Introduction
The endoplasmic reticulum (ER) is the central
compartment for producing and folding cell surface receptors and
secreted proteins. The ER is also involved in calcium balance
regulation and the biosynthesis of cholesterol and steroids
(1). Perturbations in ER homeostasis
lead to the accumulation of misfolded proteins, which activates an
adaptation response called the ER stress response, also known as
the unfolded protein response (2).
The ER stress response is mediated by three main pathways: The
inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER
kinase (PERK) and activating transcription factor 6 (ATF6)
pathways. The activation of these pathways is associated with
stress relief and ER function restoration (3).
Cancer cells are associated with various stressors,
such as hypoxia, nutrient deprivation and pH changes, which
activate cellular stress response pathways, including the ER stress
response (4). The ER stress response
is therefore crucial to cancer progression and other cancer cell
phenotypes, including invasion and migration. Li et al
(5) showed that activating ER stress
in breast cancer cells through use of Adriamycin or Tunicamycin
enhances the invasion and migration associated with heparinase.
Hypoxia-enhanced migration of breast cancer cells occurs through
the PERK-ATF4 pathway during the ER stress response (6). These results suggest that activating ER
stress in breast cancer cells enhances their invasion and
migration. The ER stress pathway is activated in breast cancer
cells even in the absence of external stress, and the activation
status is associated with cancer growth, relapse and maintenance of
a cancer stem cell population (7).
Tauroursodeoxycholic acid (TUDCA) is a taurine
conjugated form of UDCA that has been used to treat jaundice in
Asian countries and has been approved by the US Food and Drug
Administration for treating primary biliary cirrhosis (8). TUDCA acts as a chemical chaperone and
modulates several signaling pathways (9).
The present study investigated whether basal ER
stress is associated with the invasion and migration of breast
cancer using TUDCA as a chemical chaperone. TUDCA reduced the
invasiveness of the MDA-MB-231 metastatic breast cancer cell line
under normoxic and hypoxic conditions. In addition, the PERK
pathway appeared to be involved in cancer cell invasion when using
inhibitors and short hairpin RNAs (shRNAs). These results suggested
that the ER stress pathway may serve as a therapeutic target for
the development of anti-metastatic drugs and chemical chaperones,
such as TUDCA, may be candidates for anti-metastatic agents.
Materials and methods
Cell culture
MDA-MB-231 cells were purchased from the American
Type Culture Collection (Manassas, VA, USA). Cell culture and
transfection were performed as previously described (10). Sense and antisense oligonucleotides
for shRNAs targeting human PERK-299 (5′-GCGGCAGGTCATTAGTAATTA-3′)
and PERK-506 (5′-GCATGGAAACAGTTCCTTTCA-3′) were generated, annealed
and cloned into the pSUPER.puro vector (Oligoengine, Seattle, WA,
USA), according to the manufacturer's instructions. For transient
transfections of shRNAs, MDA-MB-231 cells were electroporated using
Neon® Transfection System (Thermo Fisher Scientific
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
TUDCA (Sigma-Aldrich, St. Louis, MO, USA), an ATF6 inhibitor
[4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride], an IRE1
inhibitor (4u8C) and a PERK inhibitor (GSK2606414) (Calbiochem, San
Diego, CA, USA) were used. Cell viability was evaluated using the
ADAM-MC Automatic Cell Counter (NanoEnTek, Inc., Seoul, Korea). An
MTT assay was performed using the CellTiter 96 Non-Radioactive Cell
Proliferation Assay kit (Promega, Madison, WI, USA), according to
the manufacturer's instructions.
Hypoxia treatment
Cells were exposed to hypoxia using an anaerobic
system (Thermo Scientific, Inc., Marietta, OH, USA) using mixed gas
(1% O2, 5% CO2, N2 balance).
Oxygen concentration was checked with an O2 sensor (New
Cosmos, Osaka, Japan) prior to hypoxia treatment. Cells were kept
in 37°C incubation chamber in an anaerobic system.
