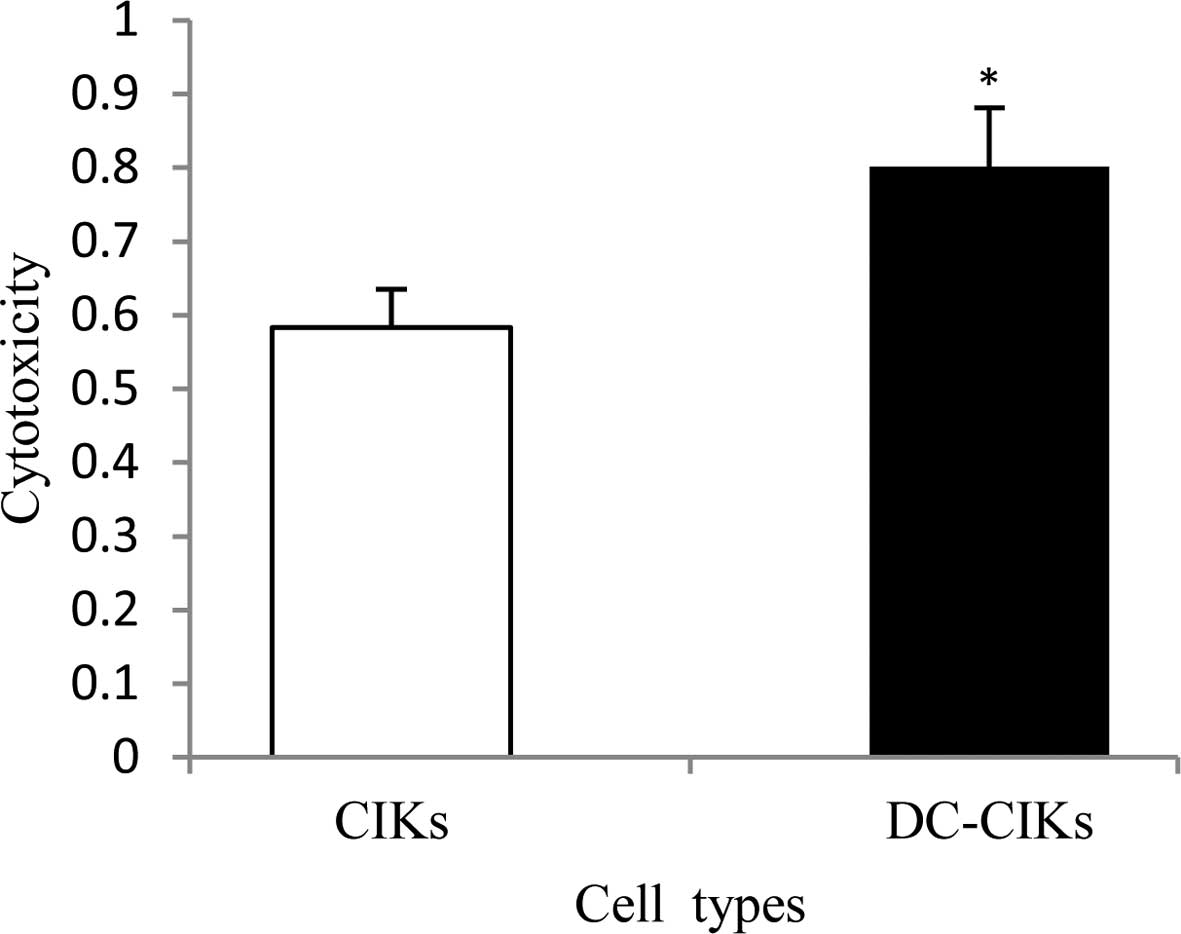
was 58 and 80%, respectively, with a significant difference
(Fig. 5).
Discussion
DCs, known to be the most powerful APCs, play a
significant role in immune response regulation, induce primary
immune responses, and potentiate the effector functions of
previously primed T lymphocytes (17–19).
Usually, tumor patients have low immune function, which leads to
tumor cells escaping autoimmune response. CIK cells are a subset of
natural killer T lymphocytes (NKTs) that are type II NKT cells
(20) with enhanced tumor cell lytic
activity (21), higher proliferation
rate (22), and relatively lower
toxicity (23). Due to these
characteristics, CIKs are extensively used in antitumor therapy in
clinics (24–26).
In this study, the influence of DCs on CIKs was
analyzed. The results revealed that DCs were suitable for
co-culture with CIKs on day 7 (Fig.
1), and that the cell quantity of DC-CIKs was significantly
improved compared with that of CIKs on day 13 (Fig. 2). Notably, the cell proliferation
quantity is related to the cytotoxicity of CIKs to tumors (27,28).
Therefore, these results strongly suggest that the antitumor
activity of DC-CIKs is enhanced compared with that of CIKs.
