Introduction
Acute myeloid leukemia (AML) is a genetically
heterogeneous disease with accumulation of acquired genetic
alterations in hematopoietic progenitor cells that disturb the
normal mechanisms of cell growth, proliferation and differentiation
(1). In recent years, there has been
an increased understanding of the role of angiogenesis in the
progression of AML (2). Although
circulating endothelial cells (CECs) and their progenitors,
endothelial progenitor cells (EPCs), were first described >30
years ago through light microscopy, the development of specific
monoclonal antibodies against these cells has only recently
provided the opportunity to investigate the pathophysiology of
these cells (3). Previously, the
immune-beads technique and/or flow cytometry were used to
investigate the significance of CECs in a variety of diseases,
including infections as well as cardiovascular, inflammatory and
autoimmune syndromes, and cancer (4).
These rare CECs (which encompass <1 cell in 1,000 circulating
blood cells) are probably derived from vessel wall turnover and are
quite stable overtime (5). The
majority of these cells exhibit characteristics of mature,
terminally differentiated cells (5).
EPCs are a sub-population of CECs that express antigens suggestive
of a stem-like or progenitor-like phenotype (6). These putative EPCs may migrate to sites
of vasculogenesis and angiogenesis, and may participate in new
blood vessel formation by stimulating vasculogenesis (7). The lack of a universal definition, a
unified phenotypic characterization and standardized methods of
detection makes comparisons very difficult, and between-studies
interpretations of EPCs should be analyzed cautiously (5).
Multiple studies have focused on CECs as a
non-invasive angiogenesis marker and their role as predictors of
the clinical response in cancer patients receiving both
antiangiogenic and standard chemotherapy for breast, lung and
hepatocellular cancer, among others (8). Furthermore, the levels of CECs have been
correlated to tumor stage and prognosis (5–16).
Notably, CECs and EPCs may be a valuable tool for
prediction of graft-versus-host disease in allo-transplantation, as
confirmed by Almici et al (9).
Furthermore, their kinetics may be helpful in monitoring the
mobilization of hematological cells prior to transplantation
(10); however, their kinetics
differs by the use of different chemotherapy agents (11). Additionally, CECs and EPCs could be
used as a novel marker for minimal residual disease in chronic
myeloid leukemia, as reported by Wu et al (12). Wierzbowska et al suggested that
endothelial cells may enhance the survival and proliferation of
leukemic blasts and mediate chemotherapy resistance in AML
(13).
The aim of the present study was to quantify CECs
and their progenitors EPCs in patients with AML by flow cytometry
at initial diagnosis and after induction chemotherapy, and to
correlate these findings with the patients' response to
treatment.
Patients and methods
Patients
The present study is a retrospective case-control
study including 40 patients with de novo AML, who presented
to the Hematology/Oncology Clinic of South Egypt Cancer Institute
(SECI), Assiut University (Assiut, Egypt) between May 2014 and
October 2015, and 20 healthy controls. The study was approved by
the Institutional Review Board of SECI. Written informed consent
was obtained from all cases and controls.
All patients were subjected to: i) A thorough
history evaluation and clinical examination, with careful
assessment of clinical signs relevant to leukemia, including
hepatomegaly, splenomegaly, lymphadenopathy, gums or skin
infiltration; ii) a complete blood test, which was performed using
fully automated blood counters and peripheral smear examinations;
iii) a bone marrow examination and cytochemistry, including
detection of myeloperoxidase, esterases, acid phosphatase and
periodic acid-Schiff; and iv) immunophenotyping using mouse
monoclonal antibodies for diagnosing AML at a 1:1,000 dilution,
including anti-cluster of differentiation (CD)34 (cat. no. 340430;
BD Biosciences, Franklin Lakes, NJ, USA), anti-CD13 (cat. no.