Invasion assay
Upper membranes of cell culture inserts (BD
Biosciences, Franklin Lakes, NJ, USA) were coated with Matrigel
diluted in Opti-MEM®I Reduced Serum Medium (1:10 ratio;
Thermo Fisher Scientific, Inc.) for 1.5 h and then rehydrated with
serum-free Dulbecco's modified Eagle's medium (DMEM) for 1 h. Next,
~2.5×104 cells suspended in serum-free DMEM were seeded
into the upper layer of cell culture inserts, and DMEM with 10%
fetal bovine serum was added to the lower chambers for 24 h at
37°C. The inserts were then removed from the medium, fixed in 100%
methanol and stained with 0.1% crystal violet dye for 10 min. The
upper surfaces of the inserts were wiped with swaps, and the
membranes were isolated from the inserts to prepare slides. The
invaded cells were observed and images were captured using an
Eclipse 80i Upright microscope (Nikon, Tokyo, Japan) and the
Image-Pro Plus software (Media Cybernetics Inc., Rockville, MD,
USA).
Wound-healing assay
The MDA-MB-231 cells were treated with either the
vehicle control (EtOH) or TUDCA, and their migration ability was
compared using the wound-healing assay. Briefly, 1×105
cells were plated into 6-well plates. Following 24 h of incubation,
the cell monolayers were scratched using a 200-µl pipette tip, and
the medium was changed for fresh medium containing the vehicle
control (EtOH) or 0.5 mM TUDCA. The widths of the scratches were
monitored for 54 h, and images were captured at 0, 6, 24 and 54 h
using a Nikon Eclipse TS100 (Nikon) inverted microscope and the
Image-Pro Plus software.
RNA purification, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total cellular RNA was extracted using the RNeasy
Mini kit (Qiagen, Valencia, CA, USA), according to the
manufacturer's instructions. RNA (1 µg) was used for cDNA synthesis
using the AccuPower RT PreMix and oligo(dT) primers (Bioneer,
Daejeon, South Korea). RT-qPCR was performed with the cDNA, Maxima
SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Inc.) and the
specific primers listed below using the CFX96 Real-Time System
(Bio-Rad, Hercules, CA, USA). The primer sequences were as follows:
MMP-7 forward, 5′-GTATGGGACATTCCTCTGATCC-3′ and reverse,
5′-CCAATGAATGAATGAATGGATG-3′; MMP-13 forward,
5′-AACCAGGTCTGGAGATATGATGA-3′ and reverse,
5′-TGTATGGGTCCGTTGAAAAA-3′; and GAPDH forward,
5′-GAAATCCCATCACCATCTTCCAGG-3′ and reverse,
5′-GAGCCCCAGCCTTCTCCATG-3′. The qPCR parameters were 5 min at 95°C,
followed by 40 cycles of 10 sec at 95°C, 10 sec at 60°C and 10 sec
at 70°C. A melting curve step (65–95°C at increments of 0.5°C) was
performed at the end of the qPCR. Relative quantification of target
gene expression was calculated by the quantitative threshold cycle
(Cq) method, using GAPDH as an endogenous reference gene for
normalization (11). RT-qPCR was
performed >3 times for each gene and representative results are
shown.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical significance was analyzed using the SPSS
version 18 software for Windows (SPSS Inc., Chicago, IL, USA).
