Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) have
been used for a long time to treat rheumatic diseases and the signs
and symptoms of inflammation. Additionally, NSAIDs have been
discussed in the last decade as potential anticancer agents
(1,2).
After the initial observation that NSAIDs could inhibit tumour
growth in the lungs and intestines of rodents, it was confirmed
that there is a substantial risk reduction for colon cancer when
patients regularly take acetylsalicylic acid (ASA). Furthermore,
evidence is accumulating that the use of NSAIDs confers a
protective effect against gastric and oesophageal cancer, and maybe
also against prostatic, ovarian and lung cancer (3). In 2012, a retrospective study showed
that the use of the NSAID ketorolac in breast cancer patients was
associated with a superior disease-free survival rate in the first
years after surgery (4).
The NSAID treatment described in the present study
follows the recommendations of Kreutz on how to apply the drugs
diflunisal, ASA and 4-aminosalicylic acid (PAS) to treat cancer
(5). The aforementioned salicylates
show a cytotoxic effect on cancer cell lines in vitro when
used in physiologic concentrations. The mechanism of action is
proposed to be NF-κB inhibition leading to cell cycle arrest and
subsequent apoptosis (6). The
anti-inflammatory effect of NSAIDs could also inhibit cancer
progression and metastasis formation, as inflammation is
hypothesised to be a possible cause (3,7,8). The first clinical data indicate that
patients with advanced solid tumours benefit from salicylate
therapy (Drevs, unpublished data).
In the present study, the beneficial effect of the
NSAID treatment was closely examined by positron emission
tomography-computed tomography (PET-CT). The latter, however, does
not always give clear results and is an expensive method.
Therefore, the effect of the therapy on the systemic region of the
tumour, the circulating tumour cells (CTCs), was simultaneously
monitored using an approach that aimed to encompass all of these
cells in the blood without loss due to enrichment procedures and
could be performed repeatedly. The detection and characterisation
of tumour-derived circulating epithelial tumour cells (CETCs) has
been a main focus of basic oncologic research in previous years.
CTCs disseminate from solid tumours, circulate in the blood or
lymphatic system and are claimed to be the cause of distant
metastases (9–11). In 2006, a direct comparison between
the enumeration of CTCs and radiological imaging in metastatic
breast cancer patients for the prediction of overall survival was
published for the first time. The study showed that CTC enumeration
is a reliable way to monitor disease progression (12). However, the isolation procedure in the
study was accompanied by a massive loss of CTCs, as the threshold
was only 5 CTCs. A method that leads to a higher yield, the
analysis of CTCs and their course by the maintrac®
approach has been shown to correlate with clinical outcome in
breast cancer patients and provides a novel analytic tool, which is
probably an alternative to invasive biopsies. The reduction or
marginal changes of CTC numbers during chemotherapy corresponds
with a good prognosis, whereas an increase corresponds with a
higher risk of metastases (13–15). The
present study shows that the dissemination of CTCs from an
epithelial tumour or from metastases can be monitored over time to
assess the response to a treatment.
Patients and methods
Patient population
Clinical data was collected from 14 patients (mean
age, 55.5 years) with advanced and heavily pre-treated epithelial
tumours who received an anti-cancer treatment in the UNIFONTIS
clinic (Tübingen, Germany) and who were followed up by PET-CT, with
additional monitoring of CTC numbers in the blood (Table I). The majority of the patients (57%)
underwent primary tumour removal. The remaining 43% of patients did
not undergo primary tumor removal due to unresectable tumors or
patient refusal. A total of 93% had histologically confirmed
distant metastases at the time of treatment.
 | Table I.Patient characteristics (n=14). |
Table I.
