Introduction
Reconstruction of alimentary tract continuity
following esophagectomy may be achieved in several ways. The
reconstruction method is able to indirectly affect cancer survival
and has an impact on postoperative dysphagia (1). For this purpose, the gastric tube or the
large or small bowel may be mobilized and transposed through the
anterior or posterior mediastinum. Each route of transposition has
advantages, but are often under debate, since the posterior route
is shorter than the anterior route, but mediastinal leakage is more
dangerous than leakage on the neck. Anastomotic leakage is a feared
complication of reconstruction with an incidence of 3–30% and a
mortality rate of 25–50% (2–7).
A gastric tube is most commonly used to aid
reconstructive surgery (3). The
anterior route of reconstruction omits the tumor bed and possible
tumor recurrence does not affect the passage of food. Anastomosis
of the esophageal stump is performed subcutaneously, which may have
an impact on the possible care and course of the leakage. Anterior
interposition of the cervical esophagus may lead to additional
damage of its blood supply and encourage the leakage as a result of
ischemic necrosis. The interval between esophagectomy and
reconstructive surgery has an obvious disadvantage for the patient
in that it excludes early oral feeding, but two-stage esophagectomy
is a relatively safe form of surgery that may reduce the risk of
critical complications in high risk patients (8,9).
Furthermore, in certain circumstances it creates the opportunity
for the esophageal stump to recover from vessel injury and
accumulate growth factors characteristic for the granulation stage
of wound healing (10).
Vascular endothelial growth factor A (VEGF-A) and
transforming growth factor β (TGF-β) are important in angiogenesis
occurring during the proliferation phase of wound healing. In
normal tissue conditions, these factors are expressed at minimal
levels. Mechanical injury and hypoxia provoke strong upregulation
of VEGF-A expression, which then correlates temporarily and
spatially with the growth of new blood vessels (10–12). VEGF
is crucial for angiogenesis, controlling blood vessel formation and
growth (13). VEGF and TGF modulate
endothelial cell proliferation and are important for the creation
of a favorable microenvironment for newly formed microvessels;
these growth factors upregulate matrix metalloproteinases, which
participate in the degradation of extracellular matrix (ECM)
components, and support endothelial cell migration, invasion and
survival (14–16). TGF-β participates in all phases of
wound healing, which includes proliferation, inflammation and ECM
remodeling. The growth factor mediates fibroblast activation,
regulates the expression of cytokines, including VEGF, and controls
the synthesis of key ECM components, such as collagen I and III
(17). The final step in the
proliferation phase is the development of granulation tissue. With
the progression of wound healing and formation of granulation
tissue, proliferation and vascularization cease, collagen synthesis
increases and the wound maintains a balance between ECM degradation
and synthesis (18–20). Subsequently, scar tissue forms and the
wound enters the remodeling stage, which lasts from several months
to years (21). However, angiogenesis
is not completely finished yet, and the tissue remains highly
vascularized. Typical features of this stage include regression and
maturation of the vascular structure and substitution of the
granulation tissue (provisional ECM) into a permanent collagenous
matrix, which guides the vessels into an optimally distributed and
functioning network (20,21). Collagen III, which is characteristic
of granulation tissue, is now extensively replaced by collagen I
(10,13–22).
Patients and methods
Study subjects
The present study comprised 27 patients out of 57
patients who underwent esophageal resection for esophageal or
gastroesophageal cancer at the Department of Gastrointestinal and
General Surgery, Wrocław Medical University (Wrocław, Poland)
between January 2007 and December 2012. Eligible inclusion criteria
included the two-stage procedure, histopathological tumor type
(squamous cell carcinoma) and anterior coloplasty or
ileo-coloplasty for reconstruction of the digestive tract. The
remaining patients did not fit the eligibility criteria (20 of them
had gastroesophageal cancer and 10 had reconstruction made of the
gastric tube). Esophagectomy with two-field lymphadenectomy was
followed by esophagostomy and gastrostomy. After a mean time of 3.3
months (range, 2–6 months) and additional radiotherapy and/or
chemotherapy that lasted 1.8–3.6 months (mean, 2.5 months),
patients underwent reconstructive surgery. The total dose of
radiotherapy was 50–60 Gy over a 5-week period. Regimes of
chemotherapy were mainly based on paclitaxel and cisplatin at a
dosage of 150 mg/m2 and 50 mg/m2,
respectively, every 14 days. The median number of chemotherapy
cycles was 4. Additionally, 12 out of the 27 eligible patients who
were treated in the same department between January 2010 and
December 2012 comprised a study group that was analyzed
immunohistochemically. The remaining patients were evaluated
retrospectively.