Significant differences between two groups were evaluated by
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TUDCA reduces invasion by MDA-MB-231
breast cancer cells
In the present study, TUDCA was used as a chemical
chaperone to investigate the role of ER stress in breast cancer
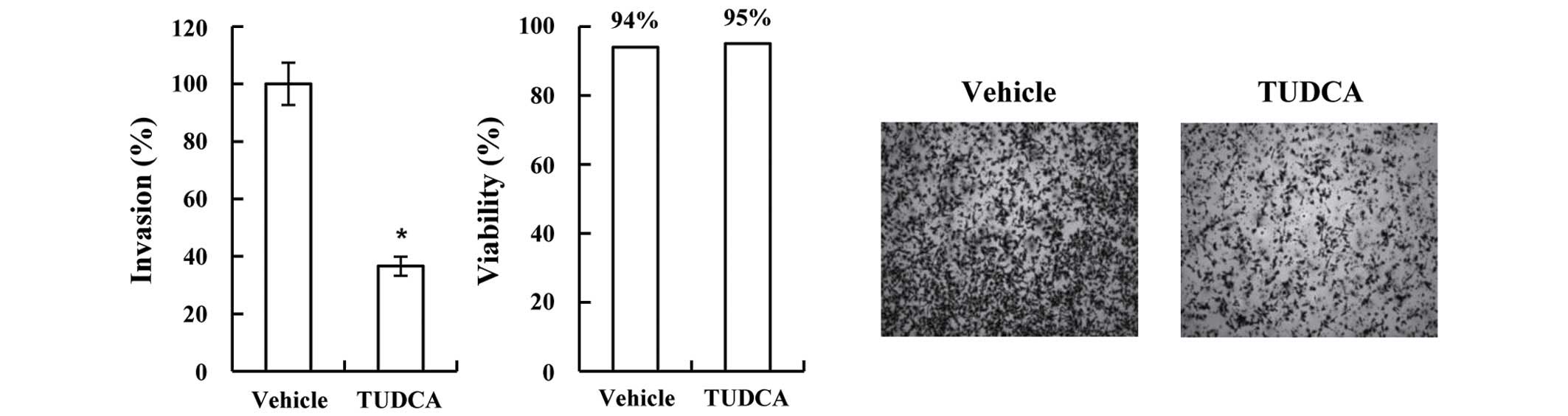
cell invasiveness. MDA-MB-231 cells were treated with TUDCA for 16
h and then used in the invasion assay with Matrigel-coated
membranes. TUDCA did not significantly modulate cell viability,
however, it did significantly reduce MDA-MB-231 cell invasion
(36.6%; P=2.122×10−6 vs. vehicle; Fig. 1). TUDCA did not significantly modulate
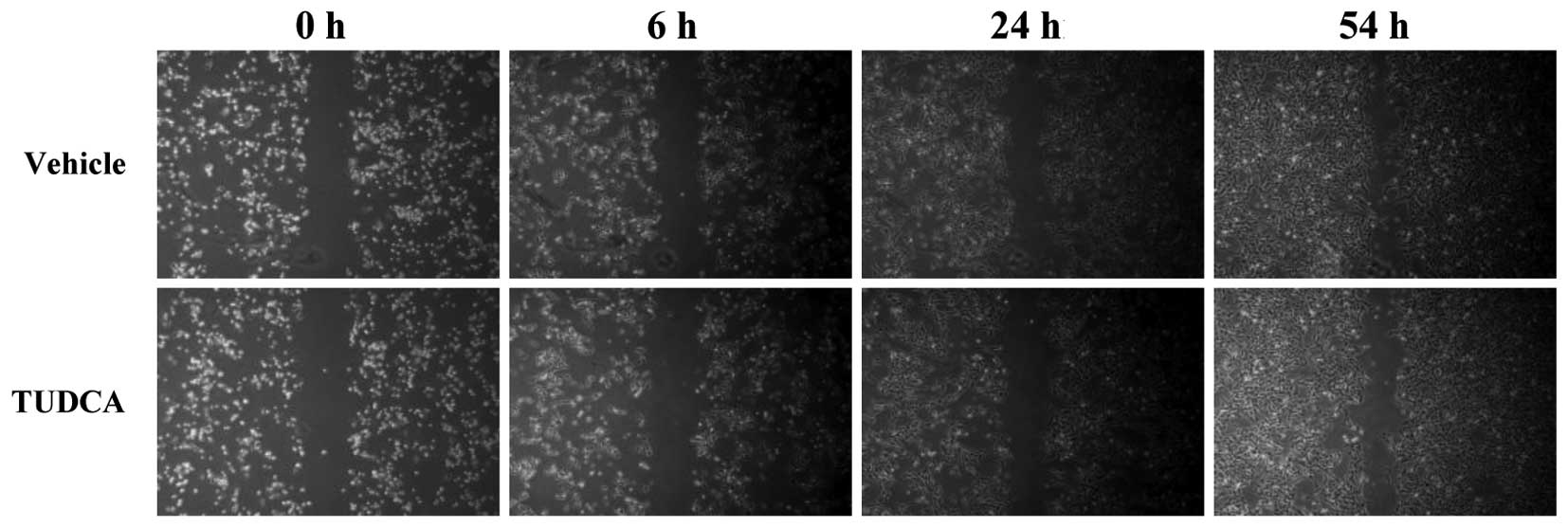
the migration or density of the MDA-MB-231 cells following a 54-h
incubation period (Fig. 2). These
results suggested that the reduced invasion following the TUDCA
treatment was not due to reduced migration or proliferation.