555394; BD Biosciences), anti-CD33 (cat. no. 340680; BD
Biosciences), anti-CD117 (cat. no. 555714; BD Biosciences),
anti-CD15 (cat. no. 332778; BD Biosciences), anti-intracellular
myeloperoxidase (cat. no. 340580; BD Biosciences), anti-CD14 (cat.
no. 550787; BD Biosciences), anti-human leukocyte antigen-antigen D
related (cat. no. 560896; BD Biosciences), anti-CD41 (cat. no.
555466; BD Biosciences), anti-CD61 (cat. no. 555753; BD
Biosciences) and anti-glycophorin A (cat. no. 340947; BD
Biosciences). Blood samples from the patients and the control group
were subjected to assessment of EPCs and CECs using flow
cytometry.
Remission induction regimens
All AML patients other than those with M3 disease
received the 3+7 protocol, which is a combination of intravenous
(IV) chemotherapy that includes 7 days of cytarabine (100
mg/m2/day by continuous infusion) and 3 days of
adriamycin (45 mg/m2, IV). AML M3 patients received the
European acute promyelocytic leukemia regimen (14), which consisted of adriamycin (45
mg/m2/day, IV) as 15–30 min infusion for 3 days (since
other anthracyclines were not available) plus cytarabine (200
mg/m2/day, IV) as 24-h continuous infusion on days 1–7,
plus all trans-retinoic acid (ATRA; 45 mg/m2/day, or 25
mg/m2/day for patients <20 years old) in 2 divided
doses, starting on day 1. The response to treatment was defined
according to the revised recommendations of the International
Working Group for Diagnosis, Standardization of Response Criteria,
Treatment Outcomes and Reporting Standards for Therapeutic Trials
in Acute Myeloid Leukemia (15).
Flow cytometric detection of CECs and
EPCs
Venous blood samples (2 ml) were collected at the
time of clinical assessment in pyrogen-free
ethylenediaminetetraacetic acid tubes. CECs and EPCs were evaluated
using a panel of mouse anti-human monoclonal antibodies at a
1:1,000 dilution: Fluorescein isothiocyanate (FITC)-labeled
anti-CD144 (cat. no. 560874; BD Biosciences), phycoerythrin
(PE)-conjugated anti-CD133 (cat. no. 130080801; Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany), peridinin chlorophyll protein
(PerCP)-conjugated anti-CD34 (cat. no. 555842; BD Biosciences) and
allophycocyanin (APC)-conjugated anti-CD45 antibodies (cat. no.
555485; BD Biosciences). Briefly, blood samples (50 µl) were
incubated with 5 µl anti-CD144, anti-CD133, anti-CD34 and anti-CD45
antibodies for 15 min at room temperature in the dark.
Subsequently, red blood cells were lysed, and resuspended in
phosphate-buffered saline. Flow cytometric analysis was performed
using a FACSCalibur flow cytometer with CellQuest Pro software (BD
Biosciences). In total, 50,000 events were analyzed, and FITC-,
PE-, PerCP-, and APC-conjugated mouse anti-human immunoglobulin G
(cat. nos. 556649, 554680, 349044, and 550854, respectively;
1:1,000 dilution; BD Biosciences) were used as isotype-matched
negative controls for each sample. The gating strategy to detect
CECs and EPCs was based on CD45 staining to exclude hematopoietic
cells. CECs were identified as cells lacking CD45 expression, which
were positive for CD144 and CD34, and negative for CD133
(CD45-/CD144+/CD34+/CD133-), while EPCs were identified as cells
that were negative for CD45, and positive for CD144, CD34 and CD133
(CD45-/CD34+CD144+/CD133+) (Fig. 1).
The number of CECs and EPCs were expressed as per 50,000 cells.
