Introduction
Gliomatosis peritonei (GP) is a rare condition that
is typically associated with immature and, less commonly, mature
teratomas and ventriculoperitoneal shunting. GP occurs commonly in
young girls and, in rare cases, can be found in males. GP is
characterized as a myriad of peritoneal nodular or miliary implants
composed of benign, mature glia (1).
As GP has an extremely low incidence rate, there is no widely
accepted guidance as to the method by which these patients should
be treated. Approximately 100 cases of GP have been described in
the literature. Since the disease was first described by Neuhaüser
in 1906 (2), it has been
well-documented that GP, when benign, does not adversely affect
patient prognosis. Glial implants associated with the disease may
remain stable for long periods of time, or may completely
disappear. The repeated measurement of tumor markers and imaging
are usually undertaken throughout follow-up. To the best of our
knowledge, only a few cases have been reported that describe the
findings of a radiological analysis (3–5). The
current study presents two cases of GP with bilateral ovarian
teratomas, describing the diagnosis, associated radiological
observations and complete clinical course of each patient.
Case report
Case 1
A 19-year-old woman was referred to The Second
Affiliated Hospital, Zhejiang University School of Medicine
(Hangzhou, China) on January 16, 2011, presenting with a 15-day
history of lower abdominal distension. During a physical
examination of the pelvis and abdomen, a large firm mass was
identified and was palpable extending up to 20 cm to the xiphoid
process. Analysis of serum tumor markers demonstrated an increase
in carcinoembryonic antigen (13.4 ng/ml; normal range, <5
ng/ml), cancer antigen (CA)125 (241.7 units/ml; normal range,
