Introduction
While stereotactic body radiotherapy (SBRT) can
effectively eradicate oligometastases, the majority of patients
with distant metastases ultimately succumb to distant metastases
(1). There is growing enthusiasm for
combining SBRT with agents that enhance antitumor immunity as an
approach to reducing the probability of subsequent distant
metastases (2,3). Ongoing studies are investigating the
combination of cytotoxic T lymphocyte-associated protein 4 (CTLA-4)
and programmed cell death protein 1 (PD-1) inhibitors with
radiation, particularly in melanoma (4).
Prior to the clinical development of CTLA-4 and PD-1
inhibitors, sunitinib was demonstrated to modulate the tumor
microenvironment and reverse the immune suppressive function of
myeloid-derived suppressor cells (MDSCs) (5). Compared with other tyrosine kinases
approved for human use, sunitinib is a relatively broad spectrum
tyrosine kinase inhibitor (6).
Sunitinib is a selective inhibitor of multiple protein tyrosine
kinases, including vascular endothelial growth factor (VEGF)
receptor types 1–3, platelet-derived growth factor receptor α and
β, c-kit, Fms-like tyrosine kinase 3, RET and colony-stimulating
factor 1 receptor (7). Sunitinib
reverses tumor-mediated immune suppression from human MDSCs
(8). Preclinical data suggests a
supra-additive antitumor effect when combining sunitinib with
radiotherapy compared to either treatment independently (9).
Based on these promising data, our group conducted a
clinical trial administering sunitinib followed by combined
sunitinib and radiation for patients with limited metastatic cancer
(10,11). With long-term follow-up, this regimen
achieved a 4-year progression-free survival rate of 34% (12). As part of this clinical study, an
increased incidence of grades 3 and 4 myelosuppression was observed
with sunitinib and radiation compared to historical controls
treated with sunitinib alone (11).
Comprehensive flow cytometric analysis demonstrated that sunitinib
reduced the population of
CD33+CD14+CD16+ monocytic MDSCs in
patients receiving sunitinib and radiotherapy (13). To complement these studies, the
current analysis was undertaken to characterize the effects of
combined sunitinib and radiation therapy on hematopoiesis.
Materials and methods
Experimental therapy
The study population consisted of 21 patients with
oligometastatic cancer (1–5 sites of metastatic cancer measuring ≤6
cm) from various primary tumors enrolled on a phase I/II trial of
sunitinib and radiotherapy between May 2007 and September 2010 with
clinical follow-up through July 2012 (NCT00463060). The
institutional review board of Mount Sinai School of Medicine (New
York, NY, USA) approved this study and all participants provided
written informed consent. For all patients, chemotherapy was
discontinued ≥21 days prior to initiating protocol therapy.
Sunitinib (25–50 mg qd) was administered on days 1–28. Radiotherapy
was delivered to all clinically apparent sites of disease with a
margin of 5–10 mm using an image-guided technique described
elsewhere (10). The radiation doses
were 40 or 50 Gy in 10 fractions from days 8–19. Further systemic
therapy consisting of either maintenance sunitinib or chemotherapy
was administered starting on day 42 at the discretion of the
treating medical oncologist.
Stratifying for the location and volume of bone
marrow irradiated, a matched-pair cohort analysis was performed on
a contemporary cohort of 21 patients with metastatic cancer who
were treated with radiation alone. These patients underwent
complete blood counts immediately prior to the start and following
the completion of radiotherapy. Baseline patient and treatment
characteristics for the two groups are described in Table I.
 | Table I.Baseline characteristics of the study
population (n=21). |
Table I.