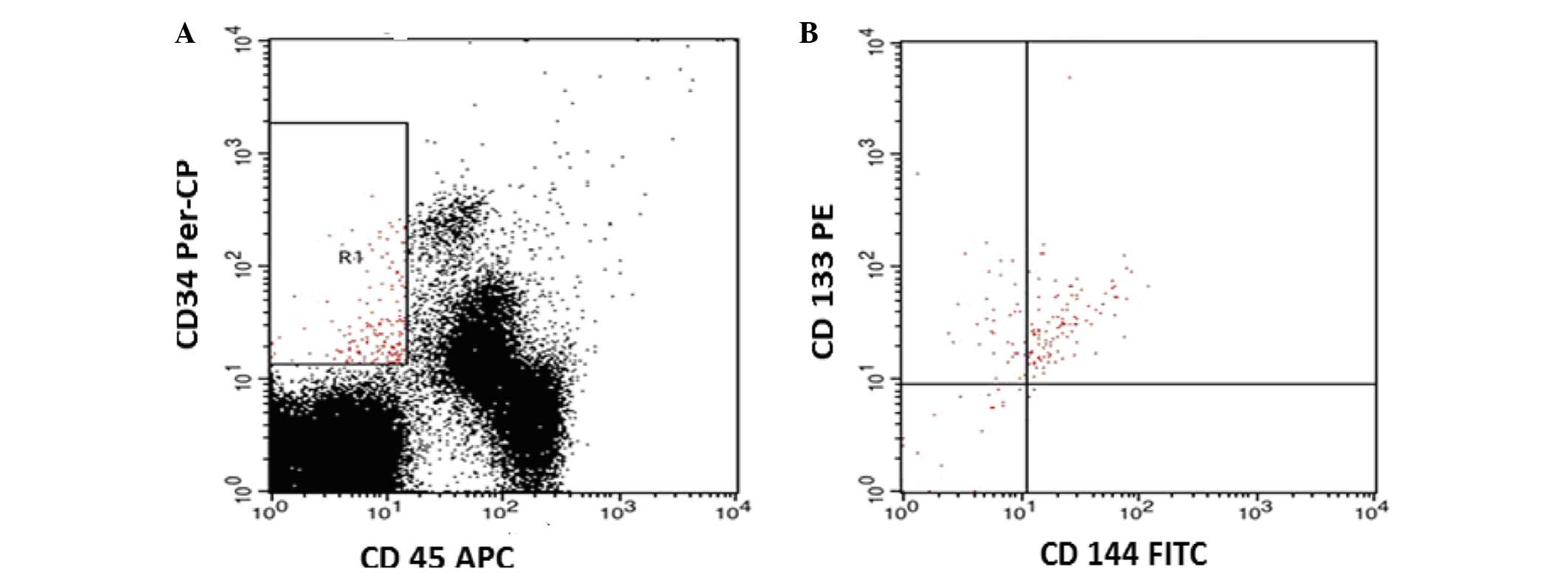 | Figure 1.Flow cytometric detection of CECs and
EPCs. (A) The analysis gate (R1) included CD34+ CD45- cells. (B)
The expression of CD144 and CD133 in the R1 gate was detected
compared with the negative isotype control (data not shown). CECs
were defined as CD45-, CD34+, CD144+ and CD133- cells, while CEPs
were identified as CD45-, CD34+, CD144+ and CD133+ cells. CECs,
circulating endothelial cells; EPCs, endothelial progenitor cells;
CD, cluster of differentiation; PerCP, peridinin chlorophyll
protein; APC, allophycocyanin; PE, phycoerythrin; FITC, fluorescein
isothiocyanate. |
Statistical analysis
Data analysis was performed with SPSS version 16
software (SPSS, Inc., Chicago, IL, USA). The statistical
differences between the groups were examined using the Mann-Whitney
U test and the Wilcoxon signed-rank test, while the t-test and the
χ2 test were used for analysis of continuous and
categorical parameters. Due to the relatively small sample size and
the requirement to indicate the uncertainty around the estimate of
the mean, the standard error (SE) was calculated as follows:
SE=SD/√N, where N is the sample size and SD is the standard
deviation. P≤0.05 was considered to indicate a statistically
significant difference. The Spearman's rank correlation coefficient
was used to examine the correlations among the different studied
parameters.