Reduced invasion due to TUDCA is
associated with reduced expression of MMP-7 and −13
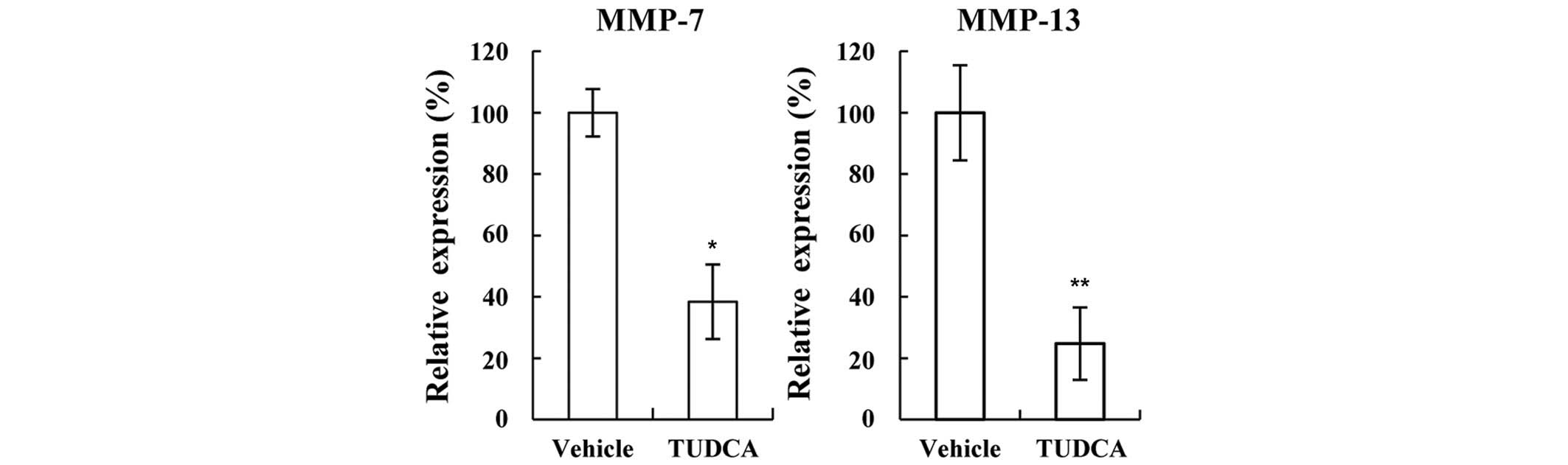
Several molecules associated with invasion were
tested using western blotting and RT-qPCR. the expression of MMP-7
and −13 was found to be significantly decreased following treatment
with TUDCA (38.4% and 24.7%, respectively; P=5.863×10−6
and P=1.006×10−5 vs. vehicle, respectively; Fig. 3), suggesting that TUDCA may reduce
invasion by decreasing MMP-7 and −13 expression. As cancer cell