Baseline characteristics of the study
population (n=21).
| Variable | Radiotherapy
alone | Radiotherapy +
sunitinib | P-value |
|---|
| Age, years |
|
|
|
| Median
(range) | 66 (28–90) | 65 (47–82) | 0.33 |
| <40,
n | 2 | 0 |
|
| 40–69,
n | 12 | 13 |
|
| ≥70,
n | 7 | 8 |
|
| Performance status,
n |
|
| 0.10 |
| 0–1 | 11 | 16 |
|
| 2–3 | 10 | 5 |
|
| Bone metastases,
n |
|
| 0.58 |
| Yes | 13 | 11 |
|
| No | 8 | 10 |
|
| Volume of bone marrow
irradiated, cm3; median (range) |
|
|
|
| Volume
receiving 10 Gy | 59 (3–220) | 35
(1–149) | 0.66 |
| Volume
receiving 15 Gy | 52 (3–201) | 29
(1–114) | 0.16 |
| Volume
receiving 20 Gy | 40 (2–191) | 23 (0–88) | 0.04 |
| Volume
receiving 30 Gy | 29 (0–168) | 11 (0–74) | 0.04 |
| Prior radiotherapy,
n |
|
| 0.33 |
|
Yes | 7 | 11 |
|
| No | 14 | 10 |
|
| Prior chemotherapy,
n |
|
| 0.72 |
|
Yes | 11 | 12 |
|
| No | 10 | 9 |
|
| Sites irradiated,
n |
|
| 0.54 |
|
Bone | 11 | 13 |
|
|
Lung | 3 | 3 |
|
|
Liver | 2 | 4 |
|
|
Visceral | 3 | 1 |
|
| Lymph
node | 2 | 2 |
|
|
Adrenal | 0 | 1 |
|
Hematological measurements
Complete blood count, differential and platelet
count were obtained prior to treatment and on days 8 and 19. For
each patient, pretreatment values served as controls for blood
levels on days 8 and 19. In the control group, complete blood
counts were obtained immediately prior to the start and following
the completion of radiotherapy. All hematological measurements were
taken at the Mount Sinai School of Medicine laboratories using the
Coulter LH 750 Hematology Analyzer (Beckman Coulter, Inc., Brea,
CA, USA), which generates a five-part differential using flow
cytometry technology based on volumetric impedance (using direct
current) and conductivity (using high frequency electromagnetic
energy and laser light scatter). This methodology has been
demonstrated to correlate well with manual slide review (14).
Bone marrow normalization
As patients in the clinical trial required radiation
to treatment sites throughout the body, the volume of bone marrow
irradiated was quantified. To standardize measurement of bone
marrow, volumes were calculated using the Eclipse Treatment
Planning System 8.0 (Varian Medical Systems, Inc., Palo Alto, CA,
USA) using a commercially available automatic segmentation
algorithm. To account for an uneven distribution of active bone
marrow throughout the body, the volume of bone marrow irradiated
was estimated by applying a correction factor that was derived by
cross referencing volumes of bone mass against anatomically
measured volumes of marrow contained within specific bones
(15). Specifically, the correction
factors were 1.0 for lumbosacral spine; 0.65 for thoracic spine;
0.5 for cervical spine, rib, sternum, scapula and iliac bone; and
0.3 for humerus, mandible, femoral head and femoral neck.
Statistical analysis
A two-sided paired t-test was used to make
comparisons between pre- and post-treatment hematological
measurements. Microsoft Excel was used to plot the differences in
changes in blood counts over time. Progression-free survival and
overall survival rates were calculated using the Kaplan-Meier
method on Stata software version 13.1 (StataCorp LP, College
Station, TX, USA). Univariate comparison between groups was
performed using the log-rank test.