Results
A total of 40 adult AML patients were included in
the present study. The sociodemographic and laboratory
characteristics of the AML patients and the controls are shown in
Table I. There was no significant
difference in the mean age or gender percentages. In total, 30% of
the patients had M2, while 22% had M4 and 22% had M3 disease.
 | Table I.Comparative analysis between patients
with acute myeloid leukemia and controls regarding sociodemographic
characteristics and several laboratory parameters. |
Table I.
Comparative analysis between patients
with acute myeloid leukemia and controls regarding sociodemographic
characteristics and several laboratory parameters.
| Parameter | Patients (n=40) | Controls (n=20) | P-value |
|---|
| Age, years
(range) | 54 (23–68) | 48 (24–56) |
0.681a |
| Gender
(male/female) | 24/16 | 13/7 |
0.091b |
| WBCs (109
cells/l) | 45.32±3.47 | 6.62±0.41 |
<0.001c |
| Platelets
(109 cells/l) | 49.39±4.83 | 231.25±15.70 |
<0.001c |
| Hemoglobin
(g/dl) | 7.49±0.25 | 12.99±0.18 |
<0.001c |
At diagnosis (baseline levels), CECs and EPCs were
significantly higher in AML patients than in the controls.
Regarding CECs and EPCs kinetics after induction chemotherapy, the
levels of these cells were significantly decreased in AML patients
compared with their levels at diagnosis, but the levels were still
significantly higher than those in the controls (Table II). After induction chemotherapy, 28
patients (70%) achieved complete response (CR) to treatment, while
12 (30%) did not achieve CR. The mean baseline levels of CECs and
EPCs in AML patients who achieved CR were significantly lower than
in those who did not achieve CR in response to induction treatment.
In addition, the levels of CECs and EPCs after induction
chemotherapy in AML patients were significantly lower in those
patients who achieved CR than in those who did not achieve CR