Furthermore, the difference in cell phenotype
between CIKs and DC-CIKs was analyzed by flow cytometry. The
results revealed that CD3+,
CD3+CD4+, CD3+CD8+ and
CD3+CD56+ T cells were significantly
increased (1.02, 1.79, 1.26 and 2.44-fold, respectively) in DC-CIKs
compared with CIKs (Fig. 3). The
CD3+ phenotype is a characteristic of T cells (29). CD3+CD4+ T cells
may induce differentiation into Th1 or Th2 cells by DCs, which
secrete IFN-γ or IL-4, IL-10, and IL-13 (18). CD3+CD8+ T cells
play an essential role in the immune response against cancers
(30).
CD3+CD56+ T cells are a subset of type II NKT
cells with non-major histocompatibility complex-restricted
tumor-killing activity (20,31). Collectively, these findings revealed
that DC-CIKs were more effective as antitumor agents than CIKs.
To investigate the cytotoxicity of CIKs and DC-CIKs
in Hela cells, cell viability analysis and MTT were used. As a
result, the DC-CIK and CIK cell viability was 96% and 98%,
respectively (Fig. 4). MTT revealed
that the CIK and DC-CIK cytotoxicity was 58% and 80%, respectively,
with a significant difference (Fig.
5). This is similar to the finding that CIK cytotoxicity was
significantly enhanced using the MTT method (1,32). Thus,
it may be assumed that DCs are beneficial to the enhancement of CIK
cytotoxicity.
In conclusion, this study is likely to be useful in
the application of DC-CIKs in antitumor therapy in the clinic. It
describes for the first time how DCs co-cultured with CIKs are of
benefit for the improvement of the CIK cell proliferation, cell
phenotype and antitumor activity in vitro.
Acknowledgements
The authors would like to thank Dr. Yueling Zhang
for revising the English in the manuscript. This study was
sponsored by the Special Fund for the Development of Strategic
Emerging Industries of Shenzhen City (no.
CYZZ20130329145313934).
References
|
1
|
Qu HQ, Zhou XS, Zhou XL and Wang J: Effect
of DC-CIK cell on the proliferation, apoptosis and differentiation
of leukemia cells. Asian Pac J Trop Med. 7:659–662. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmeel FC, Schmeel LC, Gast SM and
Schmidt-Wolf IG: Adoptive immunotherapy strategies with
cytokine-induced killer (CIK) cells in the treatment of
hematological malignancies. Int J Mol Sci. 15:14632–14648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wefers C, Lambert LJ, Torensma R and Hato
SV: Cellular immunotherapy in ovarian cancer: Targeting the stem of
recurrence. Gynecol Oncol. 137:335–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wongkajornsilp A, Wamanuttajinda V,
Kasetsinsombat K, Duangsa-ard S, Sa-ngiamsuntorn K, Hongeng S and
Maneechotesuwan K: Sunitinib indirectly enhanced anti-tumor
cytotoxicity of cytokine-induced killer cells and
CD3+CD56+ subset through the co-culturing
dendritic cells. PLoS One. 8:e789802013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Introna M, Golay J and Rambaldi A:
Cytokine induced killer (CIK) cells for the treatment of
haematological neoplasms. Immunol Lett. 155:27–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banchereau J and Palucka AK: Dendritic
cells as therapeutic vaccines against cancer. Nat Rev Immunol.
5:296–306. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thorne SH, Negrin RS and Contag CH:
Synergistic antitumor effects of immune cell-viral biotherapy.
Science. 311:1780–1784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thurner B, Haendle I, Röder C, Dieckmann
D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von
den Driesch P, et al: Vaccination with mage-3A1 peptide-pulsed
mature, monocyte-derived dendritic cells expands specific cytotoxic
T cells and induces regression of some metastases in advanced stage
IV melanoma. J Exp Med. 190:1669–1678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mody N, Dubey S, Sharma R, Agrawal U and
Vyas SP: Dendritic cell-based vaccine research against cancer.
Expert Rev Clin Immunol. 11:213–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frank MO, Kaufman J, Parveen S, Blachère
NE, Orange DE and Darnell RB: Dendritic cell vaccines containing
lymphocytes produce improved immunogenicity in patients with
cancer. J Transl Med. 12:3382014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Yang X, Li J, Ren Y, Zhang T, Zhang
C, Zhang J, Li J and Pang Y: Immune response, safety and survival
and quality of life outcomes for advanced colorectal cancer
patients treated with dendritic cell vaccine and cytokine-induced
killer cell therapy. Biomed Res Int. 2014:6038712014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan K, Li YQ, Wang W, Xu L, Zhang YJ,
Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, et al: The efficacy of
cytokine-induced killer cell infusion as an adjuvant therapy for
postoperative hepatocellular carcinoma patients. Ann Surg Oncol.