Results
Early effects of sunitinib on
hematopoiesis
The primary tumor types for patients treated with
sunitinib included lung (24%), kidney (14%), liver (14%), head and
neck squamous cell carcinoma (14%), prostate (14%), pancreas (5%),
breast (5%), melanoma (5%) and colorectal (5%). Using pretreatment
values as controls, significant changes in hematological
measurements were observed within 7 days of initiating sunitinib
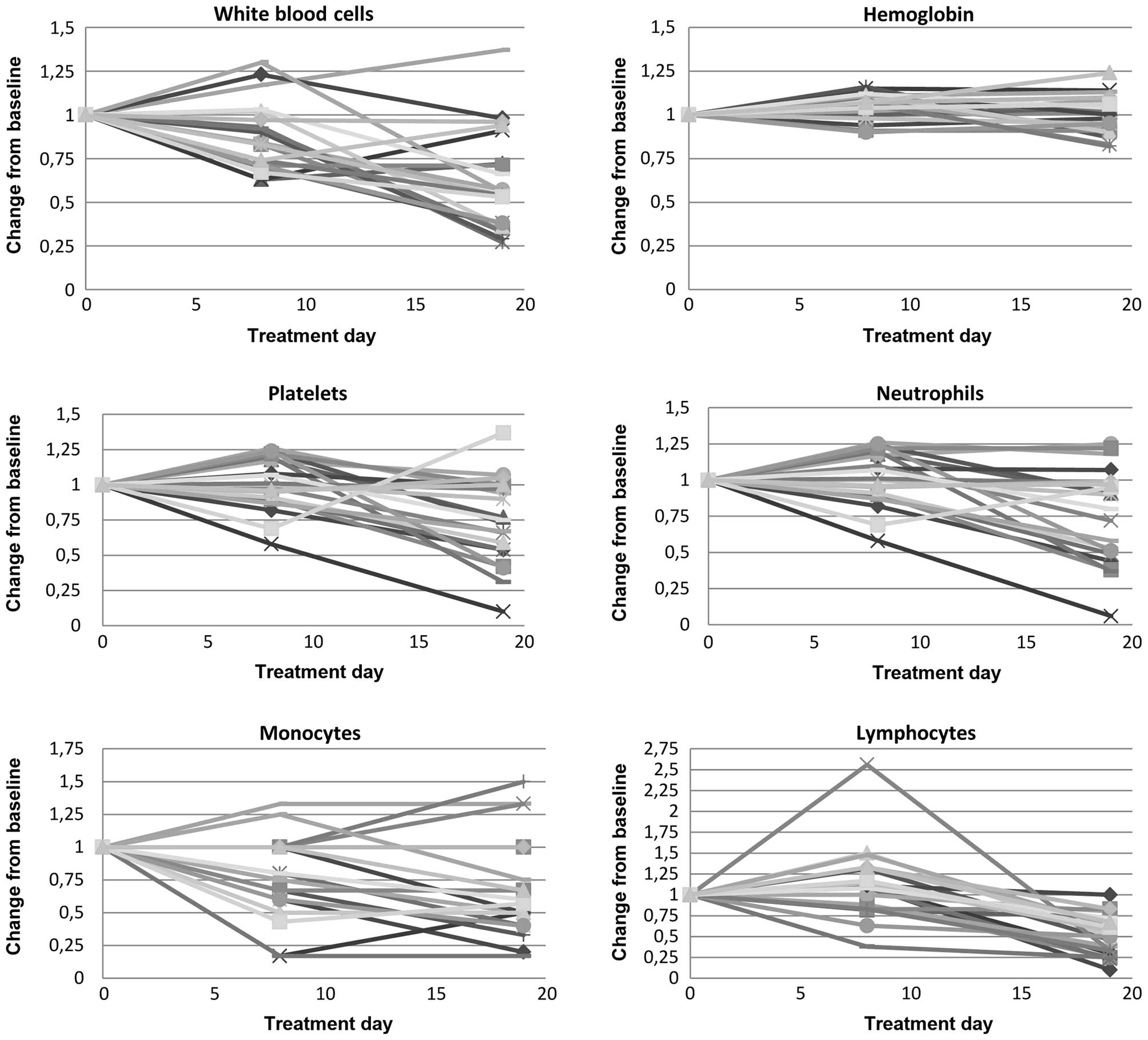
(Fig. 1 and Table II). On average, there was a 13%
reduction in leukocytes (6.72×109 vs.
5.88×109/l, P=0.02), largely due to a 30% decrease in
monocytes (0.50×109 vs. 0.35×109/l,
P<0.01) and an 18% decrease neutrophils (4.91×109 vs.