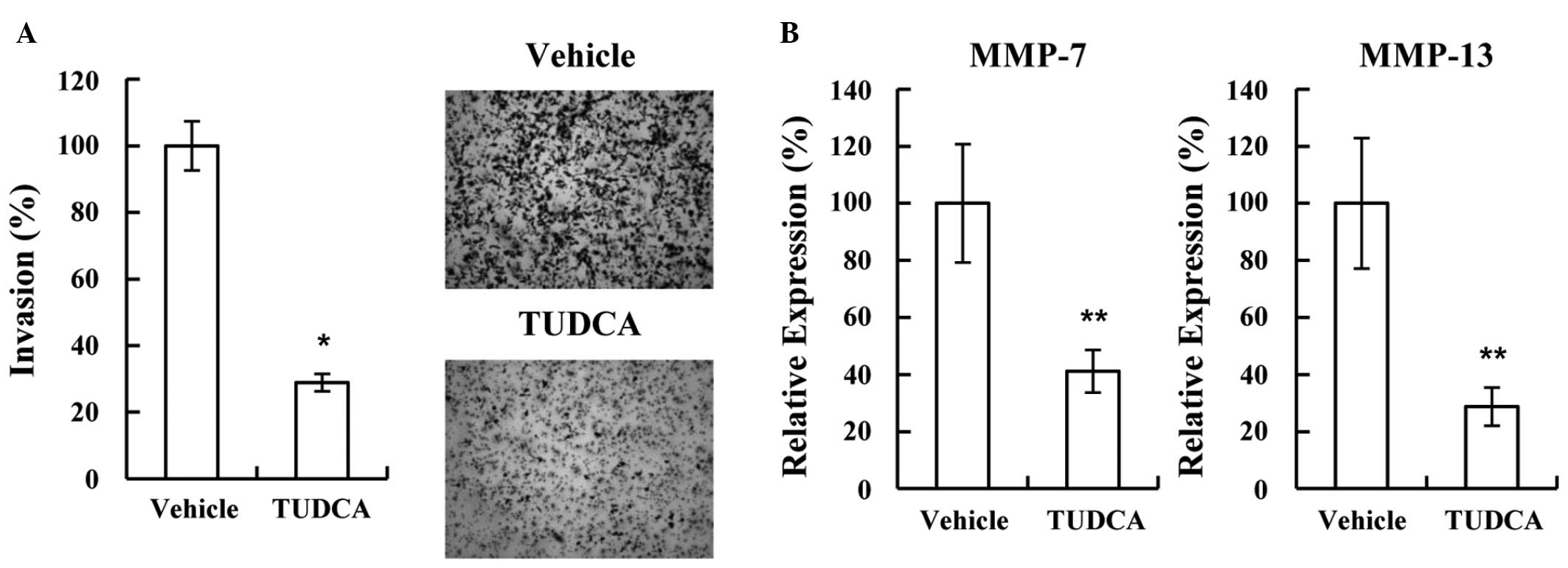
invasion is associated with hypoxia, the possibility of TUDCA
modulating invasion under hypoxic conditions was also investigated.
Consistent with the normoxic result, TUDCA significantly decreased
MDA-MB-231 cell invasion (28.9%; P=8.971×10−7 vs.
vehicle; Fig. 4A). Furthermore, the
decreased invasion was shown to be associated with decreased MMP-7
and −13 expression under hypoxic conditions (41.1% and 28.7%,
respectively; P=1.668×10−4 and P=3.569×10−4
vs. vehicle, respectively; Fig.
4B).