Baseline characteristics of the patients are
presented in Table I. The patients
were in poor general condition preoperatively. A total of 16 were
in a state of malnutrition with a body mass index (BMI) of <17
(healthy range, 18.5–24.9), while 10 patients had underlying
pulmonary insufficiency with a vital capacity or forced expiratory
volume 1 of ≤50% predicted value (normal predicted value, ≥80%). A
total of 4 patients had chronic renal failure with blood creatinine
levels between 1.5 and 2.5 mg/dl (reference value, 0.55–1.02
mg/dl), 9 had uncontrolled diabetes with fasting blood glucose
levels between 140–180 mg/dl (normal level range, 65–99 mg/dl), 8
had coronary ischemic disease, 2 of which had stents in the
coronary arteries and 1 patient had an additional stent in the
internal carotid artery, 19 patients were or used to be heavy
smokers, and 8 had more than one comorbidity.
 | Table I.Baseline characteristic of the
patients from the present study. |
Table I.
Baseline characteristic of the
patients from the present study.
|
Characteristics | Patients from
2007–2009a | Patients from
2010–2012b |
|---|
| Patients, n | 15 | 12 |
| Age range,
years | 49–77 | 47–69 |
| Age, mean ±
SEM | 59.38±1.37 | 57.16±1.90 |
| Sex,
male/female | 11/4 | 10/2 |
| BMI <17 at the
time of esophagectomy, n | 9 | 7 |
| BMI <17 at the
time of reconstruction, n | 6 | 3 |
| Chemoradiation,
n | 7 | 4 |
| Location of the
tumor within the esophagus, n |
|
|
|
Upper | 4 | 2 |
|
Middle | 7 | 6 |
|
Lower | 4 | 4 |
| Postoperative T
feature, n |
|
|
| T2 | 5 | 5 |
| T3 | 10 | 7 |
| Size of leak,
n | 2 | 2 |
| <1
cm | 1 | 1 |
| 1–1.5
cm | 1 | 1 |
| Conduit necrosis, n
(%) | 1 (6.6) | 0 (0.0) |
In all cases, a three-incision approach was applied
(right thoracotomy, laparotomy and left cervical incision).
Pyloroplasty was performed as a standard procedure following
esophagectomy. All neck anastomoses were made in an end-side to end
manner, with double-layered, interrupted suturing. Patients
remained on gastrostomy following the reconstructive surgery, until
water-soluble contrast swallow examination was routinely performed
days 6–8 post-surgery, prior to the introduction of oral intake. In
4 patients, anastomotic leakage was observed. Anastomotic leakage
was defined as discharge of saliva and or intestinal content
through a wound on the neck or as an infected neck wound incision.
The severity of the neck leakage was evaluated by contrast
examination and, in selected cases, by endoscopy.
Postoperative mortality was defined as any mortality
during hospital stay, irrespective of its length.
Microvessel density in the wall of the esophageal
stump was investigated directly after esophagectomy and 2–6 months
later, once the reconstruction had been performed.
Immunohistochemistry
Immunohistochemistry was performed to evaluate
esophageal wall specimens at the time of esophagostomy construction
and prior to anastomosis of the cervical esophagus with the colon
or ileum. For immunohistochemical examination, formalin-fixed,
paraffin-embedded tissue sections (3–5-µm thick) were
deparaffinized in two changes of xylene for 5 min each. The
sections were subsequently hydrated in decreasing concentrations of
ethanol (96, 80 and 60%) and rinsed in water. Antigen retrieval was
routinely performed by incubation in 10 mmol/l sodium citrate
buffer (pH 6.0) and heated in a microwave oven at 300, 500 and 700
W for 5 min each. Primary mouse monoclonal anti-human TGF-β
(NCL-TGF-β; Novocastra; Leica Microsystems, Inc., Buffalo Grove,
IL, USA) and rabbit polyclonal antibody against VEGF-A (071420; EMD
Millipore, Billerica, MA, USA) were used at 1:100 dilution.
Specimens were incubated with antibodies overnight at 4°C.