4.01×109/l, P<0.01). Sunitinib resulted in a marginal
increase in mean hemoglobin levels (13.2 vs. 13.9 g/dl, P<0.01).
No patients had experienced hematological toxicity of grade 3 or
above after 7 days of sunitinib treatment.
 | Table II.Effect of sunitinib, RT and combined
sunitinib + RT on circulating blood cell populations using complete
blood count. |
Table II.
Effect of sunitinib, RT and combined
sunitinib + RT on circulating blood cell populations using complete
blood count.
| Cell type | Baseline | Sunitinib alone
(day 8) | Sunitinib+RT (day
19) | RT alone
(control) |
|---|
| Leukocytes |
|
| Count,
×109/l | 6.72
(5.10–8.40) | 5.88
(4.20–7.10) | 4.03
(2.15–6.25) | 6.9
(4.74–8.36) |
|
P-value | – | 0.02 | <0.01 | 0.76 |
| Hemoglobin |
|
| Level,
g/dl | 13.2
(12.2–14.2) | 13.9
(12.5–15.5) | 13.2
(11.9–14.6) | 13.0
(11.4–14.2) |
|
P-value | – | <0.01 | 1.0 | 0.74 |
| Platelets |
|
| Count,
×109/l | 196.2
(131.5–260.0) | 192.9
(145.0–249.0) | 145.9
(90.0–192.0) | 174.3
(114.2–201.1) |
|
P-value | – | 0.71 | <0.01 | 0.20 |
| Neutrophils |
|
| Count,
×109/l | 4.91
(3.40–6.30) | 4.01
(2.55–5.55) | 2.99
(1.45–5.05) | 5.60
(3.67–7.64) |
|
P-value | – | <0.01 | <0.01 | 0.36 |
| Monocytes |
|
| Count,
×109/l | 0.50
(0.35–0.60) | 0.35
(0.25–0.40) | 0.31
(0.20–0.40) | 0.48
(0.39–0.54) |
|
P-value | – | <0.01 | <0.01 | 0.60 |
| Lymphocytes |
|
| Count,
×109/l | 1.15
(0.70–1.35) | 1.33
(0.60–1.90) | 0.65
(0.30–0.90) | 0.70
(0.35–0.94) |
|
P-value | – | 0.07 | <0.01 | <0.01 |
Effect of radiation alone on
hematopoiesis
There were no statistically significant differences
in baseline blood counts when comparing the radiation alone and
combined sunitinib and radiation cohorts. Following radiation
therapy alone, the only detectable difference was a 39% decrease in
the mean lymphocyte count compared with baseline
(1.15×109 vs. 0.70×109/l, P<0.01). Grade
2–4 lymphopenia was observed in 76% of patients; this comprised 19%
with grade 2, 52% with grade 3 and 5% with grade 4.
Effect of combined sunitinib and
radiation on erythroid, lymphoid and myeloid lineages
Hematological measurements on day 19 demonstrated
significant changes compared to pretreatment levels (Fig. 1 and Table
II). On average, there was a 40% reduction in total leukocytes
(6.72×109 vs. 4.03×109/l, P<0.01), which
included a 38% decrease in monocytes (0.50×109 vs.
0.31×109/l, P<0.01), 43% reduction in lymphocytes
(1.15×109 vs. 0.65×109/l, P<0.01) and 39%
decline in neutrophils (4.91×109 vs.
2.99×109/l, P<0.01). There was also a 26% reduction
in the mean platelet count (196.2×109 vs.
145.9×109/l, P=0.006).
Compared to sunitinib alone, combined sunitinib and
radiation demonstrated a further decrease in mean leukocyte
(P<0.01), platelet (P<0.01), neutrophil (P=0.04) and
lymphocyte counts (P<0.01), but no significant change in mean
hemoglobin level (P=0.07) or monocyte count (P=0.09). The incidence
rates of grade 3–4 leukopenia, neutropenia, thrombocytopenia and
lymphopenia were 19, 5, 10 and 57%, respectively.