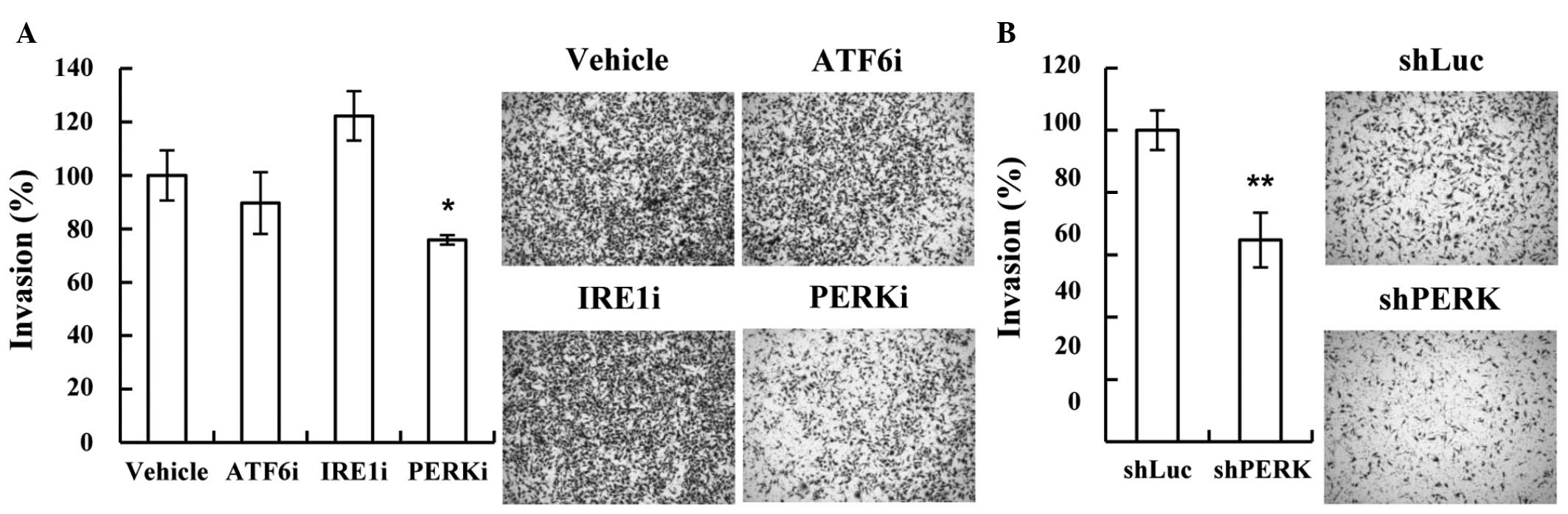
PERK in the ER stress pathway is
associated with MDA-MB-231 cell invasion
The ER stress pathway includes three downstream
pathways: ATF6, IRE1 and PERK. The present study used inhibitors of
these downstream pathways to investigate which pathway is involved
in ER stress-mediated invasion. Only the PERK inhibitor
significantly decreased the invasion of the MDA-MB-231 cells
(75.9%; P=1.197×10−3 vs. vehicle; Fig. 5A). As the PERK inhibitor may have
inhibited other signaling pathways, shRNAs targeting PERK were used
to confirm the results. shRNAs targeting different PERK mRNA
regions were prepared and used in the invasion assay. Consistent
with the results of the use of the inhibitor, depleting PERK with
the shRNAs was found to decrease MDA-MB-231 cell invasion (64.7%;
P=7.961×10−4 vs. vehicle; Fig.
5B). These results suggested that the PERK pathway may be
involved in the invasiveness of metastatic breast cancer.
Discussion
MMPs are a family of zinc-dependent endopeptidases
that are used in extracellular matrix remodeling and are associated
with embryogenic tissue remodeling, and angiogenic and pathological
processes, such as cancer cell invasion and arthritis (12). Cancer cells detach from primary
cancers and invade through the basement membrane and extracellular
matrix cleaved by MMPs. The present study found that the expression
of MMP-7 and −13 decreased significantly following TUDCA treatment.
These MMPs are involved in cell invasion in gastric cancer and
Kaposi's sarcoma (13,14). ER stress activation in breast cancer
cells using Adriamycin has been shown to enhance invasion by
activating heparinase (5); however,
this is the first study to report the involvement of the ER stress
pathway in the regulation of MMP-7 and −13 during breast cancer
cell invasion under basal conditions without an external
stimulus-activated ER stress response.
The majority of solid tumors have hypoxic regions,
due to impaired angiogenesis. Hypoxia is associated with
metastasis, particularly in patients with hypoxic tumors (15–17). The
present study showed that TUDCA reduced the invasion of MDA-MB-231
cells under hypoxic conditions. Hypoxia has been shown to activate
the ER stress pathway in cancer cells (18,19).
Therefore, TUDCA may decrease the ER stress response activated by
hypoxia, resulting in reduced invasion.