<35 units/ml) and CA19-9 (2,215.6 units/ml; normal range, <37
units/ml), whilst the α-fetoprotein level was normal (1.2 ng/ml;
normal range, <20 ng/ml).
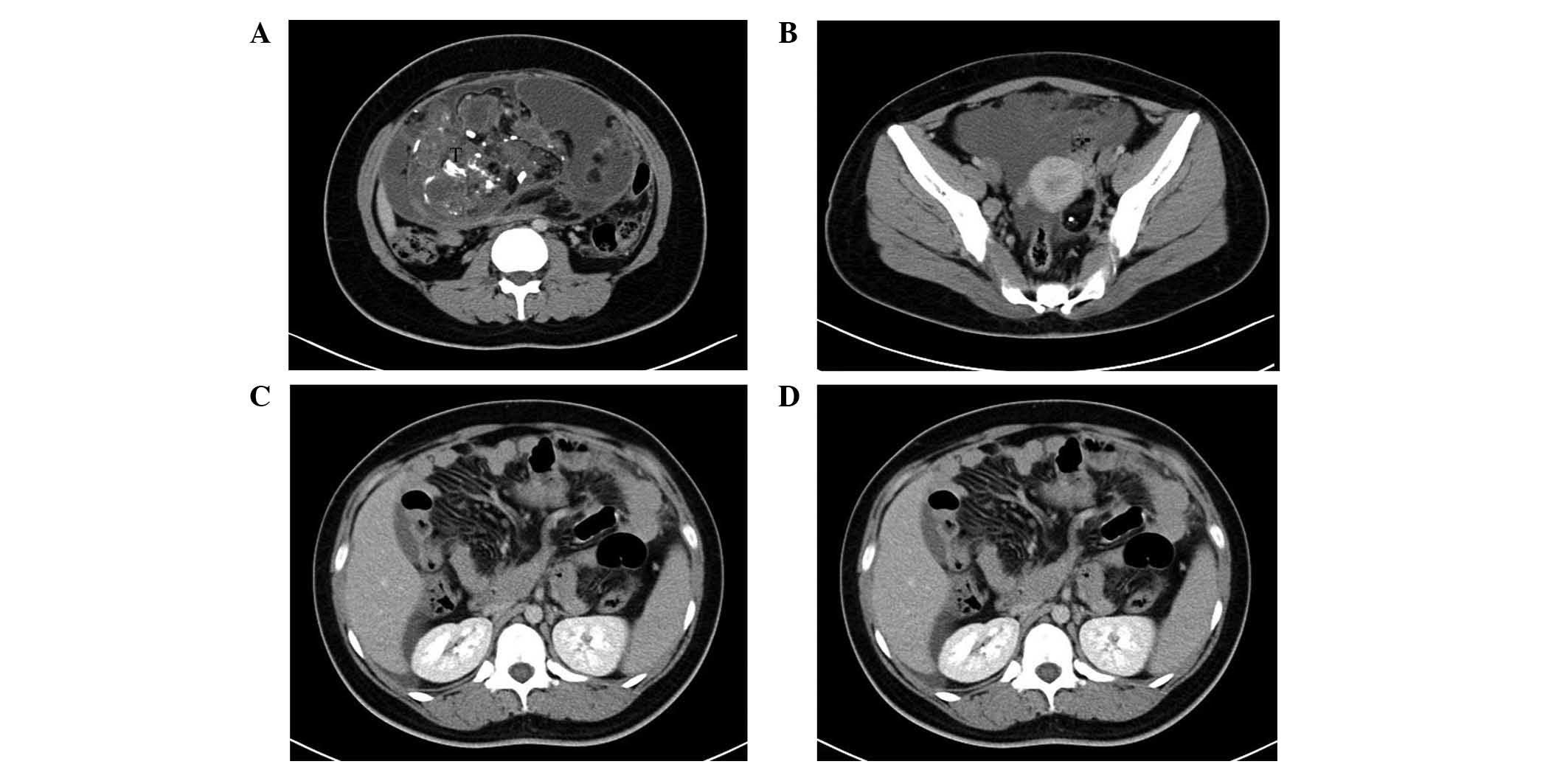
Contrast-enhanced computed tomography (CT) revealed
two cystic, solid masses in the pelvis and lower abdomen, each
containing dispersed regions of calcified and fatty components. The
two masses demonstrated similarities with bilateral ovarian
teratomas. Additionally, multiple nodules throughout the peritoneum
were associated with omental caking, and ascites was also detected
(Fig. 1A-C). Based on the results
from the CT scan and the elevated serum tumor markers, it was
suspected that the patient was presenting with malignant germ cell
tumors, specifically immature teratomas, with lymphadenopathy.
A right salpingo-oophorectomy, left tumor resection
and omentectomy were performed. The right ovarian mass was 30×25×20
cm in size, and consisted of a yellow-white solid component and
cystic areas. Two cystic masses, measuring 1×1 and 2×1 cm, were
observed in the left ovary. All tumors contained fatty components.
Numerous miliary nodules were identified in the retroperitoneum and
peritoneum, suggesting the presence of GP implants. The largest
implant was detected in the greater omentum, measuring 1 cm in
diameter, and had adhered to the right ovary. Following
histological examination, the right ovarian mass was confirmed as
an immature teratoma, and the left mass was confirmed as a mature
teratoma. Mature teratoma was characterized by well-differentiated
tissue, while immature teratoma contained immature tissue,
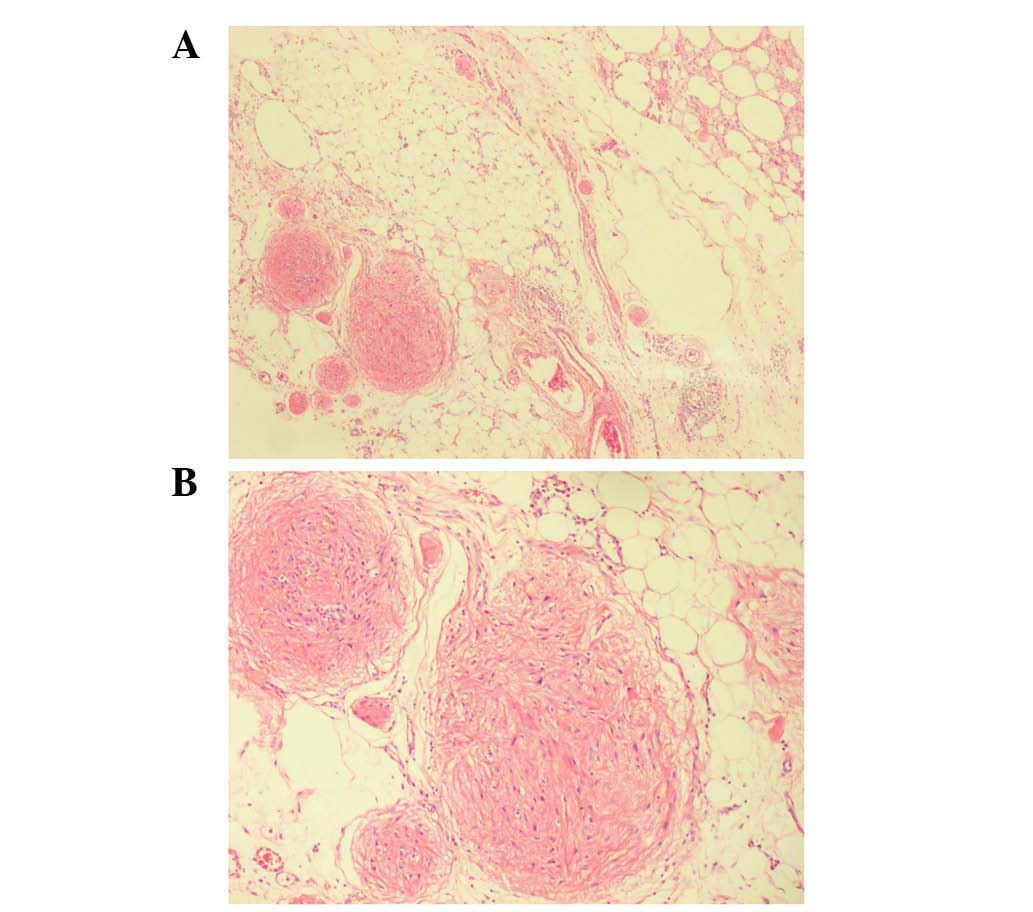
typically the neuroectodermal structures. Extensive peritoneal
implants were observed, with numerous lymphocytes and foam cells.