Anti-Rabbit or Anti-Mouse HRP-DAB Cell & Tissue Staining kits
(CTS006 and CTS002, respectively; R&D Systems, Inc.,
Minneapolis, MN, USA) were used for blocking non-specific binding,
and antibody detection and visualization. The sections were
subsequently counterstained with Mayer's hematoxylin. Omission of
the primary antibody was used as the negative control. Staining of
collagen fibers using Van Gieson's method was performed to identify
the level of newly formed collagen in granulation tissue. Stained
specimens were viewed under a light microscope (Olympus BX41;
Olympus Corporation, Tokyo, Japan), and random areas were captured
in high-powered images at a magnification of ×400. The number of
capillaries in 9 representative fields were counted for each
specimen, and the total number for each field was averaged.
VEGF-A and TGF-β staining was semiquantitatively
scored as follows: No staining, -; weak staining, +; intermediate
staining, ++; and strong staining, +++. For statistical analysis,
the values of 0, 1, 2 and 3 were respectively assigned to the
intensity of staining. Collagen formation was analyzed using ImageJ
version 1.45j software (National Institutes of Health, Bethesda,
MD, USA). The granulation index was calculated as the percentage of
collagen mapped to the whole estimated surface area.
Statistical analysis
Statistical analysis was performed with Statistica
10 software (StatSoft, Inc., Tulsa, OK, USA). Descriptive
statistics, including mean and standard error, were used to
summarize the data. Student's t-test was used for the
analysis of independent variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients characteristics
A total of 27 patients that underwent esophagectomy
between January 2007 and December 2012 were initially considered
for the study. Two patients over the age of 75 were excluded due to
postoperative mortality; each succumbed following esophagoplasty
(reconstructive second operation), with 1 mortality caused by
complications resulting from necrosis of a colonic replacement for
the esophagus and the other as a result of cardiac failure. Out of
25 patients, anastomotic leakage was observed in 4 and the mean
leakage duration was 13 days. Out of 12 patients who underwent
esophagectomy and secondary reconstruction between January 2010 and
December 2012 (the subjects of immunohistochemical examination), 2
patients developed fistulas. Leakage occurred only if the esophagus
was anastomosed with the colon. There were no leaks following
ileo-coloplasty, where the esophagus was anastomosed with the ileal
portion of the substitute. In these 2 cases, slight circumferential
necrosis of the substitute caused the leak. There were no
significant differences between microvessel density and levels of
proangiogenic factors among the patients who developed leakage in
comparison with those who healed without complication. In this
group of patients, the mean time of leakage healing was 12 days
There were no postoperative mortalities in this group.
The interval between esophagectomy and
reconstruction varied based on the period of oncological treatment,
organization of oncological therapy, the institution's schedule and
the patient's attitude towards therapy. The shortest period was 2–3
months in the majority of patients, while the longest was 6 months
in 1 patient and 5 months in 3 patients. Two patients postponed
reconstruction for subjective reasons and fear of surgery, despite
having completed radiochemotherapy for up to 3.6 months, while a
further 2 patients underwent postponed reconstruction for objective
reasons, including organization of oncological care.
Leakages were successfully treated conservatively.
Mediastinitis occurred in only 1 patient who experienced necrosis
of the conduit. During the treatment, patients were fed by
gastrostomy.