Compared to radiation alone, patients treated with
concurrent sunitinib and radiation exhibited a 40% decrease in mean
leukocyte counts (P=0.01) and a 26% reduction in mean platelet
counts (P<0.01). The effect on leukocytes was largely due to a
39% decline in mean neutrophil counts (P=0.03) and a 39% reduction
in mean monocyte counts (P<0.01). On average, there was no
effect on lymphocytes (P=0.1) and mild erythrocytosis (P=0.06) that
failed to reach statistical significance.
Potential association between outcome
and decreased absolute monocyte counts following sunitinib and
radiotherapy
In a recent analysis of patients with hepatocellular
carcinoma treated with sunitinib, a greater decrease in monocyte
count after 14 days of treatment significantly predicted
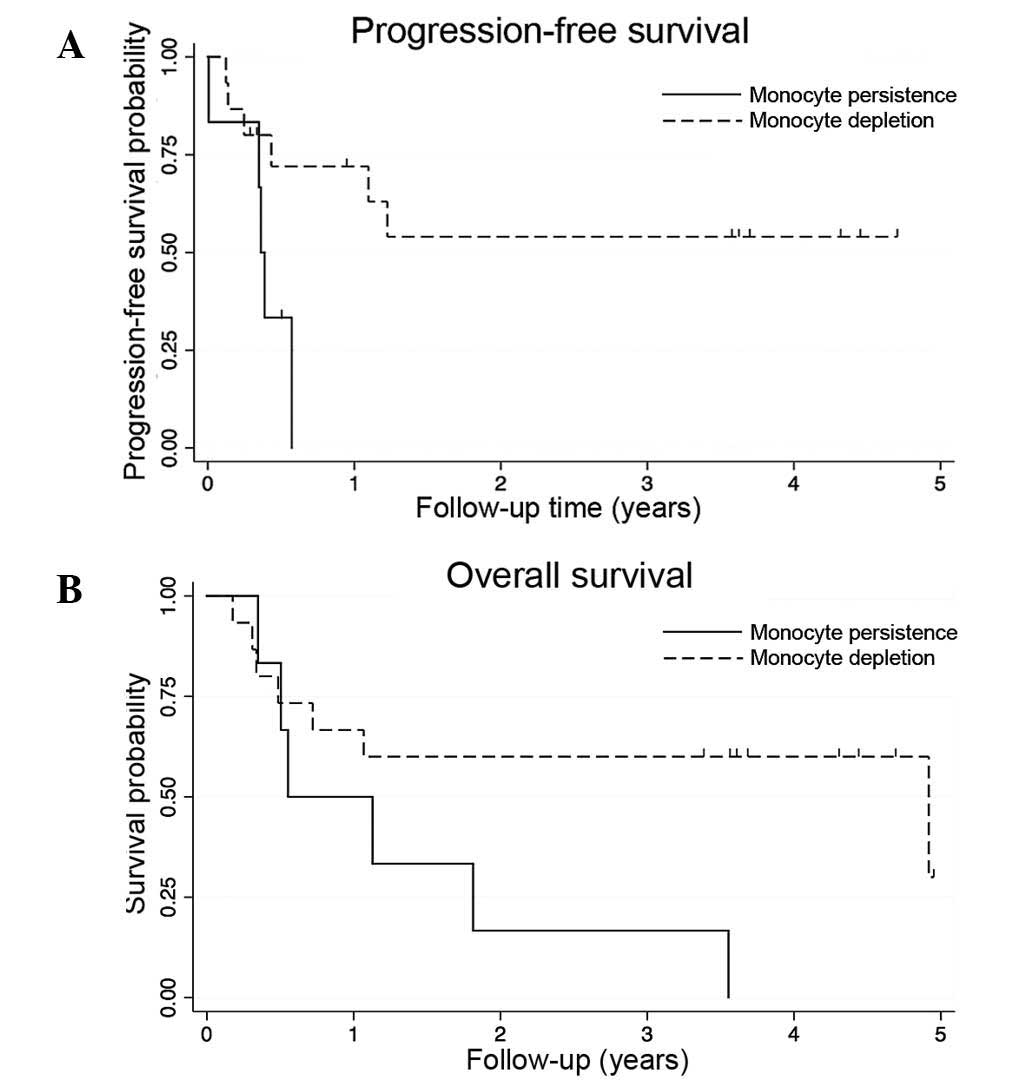
progression-free and overall survival times (16). In the current study, patients with a
greater than average decline (≥200/µl) in absolute monocyte count
following sunitinib and radiation therapy had significantly
improved rates of 2-year progression-free (54% vs. 0%, P=0.03) and
overall survival (60% vs. 17%, P=0.05). Progression-free and
overall survival curves are shown in Fig.