(Table III). There were significant
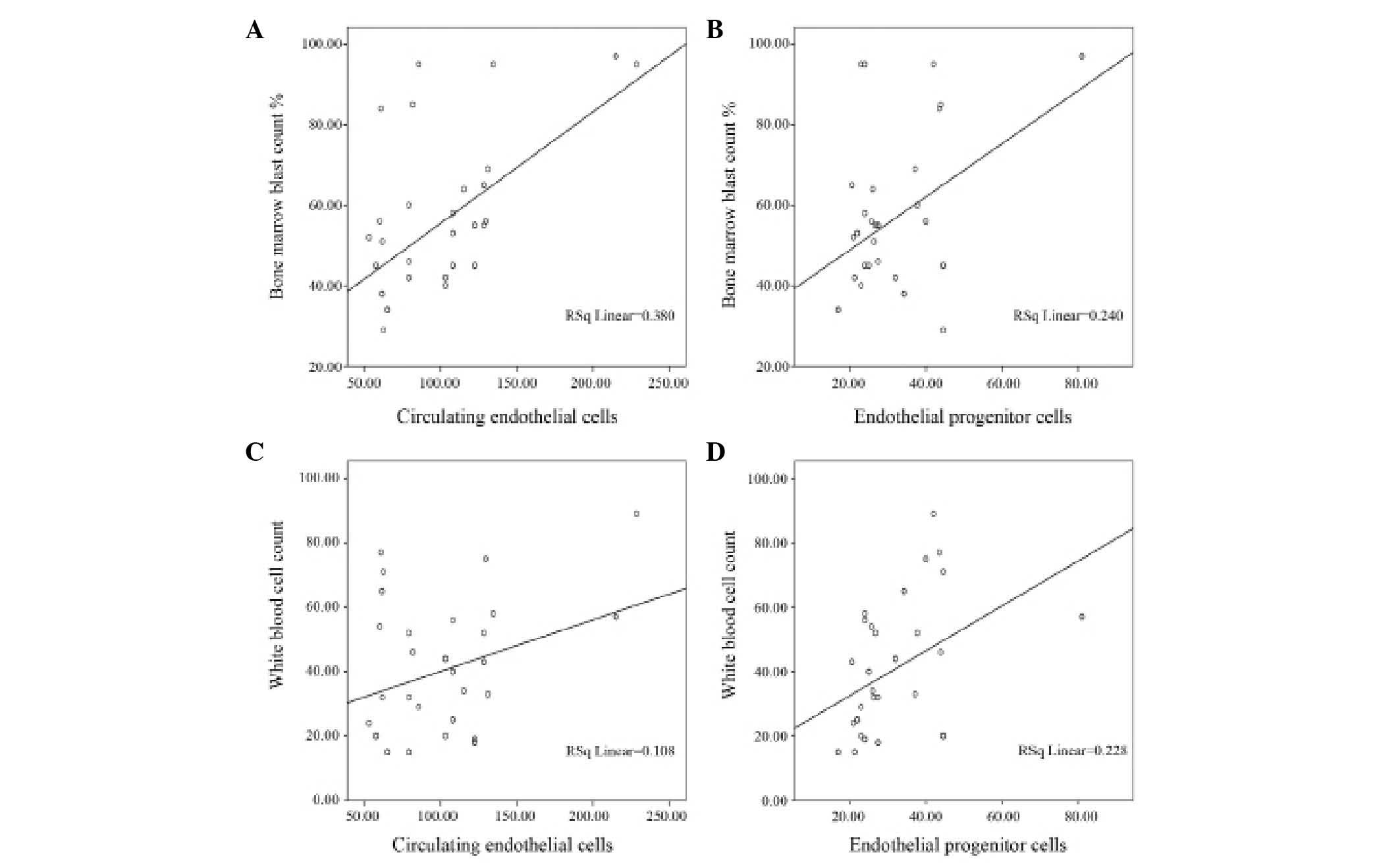
positive correlations between total leukocyte count and bone marrow
blast count with both CECs and EPCs (Fig.
2). There was no significant correlation between CEC or EPC
levels and hemoglobin levels; however, CECs were correlated with
platelet counts (Table IV). No
correlation of CECs or EPCs with patients' age, gender or
French-American-British (FAB) classification were observed.
 | Table II.CECs and EPCs in AML patients at
presentation and after induction chemotherapy vs. controls. |
Table II.
CECs and EPCs in AML patients at
presentation and after induction chemotherapy vs. controls.
| Characteristics | AML patients at
presentation (n=40) | AML patients after
induction chemotherapy (n=40) | Controls (n=20) | aP-value | bP-value | cP-value |
|---|
| CECs | 102.64±6.14 |
83.18±3.47 | 24.09±1.78 | <0.001 | <0.001 | <0.001 |
| EPCs | 32.64±1.87 | 26.67±1.18 | 4.86±0.34 | <0.001 | <0.001 | <0.001 |
 | Table III.CECs and EPCs in acute myeloid
leukemia patients at presentation and after induction chemotherapy,
and their correlation with treatment response. |
Table III.
CECs and EPCs in acute myeloid
leukemia patients at presentation and after induction chemotherapy,
and their correlation with treatment response.
| Endothelial
cells | Patients who achieved
CR (n=28) | Patients who did not
achieved CR (n=12) | P-valuea |
|---|
| CECs at
presentation | 92.66±5.72 | 125.92±14.71 | 0.011 |
| EPCs at
presentation | 29.85±1.72 | 39.15±4.32 | 0.021 |
| CECs after induction
chemotherapy | 75.13±4.58 | 93.16±5.01 | 0.005 |
| EPCs after induction
chemotherapy | 21.43±1.55 | 32.53±3.16 | 0.001 |
 | Table IV.Spearman's correlation between CECs
and EPCs in acute myeloid leukemia patients at presentation and the
investigated parameters. |
Table IV.