Immunohistochemistry
Results of statistical analysis are presented in
Table II. At the time of
reconstructive surgery, a statistically significant increase was
observed in microvessel density in all esophageal specimens
(P<0.03). A significant difference was also demonstrated in the
immunohistochemical staining intensity of TGF-β, VEGF-A and
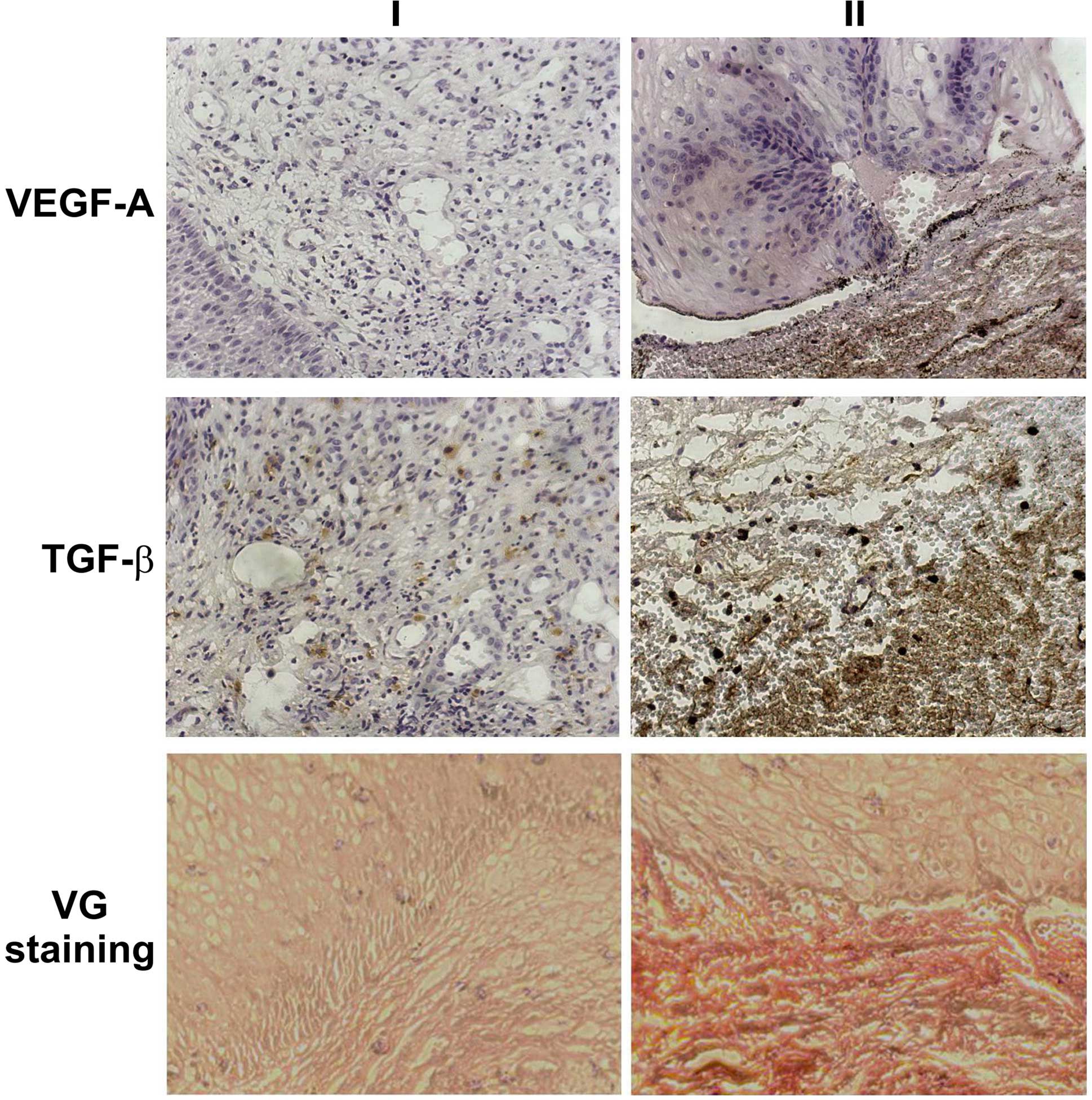
collagen fibers (P<0.05). Fig. 1
presents the differences in immunohistochemical staining in
selected microscopic images of the esophageal specimens at the time
of esophagectomy and during reconstructive surgery.
 | Table II.Statistical analysis of VEGF-A, TGF-β
and collagen staining intensity, and number of vessels in the
esophageal tissue at the time of the esophagectomy (NET) and during
reconstructive surgery (GET). |
Table II.
Statistical analysis of VEGF-A, TGF-β
and collagen staining intensity, and number of vessels in the
esophageal tissue at the time of the esophagectomy (NET) and during
reconstructive surgery (GET).