2. Due to limited sample size and the hypothesis-generating
nature of the analysis, multivariable analysis was not
attempted.
Discussion
While conventional cytotoxic chemotherapy has been
limited by a low therapeutic index, poor drug penetration through
tissue, and multi-drug resistance, molecularly targeted therapies
specific for a single signal transduction pathway are hindered by
the genetic heterogeneity within tumors and the multiple genetic
abnormalities associated with solid tumors (17–19).
Broader spectrum multi-targeted tyrosine kinase inhibitors, such as
sunitinib, offer a promising approach towards overcoming some of
these barriers to achieving a cure, by targeting multiple pathways
(6,20). One clear disadvantage of relatively
non-specific inhibitors is the toxicity resulting from ‘off target’
effects of multi-targeted agents (21). Sunitinib has been associated with
decreased leukocytes, lymphocytes, hemoglobin, monocytes,
platelets, neutrophils and increased median corpuscular volume
(16,22–24).
Clinicians are increasingly adopting more specific tyrosine kinase
inhibitors that have lower rates of hematological toxicity
(25).
The observed decrease in hematopoiesis following
sunitinib and radiation therapy in the current study provides the
clinician insight on changes in immunity. Consistent with this
notion, a rapid decrease in myeloid cells following sunitinib has
been associated with improved survival (16). An emerging body of research suggests
that sunitinib mediates antitumor immunity through bone
marrow-derived cells (8,13). For instance, flow cytometric
characterization of changes before and after sunitinib
administration suggest that the number of immunosuppressive
monocytic MDSCs and T regulatory cells decreases, while myeloid
dendritic cells increase (13,24).
In contrast to the immunosuppressive effects of
total body radiation, recent data suggests that local tumor
radiotherapy can remodel and enhance antitumor immunity (26). While wide-field radiotherapy has been
demonstrated to reduce the number of neutrophils, platelets and
lymphocytes, localized radiotherapy for metastases appears to
selectively deplete lymphocytes (27). To the best of our knowledge, the
current report is the first to evaluate the effect of a
biologically targeted agent on hematopoiesis in patients receiving
radiotherapy. In this study, the combination of sunitinib and
radiotherapy resulted in a decrease of mean platelet, myeloid and
lymphoid blood counts, with relative sparing of red blood cells.
Flow cytometry demonstrated that combined sunitinib and radiation
depleted monocytic MDSCs, T regulatory and B cells compared with
radiation alone (13). Further
studies should explore changes in plasma biomarkers contributing to
these observed changes in circulating cells.
The further development of sunitinib for
applications beyond renal cell carcinoma, pancreatic neuroendocrine
tumors and gastrointestinal stromal tumors would be enhanced by the
development of predictive biomarkers (28). Among circulating hematopoietic cells,
sunitinib selectively depletes CD14-positive monocytes that
co-express VEGF-1 or C-X-C chemokine receptor type 4 (29). More recent immune profiling suggests
that sunitinib selectively targets CD33+
CD14+ CD16+ monocytic MDSCs and
CD15+CD14− neutrophilic MDSCs (13,30). The
present results confirm the observation that a greater reduction in
monocytes following sunitinib therapy is associated with higher
progression-free and overall survival times in patients with
advanced malignancy (16). While
further confirmatory studies are required, this could serve as an
early biomarker of sunitinib efficacy.