Spearman's correlation between CECs
and EPCs in acute myeloid leukemia patients at presentation and the
investigated parameters.
| Parameter | WBCs
(×109/l) | BM blasts (%) | Hemoglobin level
(g/dl) | Platelet count
(×109/l) |
|---|
| CECs |
|
|
|
|
| r
(correlation coefficient) | 0.66 | 0.62 | 0.05 | 0.36 |
|
P-value | 0.001 | 0.001 | 0.76 | 0.022 |
| EPCs |
|
|
|
|
| r
(correlation coefficient) | 0.49 | 0.46 | 0.28 | 0.096 |
|
P-value | 0.002 | 0.003 | 0.08 | 0.556 |
Discussion
AML is a hematological malignancy of the bone marrow
characterized by a mutation in hematopoietic stem or progenitor
cells, which develops into a highly proliferative accumulation of
dysfunctional and immature myeloid cells (2). When interpreting studies on CECs and
EPCs, attention must be paid to the different definitions and
phenotypic characterization of CECs in each study, due to the
lacking of a universal definition of these cell population
(16), and to the different methods
of CECs and EPCs detection, such as flow cytometry and the
CELLSEARCH® system (16).
The cell population definition used in the present study was based
on the markers most widely accepted in flow cytometric analysis. It
is widely accepted that CD45 expression can be used to exclude
haematopoietic cells from the analysis, while endothelial cells are
identified by the expression of CD146, which is an
endothelial-specific marker, and CD31 (17). Furthermore, at the present time, the
sole antigen that appears to be expressed in EPCs and subsequently
downregulated in mature CECs is CD133 (3).
In the current study, both CECs and EPCs were higher
in AML patients than in the control group, both at diagnosis and
after induction chemotherapy, which may indicate that angiogenesis
may have a role in the maintenance of AML. This may indicate that
widespread vascular damage and disruption occur in the endothelium
of AML patients. The EPCs may increase to allow reconstitution of
the endothelial layer and to maintain re-endothelialization and
vascular repair. There was no correlation of CECs or EPCs with
patients' age, gender or FAB classification, which may indicate
that angiogenesis is a common feature in all subtypes of AML. The
positive correlations between CECs and EPCs with both total
leukocytes count and bone marrow blast count may indicate that the
levels of CECs and EPCs are correlated with the tumor mass. These
findings are in accordance with those by Wierzbowska et al,
who observed that the levels of CECs and EPCs were significantly
higher in AML patients than in the control group by a 17- and
18-fold, respectively (13). This is
in concordance with previous studies in multiple myeloma (MM),
metastatic carcinoma, myelodysplastic syndrome, gastrointestinal
stromal disease (GIST), hepatocellular carcinoma, breast cancer,
non-Hodgkin's lymphoma (NHL), myelofibrosis and chronic lymphocytic
leukemia (5–17).
In the present study, the CEC and EPC levels
decreased after induction chemotherapy compared with
pre-chemotherapy levels in AML patients, but the levels were still
higher than those in the controls. This reduction in CEC and EPC
levels may support the clinical relevance of these cells in
reflecting the tumor mass. The higher levels of CECs and EPCs in
AML patients after induction chemotherapy compared with those in
the controls may be due to the fact that these cells were measured
precisely at the time of very active bone marrow recovery, as they
were counted at the day of bone marrow aspirate performed for the
evaluation of response, usually 3–4 weeks after chemotherapy. The
EPCs may mobilize from the bone marrow with hematopoietic cells,
and these elevated mobilized EPCs possibly matured to CECs. The
lower CEC and EPC levels in patients who achieved CR compared with
patients who did not achieve CR either at presentation or after
treatment indicate that CEC and EPC levels may be used to detect
treatment response, and they could be used to reflect the level of
minimal residual disease in AML. There is evidence that EPCs are
mobilized from the bone marrow simultaneously with hematopoietic
progenitor cells (18). Endothelial
cells may enhance the survival and proliferation of leukemic blasts
and may mediate chemotherapy resistance in hematological disease,
at least at the preclinical level (19,20).