| Characteristic | Mean | SEM |
P-valuea |
|---|
| NET |
|
|
|
|
TGF-β | 1.088 | 0.077 |
|
|
VEGF-A | 0.000 | 0.000 |
|
Collagen area, % | 5.188 | 0.793 |
|
Vessels, n (range) | 19.70 (10–36) | 1.011 |
| GET directly prior
to anastomosis construction |
|
|
|
|
TGF-β | 2.094 | 0.118 | 0.001 |
|
VEGF-A | 1.325 | 0.212 | 0.001 |
|
Collagen area, % | 38.372 | 6.205 | 0.028 |
|
Vessels, n (range) | 23.19 (16–37) | 0.029 | 0.028 |
Discussion
In the Department of Gastrointestinal and General
Surgery, Wrocław Medical University, the preferred method of
surgical treatment for middle esophageal cancer extending between
the azygos vein and the lower pulmonary vein is the two-stage
procedure with esophageal reconstruction of the colon. In the
present study, approximately two thirds of the patients were in the
advanced stage of the disease, with significant dysphagia, and
nearly half of them had a BMI of <17 at the time of
esophagectomy. Nutritional status and nutritional support,
particularly by way of patients undergoing esophagectomy, are among
factors that improve perioperative mortality rate and survival
(23). Patients are at a higher risk
of complications following coloplasty (8,24).
Advanced age and prolonged duration of surgery together with
diabetes, renal failure and poor general condition are reported to
be important risk factors responsible for perioperative mortality
following esophagectomy (8,9). In the current study, there were no
mortalities subsequent to esophagectomy. Two patients succumbed to
complications following the second surgery. Reconstruction of the
esophagus (mortality rate, 7.4%) remains at an average level in
comparison with high volume institutions that report a mortality
rate <5%, although patient selection and a small study sample
appear to bias the outcomes. The leak rate tends to be higher if
the colon conduit rather than gastric tube is used, and when it is
placed in the anterior mediastinum rather than the posterior
mediastinal route (1). Patients who
succumb to complications following reconstruction surgery are
typically of an advanced age, and necrosis of the esophageal
replacement is a complication with an extremely high mortality rate
(23–25). In the series of patients who were
operated between January 2010 and December 2012, the incidence of
mortality subsequent to coloplasty was 0%. There was no
statistically significant difference between these 2 groups in
terms of as to patients age, gender, BMI or tumor size according to
the tumor-node-metastasis classification.
Using the two-stage approach and careful patient
selection for coloplasty, the patients of the present study
achieved low in-hospital mortality and a low reoperation rate.
Right thoracotomy is often favored to left thoracotomy as it offers
better approach to the esophagus and enveloping lymph nodes. In
approximately half of esophageal cancer cases, the disease develops
in the middle esophagus (1,8,9,24,25);
therefore, the optimal treatment is subtotal esophagectomy.
Intrathoracic anastomosis performed in mid-esophageal lesions is
not free of the risk of cancer-positive resections margins
(25). Certain studies have
emphasized that R0 resection in particular has an impact on
survival rate (26). None of the
patients in the present study that underwent anastomosis on the
neck after subtotal esophagectomy were positive for cancer within
the resection margin. One-stage esophagectomy and coloplasty is a
long surgery with a mean duration of 7.5 h, and has a higher
mortality rate compared with the two-stage procedure (1,8,9,24).
Furthermore, prolonged surgery duration causing
greater surgical stress is associated with acute lung injury, which
leads to systemic inflammatory response syndrome and other critical
complications, including anastomotic leakage (9).
Leakage of the cervical anastomosis remains the most
serious complication of esophageal reconstructive surgery (1,3,8,9).
The concept of a postponed anastomosis of the
cervical esophagus with the intestine provides an advantage for the
esophageal stump to accumulate growth factors and various molecules
supporting its recovery. The present study observed a significant
increase in microvessel density in all esophageal biopsies acquired
at the time of reconstructive surgery compared with probes taken
during esophagectomy (P<0.03), which was associated with
significant differences in immunohistochemical staining intensity.
The current study focused on the expression of VEGF-A and TGF-β,
which are responsible for angiogenesis and wound healing (10,13–15).
VEGF-A is an obvious choice, since is the key marker of
angiogenesis.. Besides functioning in the induction of
angiogenesis, TGF-β also possesses anti-inflammatory properties and
exhibits an inhibitory effect on collagenase activity, thus
protecting newly synthesized collagen within anastomoses (17). Bernstein et al (27) reported that topical application of
TGF-β improves radiation-impaired wounds. Transposition of the
esophageal stump to the anterior mediastinum aids the avoidance of
mediastinum contamination when leakage occurs; it also allows for
conservative treatment of the leakage, resulting in a shorter
hospital stay.