Patient characteristics (n=14).
| Characteristic | Patients, n (%) |
|---|
| Gender |
|
| Male | 2
(14) |
|
Female | 12 (86) |
| Localisation of
primary |
|
|
Cervix | 1 (7) |
|
Ovaries | 3
(21) |
|
Breasts | 4
(29) |
|
Endometrium | 1 (7) |
|
Lungs | 2
(14) |
| Skin | 1 (7) |
|
Orbits | 1 (7) |
|
Unknown | 1 (7) |
| Metastatic sites |
|
| Lymph
nodes | 8
(57) |
| Lung | 7
(50) |
| Bone | 5
(36) |
|
Brain | 3
(21) |
|
Other | 5
(36) |
| No. of metastatic
sites |
|
| 0 | 1 (7) |
| 1 | 4
(29) |
| 2 | 4
(29) |
|
>2 | 5
(36) |
Salicylate therapy
A total of 10 patients underwent treatment with
salicylate therapy. The treatment with diflunisal or other
salicylates (ASA and PAS) was applied following the recommendations
of Kreutz (5). Usually, the drugs
were administered intravenously for 4 days a week, 2 weeks in a
row. The initial dose of salicylates on the first day was 35 mg/kg,
administered intravenously. On the second to fourth days, patients
received a dose of 30 mg/kg, administered intravenously.
Intravenous application was selected to avoid gastrointestinal side
effects, which may occur in up to 20% of patients treated with
diflunisal. Salicylates were provided by OncoAdvance GmbH (Staufen,
Germany). The patients who qualified for such a treatment were in
the extreme advanced stage of the disease and had previously
undergone multiple treatments. In such cases, the off-label use is
justified in Germany. The patients were treated with salicylates
only after an extensive informative conversation with the physician
and all were required to provide written informed consent.
Metronomic low-dose chemotherapy
One patient received metronomic therapy in
combination with the administration of capecitabine and
trofosfamide combined with bevacizumab for 61 days. Metronomic
low-dose chemotherapy was administered as a long-running daily oral
application of cytostatics (capecitabine or trofosfamide) in
extremely low doses (1 g/m2 or 1 g absolute oral). In
2008, Reichle and Vogt (16)
introduced this treatment schedule in combination with
anti-inflammatory therapies and angiostatic therapies.
Response assessment by imaging
technologies
Tumour response was assessed by PET-CT. The optical
evaluation of the response to a therapy followed the Response
Evaluation Criteria In Solid Tumors (RECIST) criteria (17). PET was used to visualize the metabolic
activity of the tissue. The metabolic assessment of tumour activity
was documented, but metabolic activity data were not included in
this analysis. The PET-CT was performed by an independent facility,
the Radiological Clinic of the University Tübingen (Tübingen,
Germany).
RECIST 1.1 in brief
For the RECIST criteria, the number of target
lesions is equal to ≤2 per organ, with up to 5 in total. A complete
response (CR) is classified as the disappearance of all target
lesions. The short axis of any pathological lymph nodes must be
reduced to <10 mm. A partial response (PR) is classified as a
≥30% decrease in the sum of the diameters of the target lesions
(SLD), using the baseline sum of diameters as a reference.
Progressive disease (PD) is defined as a ≥20% increase in the SLD,
using the smallest sum on the study as a reference. The SLD must
also show an absolute increase of ≥5 mm. Stable disease (SD) is
classified as insufficient shrinkage to qualify for PR or an
insufficient increase in SLD to qualify for PD.
Quantification of CTCs and tumour cell
chemosensitivity test
The quantification of CTCs from the whole blood was
performed as previously described (14). In short, red blood cells from 1 ml
blood were lysed and the remaining white blood pellet was analysed.
Epithelial cells were detected by laser scanning cytometry using
the Olympus ScanR screening station (Olympus Corporation, Tokyo,
Japan). For staining, the cells were incubated at 4°C overnight
with a monoclonal fluorescein-isothiocyanate (FITC)-conjugated
EpCAM antibody (mouse α-human; catalog no. 130-080-301; 1:100;
Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). On the second
day, dead cells were detected with propidium iodide (PI), which is
excluded from viable cells but incorporated in dead cells. The
baseline CTC assessment for each treatment was performed prior to
(maximum, 4 months previously) or during the treatment, and the
follow-up assessment was performed at the earliest 2 weeks after
the beginning of treatment and between 1 and 6 months after the
baseline assessment. A >5-fold decrease in CTCs was considered a
‘significant decrease’, while a >5-fold increase was considered
a ‘significant increase’ in CTCs. A <5-fold increase or decrease
was considered a stable CTC course. The chemosensitivity testing of
the patients' CTCs was performed as described previously (18). CTCs among the white blood cells from
the whole blood were labelled with the FITC-conjugated EpCAM
antibody, as aforementioned. The cytotoxicity of the drug in
question was determined by exposing the cells to three different
concentrations: The physiological concentration of the drug in the
blood of a patient under treatment, plus a 10-fold higher and a
10-fold lower concentration. Dead cells could be distinguished from
living cells by PI and EpCAM antibody staining, and subsequent
quantification with the laser scanning cytometry. The
chemosensitivity rate was calculated as the ratio of dead cells to
the total cell number in the sample. The quantification of CTCs
from the whole blood and the chemosensitivity testing was performed
in the diagnostic laboratory of Dr Ulrich Pachmann (Transfusion
Medical Centre Bayreuth, Bayreuth, Germany).