The implants had no envelope, but were without cellular atypia or
mitosis (Fig. 2).
Following surgery, a chemotherapy regimen (30 mg
bleomycin on days 1–3, 90 mg etoposide on day 1 and 0.17 mg
cisplatin on day 1, for 6 cycles; interval time, 4 weeks) was
administered. Currently, 4 years after the first surgery, the
patient is alive and well, with a good prognosis and without
recurrence or metastasis. A long-term follow-up is planned at an
outpatient clinic.
Case 2
A 15-year-old woman was referred to The Second
Affiliated Hospital, Zhejiang University School of Medicine on July
17, 2013, presenting with progressively increasing fullness of the
abdomen and a significant increase in weight over the last 3 years.
The patient was subsequently referred to the Department of
Endocrinology for the appropriate treatment. Contrast-enhanced CT
detected a tumor in the lower abdomen and pelvis, measuring 10×12×9
cm; a second tumor in the left ovary, with a diameter of 3 cm, was
also revealed on the CT scan (Fig.
1D). The patient was treated with a tumorectomy. During the
surgery, bilateral tumors were found, and the larger tumor was
located in the right ovary. The tumors were removed.
Intraoperatively, tiny gray spots, measuring up to 0.5 cm in
diameter, were removed from the greater omentum and peritoneum.
During microscopic examination, the tumors appeared to consist of a
teratomatous component, exhibiting similarities to skin, intestinal
mucosa, cartilage, bones and neuroglial tissue. It was also
observed that the peritoneal implants consisted of mature glial
tissue that appeared to be well-demarcated, without mitosis or
cellular atypia. Therefore, a diagnosis of bilateral mature
teratoma and GP was confirmed. No chemotherapy was administered for
case 2. During a follow-up period of 25 months after surgery, no
notable events were recorded and the patient has a good prognosis.
A long-term follow-up is planned at an outpatient clinic.
Discussion
GP is a rare complication associated with ovarian
teratomas, with only ~100 cases recorded to date. Typical symptoms
of the disease are similar to those experienced in the present two
cases, and affected patients may occasionally develop Pseudo-Meigs'
syndrome associated with ascites and pleural effusions owing to
ovarian teratoma (6). The
pre-operative diagnosis of teratoma may be confirmed through the
combination of imaging and clinical findings. However, small GP
lesions may be easily overlooked or cause a delay in accurate
diagnosis due to the rareness of the disease.
GP is characterized by a myriad of peritoneal
nodular or miliary implants composed of mature glia (7). As mature glial cells are not aggressive,
the implants may remain stable for long periods of time, exhibiting
benign behavior. However, on rare occasions, GP may develop a
secondary malignant glial tumor (8).
The pathogenesis underlying the development of GP is not yet fully
understood. These glial nodules are traditionally confined as
peritoneal seedlings via capsular rupture from the ovarian
teratoma, although genetic evidence has demonstrated that they may
have a different genetic identity compared with ovarian tumors
(9–11). In the majority of cases, a benign
clinical course is expected for patients with GP, particularly when
undergoing cisplatin-based chemotherapy (12). In the present two cases, following
surgical resection, each patient demonstrated a positive prognosis
within a 19-month follow-up period. However, doctors should conduct
long-term follow-ups in patients presenting with GP due to the
potential for malignant transformation.