Patients of the present study qualified for
conservative treatment following the analysis of clinical data and,
if necessary, endoscopy was performed to evaluate the extent of
ischemia of the anastomotic circumference. The current study
reported that fistulas developed in 16% of cases; data from the
literature indicates that this complication may develop in 3–40% of
cases (2,28–33).
However, actual data regarding leak rates may be underestimated by
defining leakage as a condition requiring use of surgical
intervention, as one third of leaks in the chest do not require
surgery (7,31). The incidence of this complication
varies among studies. Anastomosis healing may be affected not only
by the conduit type and location of anastomosis, but also by the
preoperative status of the patient. Furthermore, according to the
literature, leakage rate following colo-esophagoplasty, including
patients with caustic strictures, remains higher than the leakage
rate following reconstruction with a gastric tube, and ranges from
6.7–46.4% (1,24,28). In
the present patients, leakage occurred only if the esophagus was
anastomosed with the colon. There was no leak among patients who
had the esophagus anastomosed with the ileum. Leakage incidence is
identified to be higher in neck anastomoses compared with
intrathoracic anastomoses (1,24,29). The
present study only included patients with esophageal cancer who
underwent two-stage surgery; the esophagus was anastomosed with the
ileum (ileo-coloplasty) or the colon (coloplasty). The present
study was not large, but the results were homogeneous. All
reconstructions were performed on the right hemi-colon, at the neck
and in a squamous cell carcinoma patient population. Similar to
other studies, the current study lacked a control group; thus,
these results may only be compared with published data (1,24,28,30–32).
The basic rules and phases of the intestinal wound
healing process are similar to the healing process of skin wounds.
However, there are key differences in the slowly progressing
proliferation and remodeling phase. While mucosal resurfacing
occurs rapidly and is completed after 7 days, the proliferation and
overlapping remodeling phase lasts for months (34). The results of the current study
confirmed this, as all esophageal specimens obtained at the time of
reconstruction had a significantly larger vascular network, a high
granulation index and the growth factors essential for the
proliferation and early remodeling phase.
Rijcken et al (34) reported that recovery of the vascular
network and increased angiogenesis serve an essential role in the
process of anastomosis healing. In the present study, all
esophageal specimens obtained following esophagectomy exhibited
lower levels of VEGF-A and TGF-β compared with those obtained at
the time of esophageal anastomosis formation. VEGF-A and TGF-β
levels were associated with vascular net density and were
statistically higher in esophageal stumps at the time of
reconstruction, although the vascular network of the esophageal
stump may be damaged while being translocated to the anterior
mediastinum during the first surgical stage.
The current study also noted that the new vessel
network and existing key growth factors overlapped with the further
proliferative phase of wound healing and promoted a favorable
environment for healing anastomoses constructed up to several
months later. This finding is in agreement with Ishii et al
(35), who reported that following
VEGF-A application, microangiographic analysis of biopsies of
colonic anastomoses in rats revealed significantly higher capillary
counts and granulation tissue formation in comparison with
saline-treated controls (35–37). The present study demonstrated that
growth factors from the esophageal stump were physiologically
present and anastomosis healing took place in a naturally-created
environment. We hypothesize that in two-stage reconstruction, the
ischemic tissue is under direct influence of growth factors already
synthesized and delivered by the esophageal tissue that promote its
vascularization. Therefore, in such an environment, even if the
terminal circumference of the colonic transplant suffers from
ischemia and esophago-intestinal fistula occurs, the tissue heals
more easily. The mean time of fistula healing in the current study
was 13 days, and none of the patients who developed the leak
required any surgical intervention. All stumps were in their
proliferative phase of wound healing, which indicates that
proangiogenic VEGF-A and TGF-β were already highly expressed in the
anastomosed tissue and did not require delivery to the healing
site. A number of clinical trials have failed to successfully
complete wound healing following topical application of exogenous
VEGF (37–39).
The current study has presented a novel surgical
approach for the performance of difficult anastomoses. In general,
tension-free suturing and excellent blood perfusion lead to primary
spontaneous healing of anastomoses (2–5,36). In pathological conditions, including
systemic or local severe inflammation and ischemia, the healing
process may be impaired and result in the occurrence of fistulas or
fibrosis, subsequently leading to stenosis of the site of the
anastomosis. In such conditions, anastomosis may be postponed and
performed in a second surgery. Postponed esophageal anastomosis has
an obvious disadvantage; the prolonged time of feeding omitting the
natural way of oral food intake. Nevertheless, it may be reserved
for select patients, particularly in the era of neoadjuvant
chemoradiation.