Statistical analysis
All statistical analysis was performed using
SigmaPlot software (version 13.0; Systat Software GmbH, Erkrath,
Germany). Data was analyzed using Pearson's χ2 test with
4 degrees of freedom. P<0.05 was considered to indicate a
statistically significant difference.
Results
Chemosensitivity of circulating tumour
cells
For the present study, 25 patients were screened
using the inclusion criteria. For inclusion in the study, the
patients had to have at least one chemosensitivity testing, at
least one medical treatment followed by PET-CT response assessment
and at least two CTC counts, the first one (baseline) shortly prior
to or shortly after the beginning of treatment and the second one
(follow-up) between 1 and 6 months later. In total, 14 patients met
the inclusion criteria. The in vitro sensitivity of the CTCs
to salicylates, selected cytostatics and tyrosine kinase inhibitors
(TKIs) was tested for each patient individually. Diflunisal was
found to be an effective cytotoxic agent in 11 patients (mean
chemosensitivity-index, 86%) and PAS was found to be an effective
cytotoxic agent in 8 patients (mean chemosensitivity-index, 73%),
as shown in the results of the in vitro chemosensitivity
test (Table II). The cytostatic
drugs used in these patients (cisplatin, capecitabine,
trofosfamide, paclitaxel, docetaxel and carboplatin) showed high
cytotoxic activity ranging from 40 to 95%. The TKIs pazopanib and
imatinib also showed cytotoxic activity of 95% in 2 patients.
 | Table II.CTC chemosensitivity assays
(performed, n=13). |
Table II.
CTC chemosensitivity assays
(performed, n=13).
| Treatment | Mean chemosensitivity
rate (patients, n) |
|---|
| Diflunisal | 86%
(11) |
| PAS | 73% (8) |
| Cisplatin | 76% (4) |
| Capecitabine | 40% (4) |
| Trofosfamide | 55% (4) |
| Paclitaxel | 60% (1) |
| Docetaxel | 95% (1) |
| Carboplatin | 80% (1) |
| Pazopanib | 95% (1) |
| Imatinib | 95% (1) |
Response assessment by PET-CT
The treatment response data of all patients assessed
by PET-CT was collected. A total of 21 treatments were assessed
(Table III). Additionally, the
patient's condition was assessed without previous treatment 6 times
(Table III). The median age of the
patients was 55.5 years. PET-CTs that were performed no earlier
than 2 weeks after the beginning of treatment and no later than 4
months after the treatment were included in the evaluation. CR to a
treatment was observed in 1 case after diflunisal treatment. PR to
a treatment was observed in 5 cases, three times after treatment
with cytostatic drugs in combination with bevacizumab, once after
treatment with diflunisal and once after a combined treatment with
ASA, tamoxifen and bevacizumab. SD after treatment with diflunisal
or other salicylates was observed in 7 cases. SD after treatment
with cytostatic drugs was observed in 1 case and SD without
previous treatment was observed in 1 case. The objective response
rate, which is the sum of the CR and PR rates, was 22%. PD
following a treatment was observed in 7 cases and a PD without
previous treatment was documented 5 times.
 | Table III.Response assessment. |
Table III.