In the present study, TUDCA was shown to act as a
chemical chaperone that reduces the invasion of the MDA-MB-231
metastatic breast cancer cell line by decreasing the basal ER
stress response, suggesting that the ER stress pathway may be
involved in breast cancer cell invasion, as well as survival
against stressors such as hypoxia, glucose starvation and pH
changes. Furthermore, TUDCA was found to reduce breast cancer cell
invasion under hypoxic conditions, which suggested that the ER
stress pathway may be a good therapeutic target for cancer
metastasis, and that chemical chaperones, such as TUDCA, may be
useful for that purpose. TUDCA is not currently approved by the
Food and Drug Administration, but UDCA, which is approved, is
metabolized to TUDCA in the liver.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (DIRAMS) grant funded by the Korea government
(MSIP) (grant nos. 50597-2014 and 50491-2015).
References
|
1
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JH, Walter P and Yen TS: Endoplasmic
reticulum stress in disease pathogenesis. Annu Rev Pathol.
3:399–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Healy SJ, Gorman AM, Mousavi-Shafaei P,
Gupta S and Samali A: Targeting the endoplasmic reticulum-stress
response as an anticancer strategy. Eur J Pharmacol. 625:234–246.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu H, Huang YY, Pu LJ, Zhang XD,
Jiang CC and Jiang ZW: Suppression of endoplasmic reticulum
stress-induced invasion and migration of breast cancer cells
through the downregulation of heparanase. Int J Mol Med.
31:1234–1242. 2013.PubMed/NCBI
|
|
6
|
Nagelkerke A, Bussink J, Mujcic H, Wouters
BG, Lehmann S, Sweep FC and Span PN: Hypoxia stimulates migration
of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded
protein response. Breast Cancer Res. 15:R22013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen X, Iliopoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1α pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wimmer R, Hohenester S, Pusl T, Denk GU,
Rust C and Beuers U: Tauroursodeoxycholic acid exerts
anticholestatic effects by a cooperative cPKC alpha-/PKA-dependent
mechanism in rat liver. Gut. 57:1448–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vang S, Longley K, Steer CJ and Low WC:
The unexpected uses of urso- and tauroursodeoxycholic acid in the
treatment of non-liver diseases. Glob Adv Health Med. 3:58–69.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han YK, Lee JH, Park GY, Chun SH, Han JY,
Kim SD, Lee J, Lee CW, Yang K and Lee CG: A possible usage of a
CDK4 inhibitor for breast cancer stem cell-targeted therapy.
Biochem Biophys Res Commun. 430:1329–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer-roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye Y, Zhou X, Li X, Tang Y, Sun Y and Fang
J: Inhibition of epidermal growth factor receptor signaling
prohibits metastasis of gastric cancer via downregulation of MMP7
and MMP13. Tumour Biol. 35:10891–10896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Wang S, Lu L and Wei G: MiR99a
modulates MMP7 and MMP13 to regulate invasiveness of Kaposi's
sarcoma. Tumour Biol. 35:12567–12573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chia SK, Wykoff CC, Watson PH, Han C, Leek
RD, Pastorek J, Gatter KC, Ratcliffe P and Harris AL: Prognostic
significance of a novel hypoxia-regulated marker, carbonic
anhydrase IX, in invasive breast carcinoma. J Clin Oncol.
19:3660–3668. 2001.PubMed/NCBI
|
|
16
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
17
|
Rademakers SE, Span PN, Kaanders JH, Sweep
FC, van der Kogel AJ and Bussink J: Molecular aspects of tumour
hypoxia. Mol Oncol. 2:41–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, László C, Liu Y, Liu W, Chen X,
Evans SC and Wu S: Regulation of G(1) arrest and apoptosis in
hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation.
Neoplasia. 12:61–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rouschop KM, Dubois LJ, Keulers TG, van
den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M
and Wouters BG: PERK/eIF2α signaling protects therapy resistant
hypoxic cells through induction of glutathione synthesis and
protection against ROS. Proc Natl Acad Sci USA. 110:4622–4627.
2013. View Article : Google Scholar : PubMed/NCBI
|