Although the present study provides novel
observations, it has numerous limitations. Firstly, the sample size
was extremely limited and the patient population was highly
heterogeneous. Therefore, the potential association between
monocyte depletion and improved outcome should be considered as
hypothesis-generating. Secondly, observations of selective effects
on circulating cells in peripheral blood may not necessarily
reflect biology in the tumor bed (30). Arguably, this problem may be less
relevant when all areas of gross disease are ablated with surgery
or radiation. Finally, the clinical relevance of combined sunitinib
and radiation has decreased as interest in combining angiogenesis
inhibitors with radiotherapy has waned (31). However, lessons learned from the
experience of combining radiotherapy with novel agents, including
sunitinib, may inform future studies combining radiotherapy with
immunotherapy for advanced cancer (4,32).
In conclusion, the present study reports changes in
hematopoiesis following sunitinib and radiotherapy that supplement
previously reported flow cytometric data.
Acknowledgements
This study was supported in part by research funding
from Pfizer Inc. (New York, NY, USA) awarded to Dr Johnny Kao. Dr
Johnny Kao is a William Harris Research Fellow.
References
|
1
|
Salama JK, Hasselle MD, Chmura SJ, Malik
R, Mehta N, Yenice KM, Villaflor VM, Stadler WM, Hoffman PC, Cohen
EE, et al: Stereotactic body radiotherapy for multisite
extracranial oligometastases: Final report of a dose escalation
trial in patients with 1 to 5 sites of metastatic disease. Cancer.
118:2962–2970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Twyman-Saints Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck
M, Sung M, Schwartz M, Divino CM, Pan PY and Chen SH: The novel
role of tyrosine kinase inhibitor in the reversal of immune
suppression and modulation of tumor microenvironment for
immune-based cancer therapies. Cancer Res. 69:2514–2522. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karaman MW, Herrgard S, Treiber DK,
Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI,
Edeen PT, et al: A quantitative analysis of kinase inhibitor
selectivity. Nat Biotechnol. 26:127–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faivre S, Demetri G, Sargent W and Raymond
E: Molecular basis for sunitinib efficacy and future clinical
development. Nat Rev Drug Discov. 6:734–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko JS, Zea AH, Rini BI, Ireland JL, Elson
P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al:
Sunitinib mediates reversal of myeloid-derived suppressor cell
accumulation in renal cell carcinoma patients. Clin Cancer Res.
15:2148–2157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schueneman AJ, Himmelfarb E, Geng L, Tan
J, Donnelly E, Mendel D, McMahon G and Hallahan DE: SU11248
maintenance therapy prevents tumor regrowth after fractionated
irradiation of murine tumor models. Cancer Res. 63:4009–4016.
2003.PubMed/NCBI
|
|
10
|
Kao J, Packer S, Vu HL, Schwartz ME, Sung
MW, Stock RG, Lo YC, Huang D, Chen SH and Cesaretti JA: Phase 1
study of concurrent sunitinib and image-guided radiotherapy
followed by maintenance sunitinib for patients with
oligometastases: Acute toxicity and preliminary response. Cancer.
115:3571–3580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong CC, Ko EC, Sung MW, Cesaretti JA,
Stock RG, Packer SH, Forsythe K, Genden EM, Schwartz M, Lau KH, et
al: Phase II trial of concurrent sunitinib and image-guided
radiotherapy for oligometastases. PLoS One. 7:e369792012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kao J, Chen CT, Tong CC, Packer SH,
Schwartz M, Chen SH and Sung MW: Concurrent sunitinib and
stereotactic body radiotherapy for patients with oligometastases:
Final report of a prospective clinical trial. Target Oncol.