When comparing the radiological data from the
present cases with previously published cases, notable findings
have been observed: i) Although the teratomas in the present cases
were localized on each ovary (bilateral), the majority of cases
within the literature reported unilateral teratomas, without a
particular preference as to which ovary was affected; up until now,
only one case of bilateral teratoma had been reported in the
literature (13). ii) Although the
radiological data regarding GP in the current literature is scarce,
the radiological findings in the present two cases appear to be
consistent with previous observations. The CT scans from the
current study revealed multiple nodules throughout the peritoneum,
with associated omental caking and ascites. The peritoneal implants
were miliary, measured 0.3–1.2 cm in size, and were well-marginated
and markedly enhanced (4,5,14). The
findings in the two present cases are similar to previous
observations of peritoneal tuberculosis and peritoneal
dissemination of malignant tumors (5). Therefore, with regard to the
identification of ovarian masses, imaging analysis of peritoneal
dissemination alone cannot aid the differentiation of benign
implants from diffuse peritoneal malignant seeding. However,
radiological analysis may provide important information regarding
tumor staging and the requirement for surgery. Therefore, in
patients with bilateral ovarian teratomas, GP should be considered
as a possible diagnosis by radiologists and relative radiological
investigations are warranted.
In summary, the current study reports two cases
presenting with bilateral ovarian teratomas with GP. These cases
suggest that: i) In patients with ovarian teratoma, a possible
diagnosis of GP should be considered, particularly in individuals
presenting with bilateral teratomas; ii) although radiology may
provide biological and histopathological information regarding GP
status, the radiological differential diagnosis of GP is
challenging due to the rareness of the disease; and iii) the
follow-up of patients with GP should not be discontinued following
the initial diagnosis and treatment, even if symptoms are
alleviated or disappear.
References
|
1
|
Nogales FF, Dulcey I and Preda O: Germ
cell tumors of the ovary: An update. Arch Pathol Lab Med.
138:351–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neuhaüser H: On teratoid tumors of the
ovary. Arch Gynaek. 79:696–719. 1906.(In German).
|
|
3
|
Brammer HM III, Buck JL, Hayes WS, Sheth S
and Tavassoli FA: From the archives of the AFIP. Malignant germ
cell tumors of the ovary: Radiologic-pathologic correlation.
Radiographics. 10:715–724. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kishimoto K, Ito K, Furukawa M, Ogasawara
N, Matsunaga N, Nawata S, Ogata H and Kato H: Immature teratoma
with gliomatosis peritonei associated with pregnancy. Abdom
Imaging. 27:96–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamoto D, Ishigami K, Yoshimitsu K, Irie
H, Tajima T, Asayama Y, Hirakawa M, Ushijima Y, Nishihara Y, Amada
S, et al: Gliomatosis peritonei associated with immature ovarian
teratoma: A mimicker of peritoneal dissemination of malignant
diseases. J Comput Assist Tomogr. 31:317–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan J, McClennan BL, Qureshi S, Martell
M, Iyer A and Bokhari SJ: Meigs syndrome and gliomatosis peritonei:
A case report and review of literature. Gynecol Oncol. 98:313–317.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menéndez-Sánchez P, Villarejo-Campos P,
Padilla-Valverde D, Murillo-Lázaro C and Martín-Fernández J:
Gliomatosis peritonei: Recurrence, treatment and surveillance. Cir
Cir. 79:256–259. 2011.PubMed/NCBI
|
|
8
|
Dadmanesh F, Miller DM, Swenerton KD and
Clement PB: Gliomatosis peritonei with malignant transformation.
Mod Pathol. 10:597–601. 1997.PubMed/NCBI
|
|
9
|
Ferguson AW, Katabuchi H, Ronnett BM and
Cho KR: Glial implants in gliomatosis peritonei arise from normal
tissue, not from the associated teratoma. Am J Pathol. 159:51–55.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwan MY, Kalle W, Lau GT and Chan JK: Is
gliomatosis peritonei derived from the associated ovarian teratoma?
Hum Pathol. 35:685–688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Best DH, Butz GM, Moller K, Coleman WB and
Thomas DB: Molecular analysis of an immature ovarian teratoma with
gliomatosis peritonei and recurrence suggests genetic independence
of multiple tumors. Int J Oncol. 25:17–25. 2004.PubMed/NCBI
|
|
12
|
Liang L, Zhang Y, Malpica A, Ramalingam P,
Euscher ED, Fuller GN and Liu J: Gliomatosis peritonei: a
Clinicopathologic and immunohistochemical study of 21 cases. Mod
Pathol. 28:1613–1620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roscher AA, Weinstein EC and Powsner L:
Giant teratomas with benign glial abdominal seeding, mimicking
diffuse abdominal carcinomatosis. Int Surg. 60:461–465.
1975.PubMed/NCBI
|
|
14
|
England RA, deSouza NM and Kaye SB:
Gliomatosis peritonei: MRI appearances and its potential role in
follow up. Br J Radiol. 80:e101–e104. 2007. View Article : Google Scholar : PubMed/NCBI
|