In conclusion, two-staged esophagectomy enables
high-risk patients to recover from surgical stress after
esophagectomy and to avoid critical complications. Postponed
cervical anastomosis conducted with the colon replacement is not
free from leak occurrence; however, the esophageal stump, which
accumulated proangiogenic factors and acquired novel microvessel
networks, heals more easily.
References
|
1
|
Urschel JD, Urschel DM, Miller JD, Bennett
WF and Young JE: A meta-analysis of randomized controlled trials of
route of reconstruction after esophagectomy for cancer. Am J Surg.
182:470–475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feith M, Gillen S, Schuster T, Theisen J,
Friess H and Gertler R: Healing occurs in most patients that
receive endoscopic stents for anastomotic leakage; dislocation
remains a problem. Clin Gastroenterol Hepatol. 9:202–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schubert D, Dalicho S, Flohr L, Benedix F
and Lippert H: Management of postoperative complications following
esophagectomy. Chirurg. 83:712–718. 2012.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Böhm G, Mossdorf A, Klink C, Klinge U,
Jansen M, Schumpelick V and Truong S: Treatment algorithm for
postoperative upper gastrointestinal fistulas and leaks using
combined vicryl plug and fibrin glue. Endoscopy. 42:599–602. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reavis KM: The esophageal anastomosis: How
improving blood supply affects leak rate. J Gastrointest Surg.
13:1558–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarela AI, Tolan DJ, Harris K, Dexter SP
and Sue-Ling HM: Anastomotic leakage after esophagectomy for
cancer: A mortality-free experience. J Am Coll Surg. 206:516–523.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
CoolsLartigue J, Andalib A, AboAlsaud A,
Gowing S, Nguyen M, Mulder D and Ferri L: Routine contrast
esophagram has minimal impact on the postoperative management of
patients undergoing esophagectomy for esophageal cancer. Ann Surg
Oncol. 21:2573–2579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sueyoshi S, Yamana H, Fujita H, Tanaka T,
Toh U, Kubota M, Tanaka Y, Mine T, Sasahara H and Shirouzu K:
Radical esophagectomy and secondary anastomosis for high-risk
patients with intrathoracic esophageal carcinoma. Jpn J Thorac
Cardiovasc Surg. 48:683–687. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morita M, Nakanoko T, Kubo N, Fujinaka Y,
Ikeda K, Egashira A, Saeki H, Uchiyama H, Ohga T, Kakeji Y, et al:
Two-staged operation for high-risk patients with thoracic
esophageal cancer: An old operation revisited. Ann Surg Oncol.
18:2613–2621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eming SA, Brachvogel B, Odorisio T and
Koch M: Regulation of angiogenesis: Wound healing as a model. Prog
Histochem Cytochem. 42:115–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma RA, Harris AL, Dalgleish AG,
Steward WP and O'Byrne KJ: Angiogenesis as a biomarker and target
in cancer chemoprevention. Lancet Oncol. 2:726–732. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yazdani S, Kasajima A, Tamaki K, Nakamura
Y, Fujishima F, Ohtsuka H, Motoi F, Unno M, Watanabe M, Sato Y and
Sasano H: Angiogenesis and vascular maturation in neuroendocrine
tumors. Hum Pathol. 45:866–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao P, Kodra A, TomicCanic M, Golinko MS,
Ehrlich HP and Brem H: The role of vascular endothelial growth
factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim IY, Kim MM and Kim SJ: Transforming
growth factor-beta: Biology and clinical relevance. J Biochem Mol
Biol. 38:1–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wietecha MS and DiPietro LA: Therapeutic
approaches to the regulation of wound angiogenesis. Adv Wound Care
(New Rochelle). 2:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eming SA and Hubbell JA: Extracellular
matrix in angiogenesis: Dynamic structures with translational
potential. Exp Dermatol. 20:605–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schultz GS and Wysocki A: Interactions
between extracellular matrix and growth factors in wound healing.
Wound Repair Regen. 17:153–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pakyari M, Farrokhi A, Maharlooei MK and
Ghahary A: Critical role of transforming growth factor beta in
different phases of wound healing. Adv Wound Care (New Rochelle).
2:215–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Egginton S and Gaffney E: Tissue capillary
supply-it's quality not quantity that counts! Exp Physiol.