Response assessment.
| Patient no. | Treatment | PET-CT | CTCs |
|---|
| 1 | Diflunisal | PR | Decrease |
| 3 | Paclitaxel,
Bevacizumab | PR | Decrease |
|
| Paclitaxel | PD | Decrease |
|
| Doxorubicin,
Cyclophosphamide, |
|
|
|
| Bevacizumab | SD | Stable course |
| 4 | Diflunisal | PD | Decrease |
|
| None | PD | Increase |
| 7 | Diflunisal | SD | Stable course |
|
| Diflunisal | SD | Stable course |
| 8 | Diflunisal | SD | Stable course |
|
| None | PD | Stable course |
|
| PAS | PD | Decrease |
| 10 | Diflunisal | SD | Decrease |
|
| PAS | SD | Stable course |
|
| None | PD | Increase |
|
| None | SD | Stable course |
| 11 | Diflunisal | CR | Decrease |
|
| None | PD | Increase |
|
| ASA, bevacizumab,
tamoxifen | PR | Stable course |
| 12 | Diflunisal | SD | Stable course |
| 19 | Diflunisal | PD | Increase |
|
| Trofosfamide,
Bevacizumab | PR | Decrease |
| 20 | Trofosfamide,
bevacizumab, zoledronic acid | PR | Decrease |
| 21 | Diflunisal | SD | Decrease |
| 22 | Gefitinib | PD | Decrease |
| 23 | Diflunisal | PD | Increase |
|
| Diflunisal | PD | Stable course |
| 25 | None | PD | Increase |
Treatment monitoring by quantification
of CTCs
An increase in cell numbers is assumed to correspond
to high tumour activity and poor prognosis (13). The results from the present study
showed that the behaviour of CTC numbers correlates well with the
imaging response. The positive imaging response (CR or PR)
correlated with a CTC reduction in 5/6 (83%; P=0.030) of cases. The
assessment of a SD correlated with a stable CTC course in 7/9 cases
(78%; P<0.001). The assessment of a PD revealed no correlation
with an increase in cell numbers in 6/12 (50%; P=0.368) of cases
(Table IV). The χ2 test
confirmed that PET-CT outcome and CTC course were significantly
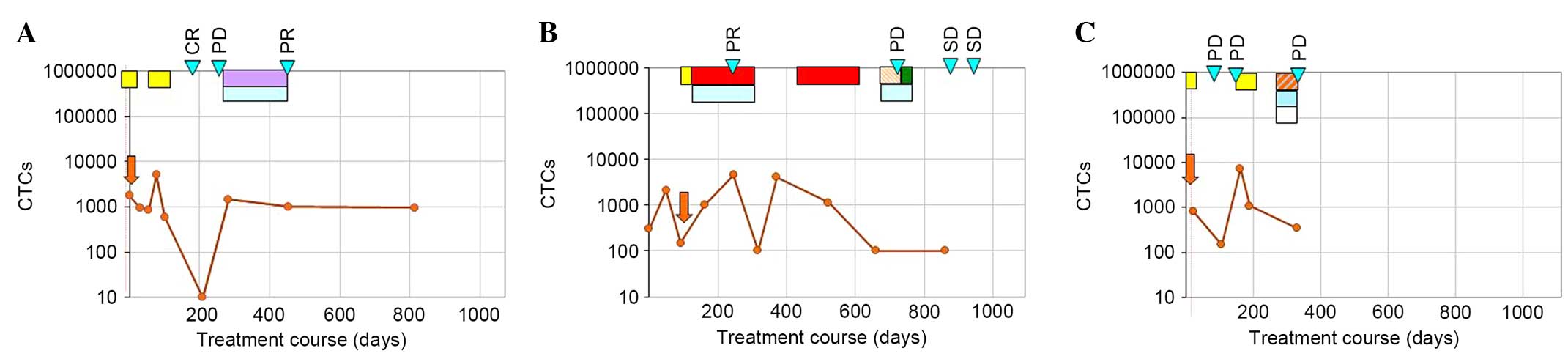
correlated (P=0.0018). Fig. 1 shows
three examples of individual treatment courses. CTC quantity was
measured during the treatment periods and for follow-up. A patient
who was suffering from an endocervical carcinoma started with
stable CTC numbers during the initial diflunisal treatment
(Fig. 1A). The following decrease
from 600 to 0 cells was accompanied by a CR, and the following rise
was accompanied by PD. Thereafter, a tamoxifen/bevacizumab
treatment resulted in a PR, which was accompanied by stable CTC
numbers (Fig. 1A). The patient in
Fig. 1B exhibited rising CTC numbers
during the paclitaxel/bevacizumab treatment, but then falling
tumour cell numbers indicated successful treatment. Rising cell
numbers during paclitaxel treatment have been previously observed
and are assumed to originate from enhanced cell dissemination from
the primary tumour and most probably also from metastases (19). The treatment success was also
reflected by a PR. After the chemotherapy, cell numbers were rising
and could be again reduced by paclitaxel therapy. However, 3 months
later, PD was observed despite previously falling numbers. Whether
a repeated increase in cell numbers, as had been observed prior to
the previous progression, had occurred could not be evaluated,
since the next analysis was performed only after an additional
treatment. Thereafter a stable CTC course resulting from treatments
with doxorubicin/bevacizumab and cyclophosphamide/bevacizumab
treatment was reflected by findings of SD in PET-CT. Furthermore,
the CTC course of a breast cancer patient (Fig. 1C) showed a decrease in the number of
CTCs after a 4-week diflunisal treatment. Subsequent rising CTC
numbers were accompanied by PD. Another diflunisal treatment (for 4
weeks) and the following low-dose chemotherapy resulted in a
decrease in CTC numbers, while the disease continued to progress,
as indicated in Fig. 1C. Again, it
was assumed that this was an incomplete response characterized by a
positive response on the CTC level, but PD observed by the imaging
technique.