The present results are in concordance with other
studies. In a study of imatinib-resistant GIST patients, the
authors detected changes in CECs, which differed between patients
with clinical benefit and those with progressive disease (21). Another study of low-dose
cyclophosphamide administered continuously in combination with
celecoxib in adult patients with relapsed or refractory aggressive
NHL demonstrated that CECs and EPCs declined and remained low in
responders (22). Similarly, CEC and
EPCs were observed to be correlated with disease activity (serum M
protein and β2 microglobulin) and with response to thalidomide
therapy in MM (23). Conversely,
CECs/EPCs did not prove to be useful pharmacodynamic biomarkers
(24). In a phase I study of
enzastaurin (a protein kinase Cβ inhibitor) administered in
combination with gemcitabine and cisplatin to patients with
advanced tumors, the single agent enzastaurin had no effect on any
of the angiogenesis biomarkers analyzed (CECs and messenger RNA
expression of CD133 and CD146 in peripheral blood) (25).
In conclusion, the present study revealed that CEC
levels are higher in AML and correlate with disease status and
response to treatment. Further investigation should be undertaken
to better determine their predictive value and implication in AML
management.
References
|
1
|
Fröhling S, Scholl C, Gilliland DG and
Levine RL: Genetics of myeloid malignancies: Pathogenetic and
clinical implications. J Clin Oncol. 23:6285–6295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trujillo A, McGee C and Cogle CR:
Angiogenesis in acute myeloid leukemia and opportunities for novel
therapies. J Oncol. 2012:1286082012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
George F, Brisson C, Poncelet P, Laurent
JC, Massot O, Arnoux D, Ambrosi P, Klein-Soyer C, Cazenave JP and
Sampol J: Rapid isolation of human endothelial cells from whole
blood using S-Endo1 monoclonal antibody coupled to immuno-magnetic
beads: Demonstration of endothelial injury after angioplasty.
Thromb Haemost. 67:147–153. 1992.PubMed/NCBI
|
|
4
|
Blann AD, Woywodt A, Bertolini F, Bull TM,
Buyon JP, Clancy RM, Haubitz M, Hebbel RP, Lip GY, Mancuso P, et
al: Circulating endothelial cells. Biomarker of vascular disease.
Thromb Haemost. 93:228–235. 2005.PubMed/NCBI
|
|
5
|
Bertolini F, Shaked Y, Mancuso P and
Kerbel RS: The multifaceted circulating endothelial cell in cancer:
Towards marker and target identification. Nat Rev Cancer.
6:835–845. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fadini GP, Losordo D and Dimmeler S:
Critical reevaluation of endothelial progenitor cell phenotypes for
therapeutic and diagnostic use. Circ Res. 110:624–637. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almici C, Skert C, Verardi R, Di Palma A,
Bianchetti A, Neva A, Braga S, Malagola M, Turra A, Marini M and
Russo D: Changes in circulating endothelial cells count could
become a valuable tool in the diagnostic definition of acute
graft-versus-host disease. Transplantation. 98:706–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szmigielska-Kapłon A, Krawczyńska A,
Czemerska M, Pluta A, Cebula-Obrzut B, Szmigielska K, Smolewski P,
Robak T and Wierzbowska A: Circulating endothelial cell kinetics
and their potential predictive value during mobilization procedure.