20:4305–4311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laport GG, Sheehan K, Baker J, Armstrong
R, Wong RM, Lowsky R, Johnston LJ, Shizuru JA, Miklos D, Arai S, et
al: Adoptive immunotherapy with cytokine-induced killer cells for
patients with relapsed hematologic malignancies after allogeneic
hematopoietic cell transplantation. Biol Blood Marrow Transplant.
17:1679–1687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao L, Run-Ming J and Yi J: T-Bet
mediated anti-neoplastic effects of dendritic cell-cytokine induced
killer cells in vitro. Iran J Pediatr. 22:43–51. 2012.PubMed/NCBI
|
|
16
|
Pan Y, Tao Q, Wang H, Xiong S, Zhang R,
Chen T, Tao L and Zhai Z: Dendritic cells decreased the concomitant
expanded Tregs and Tregs related IL-35 in cytokine-induced killer
cells and increased their cytotoxicity against leukemia cells. PLoS
One. 9:e935912014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lenahan C and Avigan D: Dendritic cell
defects in patients with cancer: mechanisms and significance.
Breast Cancer Res. 8:1012006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Wei Y, He J, Cui G, Zhu Y, Lu C,
Ding Y, Xue R, Bai L, Uede T, et al: Natural killer T cells play a
necessary role in modulating of immune-mediated liver injury by gut
microbiota. Sci Rep. 4:72592014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Datta J, Terhune JH, Lowenfeld L, Cintolo
JA, Xu S, Roses RE and Czerniecki BJ: Optimizing dendritic
cell-based approaches for cancer immunotherapy. Yale J Biol Med.
87:491–518. 2014.PubMed/NCBI
|
|
20
|
Gütgemann S, Frank S, Strehl J and
Schmidt-Wolf IG: Cytokine-induced killer cells are type II natural
killer T cells. Ger Med Sci. 5:Doc072007.PubMed/NCBI
|
|
21
|
Margolin KA, Negrin RS, Wong KK,
Chatterjee S, Wright C and Forman SJ: Cellular immunotherapy and
autologous transplantation for hematologic malignancy. Immunol Rev.
157:231–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linn YC and Hui KM: Cytokine-induced
killer cells: NK-like T cells with cytotolytic specificity against
leukemia. Leuk Lymphoma. 44:1457–1462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: first report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jäkel CE, Vogt A, Gonzalez-Carmona MA and
Schmidt-Wolf IG: Clinical studies applying cytokine-induced killer
cells for the treatment of gastrointestinal tumors. J Immunol Res.
2014:8972142014.PubMed/NCBI
|
|
25
|
Zhang Y, Xia L, Zhang Y, Wang Y, Lu X, Shi
F, Liu Y, Chen M, Feng K, Zhang W, et al: Analysis of adverse
events following the treatment of autologous cytokine-induced
killer cells for adoptive immunotherapy in malignant tumour
sufferers. Expert Opin Biol Ther. 15:481–493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Liu XY, Zhang T, Zhang XF, Zhao
L, Long F, Liu ZK and Wang EH: The dual-functional capability of
cytokine-induced killer cells and application in tumor immunology.
Hum Immunol. 76:385–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bach M, Schimmelpfennig C and Stolzing A:
Influence of murine mesenchymal stem cells on proliferation,
phenotype, vitality, and cytotoxicity of murine cytokine-induced
killer cells in coculture. PLoS One. 9:e881152014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan WC and Linn YC: A comparison between
cytokine- and bead-stimulated polyclonal T cells: the superiority
of each and their possible complementary role. Cytotechnology. Dec
7–2014.(Epub ahead of print). PubMed/NCBI
|
|
29
|
Wang M, Cao JX, Pan JH, Liu YS, Xu BL, Li
D, Zhang XY, Li JL, Liu JL, Wang HB and Wang ZX: Adoptive
immunotherapy of cytokine-induced killer cell therapy in the
treatment of non-small cell lung cancer. PLoS One. 9:e1126622014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salaun B, Yamamoto T, Badran B,
Tsunetsugu-Yokota Y, Roux A, Baitsch L, Rouas R, Fayyad-Kazan H,
Baumgaertner P, Devevre E, et al: Differentiation associated
regulation of microRNA expression in vivo in human CD8+
T cell subsets. J Transl Med. 9:442011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmeel LC, Schmeel FC, Coch C and
Schmidt-Wolf IG: Cytokine-induced killer (CIK) cells in cancer
immunotherapy: report of the international registry on CIK cells
(IRCC). J Cancer Res Clin Oncol. 141:839–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue CM, Chen C, Xu J, et al: Influence of
some traditional Chinese medicines (TCMS) on cytokine-induced
killer cells proliferation and anti-tumor features in vitro. Int J
Res Ayurveda Pharm. 4:228–232. 2013. View Article : Google Scholar
|