9:145–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen HM, Ma G, Gildener-Leapman N,
Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M,
Sung M, et al: Myeloid derived suppressor cells as an immune
parameter in patients with concurrent sunitinib and stereotactic
body radiotherapy. Clin Cancer Res. 21:4073–4085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chin-Yee IH, Keeney M, Johnson K, Brown W,
Wolfe N and Kaplan S: White blood cell flagging rates of the
Coulter LH 750 analyzer compared with the Coulter Gen.s Hematology
Analyzer. Laboratory Hematology. 7:211–216. 2001.
|
|
15
|
Ellis RE: The distribution of active bone
marrow in the adult. Phys Med Biol. 5:255–258. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu AX, Duda DG, Ancukiewicz M, di Tomaso
E, Clark JW, Miksad R, Fuchs CS, Ryan DP and Jain RK: Exploratory
analysis of early toxicity of sunitinib in advanced hepatocellular
carcinoma patients: Kinetics and potential biomarker value. Clin
Cancer Res. 17:918–927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez-Garcia I, Solé RV and Costa J:
Metapopulation dynamics and spatial heterogeneity in cancer. Proc
Natl Acad Sci USA. 99:13085–13089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tannock IF: Conventional cancer therapy:
Promise broken or promise delayed? Lancet. 351(Suppl 2): SII9–SI16.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morabito A, De Maio E, Di Maio M, Normanno
N and Perrone F: Tyrosine kinase inhibitors of vascular endothelial
growth factor receptors in clinical trials: Current status and
future directions. Oncologist. 11:753–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Norden-Zfoni A, Desai J, Manola J, Beaudry
P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, et al:
Blood-based biomarkers of SU11248 activity and clinical outcome in
patients with metastatic imatinib-resistant gastrointestinal
stromal tumor. Clin Cancer Res. 13:2643–2650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rini BI, Choueiri TK, Elson P, Khasawneh
MK, Cotta C, Unnithan J, Wood L, Mekhail T, Garcia J, Dreicer R and
Bukowski RM: Sunitinib-induced macrocytosis in patients with
metastatic renal cell carcinoma. Cancer. 113:1309–1314. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Cruijsen H, van der Veldt AA, Vroling
L, Oosterhoff D, Broxterman HJ, Scheper RJ, Giaccone G, Haanen JB,
van den Eertwegh AJ, Boven E, et al: Sunitinib-induced myeloid
lineage redistribution in renal cell cancer patients: CD1c+
dendritic cell frequency predicts progression-free survival. Clin
Cancer Res. 14:5884–5892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Formenti SC and Demaria S: Combining
radiotherapy and cancer immunotherapy: A paradigm shift. J Natl
Cancer Inst. 105:256–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mac Manus M, Lamborn K, Khan W, Varghese
A, Graef L and Knox S: Radiotherapy-associated neutropenia and
thrombocytopenia: Analysis of risk factors and development of a
predictive model. Blood. 89:2303–2310. 1997.PubMed/NCBI
|
|
28
|
Leighl NB: Sunitinib: The next advance in
small-cell lung cancer? J Clin Oncol. 33:1637–1639. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zurita AJ, Khajavi M, Wu HK, Tye L, Huang
X, Kulke MH, Lenz HJ, Meropol NJ, Carley W, DePrimo SE, et al:
Circulating cytokines and monocyte subpopulations as biomarkers of
outcome and biological activity in sunitinib-treated patients with
advanced neuroendocrine tumours. Br J Cancer. 112:1199–1205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ko JS, Rayman P, Ireland J, Swaidani S, Li
G, Bunting KD, Rini B, Finke JH and Cohen PA: Direct and
differential suppression of myeloid-derived suppressor cell subsets
by sunitinib is compartmentally constrained. Cancer Res.
70:3526–3536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: Ipilimumab versus placebo after radiotherapy in
patients with metastatic castration-resistant prostate cancer that
had progressed after docetaxel chemotherapy (CA184-043): A
multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.
15:700–712. 2014. View Article : Google Scholar : PubMed/NCBI
|