95:971–979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wietecha MS, Cerny WL and DiPietro LA:
Mechanisms of vessel regression: Toward an understanding of the
resolution of angiogenesis. Curr Top Microbiol Immunol. 367:3–32.
2013.PubMed/NCBI
|
|
22
|
Tirziu D and Simons M: Endothelium as
master regulator of organ development and growth. Vascul Pharmacol.
50:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
LlopTalaveron JM, FarranTeixidor L,
BadiaTahull MB, VirgiliCasas M, LeivaBadosa E, Galán-Guzmán MC,
Miró-Martin M and Aranda-Danso H: Artificial nutritional support in
cancer patients after esophagectomy: 11 years of experience. Nutr
Cancer. 66:1038–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamai Y, Hihara J, Emi M, Aoki Y and Okada
M: Esophageal reconstruction using the terminal ileum and right
colon in esophageal cancer surgery. Surg Today. 42:342–350. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang HT, Wang F, Shen L, Xia CQ, Lu CX
and Zhong CJ: Clinical outcome of middle thoracic esophageal cancer
with intrathoracic or cervical anastomosis. Thorac Cardiovasc Surg.
63:328–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McK Manson J and Beasley WD: A personal
perspective on controversies in the surgical management of
oesophageal cancer. Ann R Coll Surg Engl. 96:575–578. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bernstein EF, Harisiadis L, Salomon G,
Norton J, Sollberg S, Uitto J, Glatstein E, Glass J, Talbot T,
Russo A, et al: Transforming growth factor-beta improves healing of
radiation-impaired wounds. J Invest Dermatol. 97:430–434. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Markar SR, Arya S, Karthikesalingam A and
Hanna GB: Technical factors that affect anastomotic integrity
following esophagectomy: Systemic review and meta-analysis. Ann
Surg Oncol. 20:4274–4281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yasuda T and Shiozaki H: Esophageal
reconstruction with colon tissue. Surg Today. 41:745–753. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paul S and Altorki NJ: Outcomes in the
management of esophageal cancer. Surg Oncol. 110:599–610. 2014.
View Article : Google Scholar
|
|
31
|
Saeki H, Morita M, Tsuda Y, Hidaka G,
Kasagi Y, Kawano H, Otsu H, Ando K, Kimura Y, Oki E, et al:
Multimodal treatment strategy for clinical T3 thoracic esophageal
cancer. Ann Surg Oncol. 20:4267–4273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morita M, Otsu H, Kawano H, Kumashiro R,
Taketani K, Kimura Y, Saeki H, Ando K, Ida S, Oki E, et al:
Advances in esophageal surgery in elderly patients with thoracic
esophageal cancer. Anticancer Res. 33:1641–1647. 2013.PubMed/NCBI
|
|
33
|
Schaible A, Sauer P, Hartwig W, Hackert T,
Hinz U, Radeleff B, Büchler MW and Werner J: Radiologic versus
endoscopic evaluation of the conduit after esophageal resection: A
prospective, blinded, intraindividually controlled diagnostic
study. Surg Endosc. 28:2078–2085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rijcken E, Sachs L, Fuchs T, Spiegel HU
and Neumann PA: Growth factors and gastrointestinal anastomotic
healing. J Surg Res. 187:202–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishii M, Tanaka E, Imaizumi T, Sugio Y,
Sekka T, Tanaka M, Yasuda M, Fukuyama N, Shinozaki Y, Hyodo K, et
al: Local VEGF administration enhances healing of colonic
anastomoses in a rabbit model. Eur Surg Res. 42:249–257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: Molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brudno Y, EnnettShepard AB, Chen RR,
Aizenberg M and Mooney DJ: Enhancing microvascular formation and
vessel maturation through temporal control over multiple
pro-angiogenic and pro-maturation factors. Biomaterials.
34:9201–9209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Barrientos S, Brem H, Stojadinovic O and
Tomic-Canic M: Clinical application of growth factors and cytokines
in wound healing. Wound Repair Regen. 22:569–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grommes J, Binnebösel M, Klink CD, von
Trotha KT, Schleimer K, Jacobs MJ, Neumann UP and Krones CJ:
Comparison of intestinal microcirculation and wound healing in a
rat model. J Invest Surg. 26:46–52. 2013. View Article : Google Scholar : PubMed/NCBI
|