J Clin Apher. 28:341–348. 2013.PubMed/NCBI
|
|
11
|
Ali AM, Ueno T, Tanaka S, Takada M,
Ishiguro H, Abdellah AZ and Toi M: Determining circulating
endothelial cells using cellsearch system during preoperative
systemic chemotherapy in breast cancer patients. Eur J Cancer.
47:2265–2272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu JY, Huang L, Zhou JF, Pei RZ, Ma JX,
Zhang PS, Liu XH, Du XH, Chen D, Sha KY, et al: Expression of
BCR/ABL fusion gene in circulating endothelial cells from chronic
myelogenous leukemia patients and its clinical significance.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:927–931. 2014.(In Chinese).
PubMed/NCBI
|
|
13
|
Wierzbowska A, Robak T, Krawczyńska A,
Wrzesień-Kuś A, Pluta A, Cebula B and Smolewski P: Circulating
endothelial cells in patients with acute myeloid leukemia. Eur J
Haematol. 75:492–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanz MA, Grimwade D, Tallman MS, Lowenberg
B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H,
et al: Management of acute promyelocytic leukemia: Recommendations
from an expert panel on behalf of the European LeukemiaNet. Blood.
113:1875–1891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the international working
group for diagnosis, standardization of response criteria,
treatment outcomes and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mancuso P, Antoniotti P, Quarna J, Calleri
A, Rabascio C, Tacchetti C, Braidotti P, Wu HK, Zurita AJ, Saronni
L, et al: Validation of a standardized method for enumerating
circulating endothelial cells and progenitors: Flow cytometry and
molecular and ultrastructural analyses. Clin Cancer Res.
15:267–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chorváth B and Sedlák J: Hematopoietic
cell differentiation antigens (CD system 1997). Cancer research
relevance. Neoplasma. 45:273–276. 1998.PubMed/NCBI
|
|
18
|
Heissig B, Hattori K, Dias S, Friedrich M,
Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et
al: Recruitment of stem and progenitor cells from the bone marrow
niche requires MMP-9 mediated release of kit-ligand. Cell.
109:625–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shain KH, Landowski TH and Dalton WS: The
tumor microenvironment as a determinant of cancer cell survival: A
possible mechanism for de novo drug resistance. Curr Opin Oncol.
12:557–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damiano JS, Cress AE, Hazlehurst LA, Shtil
AA and Dalton WS: Cell adhesion mediated drug resistance (CAM-DR):
Role of integrins and resistance to apoptosis in human myeloma cell
lines. Blood. 93:1658–1667. 1999.PubMed/NCBI
|
|
21
|
Norden-Zfoni A, Desai J, Manola J, Beaudry
P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, et al:
Blood-based biomarkers of SU11248 activity and clinical outcome in
patients with metastatic imatinib-resistant gastrointestinal
stromal tumor. Clin Cancer Res. 13:2643–2650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buckstein R, Kerbel RS, Shaked Y, Nayar R,
Foden C, Turner R, Lee CR, Taylor D, Zhang L, Man S, et al:
High-dose celecoxib and metronomic ‘low-dose’ cyclophosphamide is
an effective and safe therapy in patients with relapsed and
refractory aggressive histology non-Hodgkin's lymphoma. Clin Cancer
Res. 12:5190–5198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Vakil V, Braunstein M, Smith EL,
Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U,
et al: Circulating endothelial progenitor cells in multiple
myeloma: Implications and significance. Blood. 105:3286–3294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown AP, Citrin DE and Camphausen KA:
Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis
Rev. 27:415–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rademaker-Lakhai JM, Beerepoot LV, Mehra
N, Radema SA, van Maanen R, Vermaat JS, Witteveen EO, Visseren-Grul
CM, Musib L, Enas N, et al: Phase I pharmacokinetic and
pharmacodynamic study of the oral protein kinase C beta-inhibitor
enzastaurin in combination with gemcitabine and cisplatin in
patients with advanced cancer. Clin Cancer Res. 13:4474–4481. 2007.
View Article : Google Scholar : PubMed/NCBI
|