 | Table IV.Contingency table PET-CT and CTC
course. |
Table IV.
Contingency table PET-CT and CTC
course.
|
| CTC reduction | Stable CTC
course | Increased CTC |
|---|
|
|
|
|
|
|---|
| PET-CT outcome | n | χ2 | n | χ2 | n | χ2 | P-value | Sum, n |
|---|
| CR + PR | 5 | 2.67 | 1 | 0.67 | 0 | 1.33 | 0.030 | 6 |
| SD | 2 | 0.76 | 7 | 3.33 | 0 | 2.00 | 0.001 | 9 |
| PD | 4 | 0.16 | 2 | 1.34 | 6 | 4.17 | 0.368 | 12 |
| Sum | 11 |
| 10 |
| 6 |
|
| 27 |
The principles outlined in the Declaration of
Helsinki and the German Data Protection Act were followed during
data collection. Informed consent for the publication of clinical
data and CTC count data was obtained from the participants
involved.
Discussion
The majority of patients who seek an integrative
treatment at the UNIFONTIS clinic are heavily pre-treated and have
already switched from curative to palliative care, or they refuse
any treatment with cytostatic agents. The integrative and
personalized treatment combines medical treatment with non-medical
and naturopathic treatments. The present study assessed the
treatment response in 14 patients with advanced epithelial tumours.
Treatments were combined treatments with salicylates, cytostatics,
bevacizumab, tamoxifen and zoledronic acid.
Overall, 6 patients experienced tumour remission
(complete or partial). With respect to the usually poor prognosis
of the patients in the palliative situation, this is a reasonable
success. The present data confirmed the anticancer effect of
salicylates, since disease remission (complete and partial) could
be observed after diflunisal treatment. However, further studies
with a higher number of cases are required to establish the role of
salicylates as anticancer drugs.
It has previously been shown that in cancers,
systemic treatment affects CTC numbers and can reduce them
(20,21). However, the quantification of CTCs is
not generally accepted to be a biomarker for treatment results. The
present study provides a notable finding: Positive response
assessments by imaging techniques (PR and CR) were generally
reflected by a reduction in CTC numbers (83%). Likewise the finding
of SD was often accompanied by stable CTC numbers (78%). However,
the PD assessment by PET-CT was not always accompanied by an
increase in cell numbers. In the latter case, a reduction of CTCs
or stable CTCs could be observed after treatment, while the solid
tumour/metastases were clearly growing. This may reflect a response
to a medical treatment limited to the CTCs, probably due to
insufficient access of the drugs to the respective solid
tumour/metastases. The insufficient access is most probably the
result of the well-known fact that the majority of tumours exhibit
high intra-tumoural pressure, thus creating an interstitial flow in
a direction from the tumour to the healthy tissue, which is a major
cause of therapeutic failure and treatment resistance (22).
The present study concludes that the analysis of CTC
quantities, in addition to the standard procedures for follow-up
monitoring, may allow the physician to differentiate between
treatments that are clearly not working (no response from the CTCs
and a growing tumour mass) and those in which the drugs cannot
sufficiently be delivered to the cells inside the tumour mass. This
would imply that in this case, actions to reduce the tumour
pressure may improve the treatment outcomes and thus provide a more
personalized treatment to the patient. The results of the present
study are consistent with the findings of Yu and Cristofanilli
(23) who suggested that CTC
enumeration may be used to monitor treatment along with imaging
techniques. The authors identified a correlation between the two
different monitoring modalities in advanced malignancies (23). As a result of the present study, a
combined analysis of imaging and CTC quantification appears to be
more sensitive than imaging alone and is therefore warranted for
the individual treatment monitoring of patients with solid
tumours.
Acknowledgements
The authors would like to thank Ms. Erika Schill and
Ms. Laura Redel of the Transfusion Medical Centre Bayreuth
(Bayreuth, Germany) for providing technical assistance. English
language proofreading of the original manuscript was performed by
24translate GmbH (Hamburg, Germany).
References
|
1
|
Thun MJ, Henley SJ and Patrono C:
Nonsteroidal Anti-inflammatory drugs as anticancer agents:
Machanistic, pharmarcologic and clinical issues. J Natl Cancer
Inst. 94:252–266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claudius AK, Kankipati CS, Kilari RS,
Hassan S, Guest K, Russell ST, Perry CJ, Stark LA and Nicholl ID:
Identification of aspirin analogues that repress NF-κB signalling
and demonstrate anti-proliferative activity towards colorectal
cancer in vitro and in vivo. Ocol Rep. 32:1670–1680. 2014.
|
|
3
|
Ulrich CM, Bigler J and Potter JD:
Non-steroidal anti-inflammatory drugs for cancer prevention:
Promise, perils and pharmacogenetics. Nat Rev Cancer. 6:130–140.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Retsky M, Rogers R, Demicheli R, Hrushesky
WJ, Gukas I, Vaidya JS, Baum M, Forget P, Dekock M and Pachmann K:
NSAID analgesic ketorolac used perioperatively may suppress early
breast cancer relapse: Particular relevance to triple negative
subgroup. Breast Cancer Res Treat. 134:881–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kreutz W: Diflunisal for the treatment of
cancer. German Patent WO/2005/016354. Filed August 18, 2004. Issued
February 24. 2005.
|
|
6
|
Bock JM, Menon SG, Goswami PC, Sinclair
LL, Bedford NS, Domann FE and Trask DK: Relative non-steriodal
anti-inflammatory drug (NSAID) antiproliferative activity is
mediated through p21-induced G1 arrest and E2F inhibition. Mol
Carcinog. 46:857–864. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang RA, Lu YY and Fan DM: Reasons for
cancer metastasis: A holistic perspective. Mol Clin Oncol.
3:1199–1202. 2014.
|
|
9
|
Plaks V, Koopman CD and Werb Z:
Circulating Tumor Cells. Science. 341:1186–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaorav GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2002.
View Article : Google Scholar
|
|
12
|
Budd GT, Cristofanilli M, Ellis MJ,
Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV,
Terstappen LW and Hayes DF: Circulating tumor cells versus
imaging-predicting overall survival in metastatic breast cancer.
Clin Cancer Res. 12:6403–6409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pachmann K, Camara O, Kavallaria A,
Krauspe S, Malarski N, Gajda M, Kroll T, Jörke C, Hammer U,
Altendorf-Hofmann A, et al: Monitoring the response of circulating
epithelial tumor cells to adjuvant chemotherapy in breast cancer
allows detection of patients at risk of early relapse. J Clin
Oncol. 26:1208–1215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pachmann K, Dengler R, Lobodasch K,
Fröhlich F, Kroll T, Rengsberger M, Schubert R and Pachmann U: An
increase in cell number at completion of therapy may develop as an
indicator of early relapse: Quantification of CETC for monitoring
of adjuvant therapy in breast cancer. J Cancer Res Clin Oncol.
134:59–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pachmann K, Camara O, Kavallis A,
Schneider U, Schünemann S and Höffken K: Quantification of the
response of circulating epithelial cells to neoadjuvant treatment
for breast cancer: A new tool for treatment monitoring. Breast
Cancer Res. 7:R975–R979. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reichle A and Vogt T: Systems Biology: A
Therapeutic Target for Tumor Therapy. Cancer Microenviron.
1:159–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rüdiger N, Stein EL, Schill E, Spitz G,
Rabenstein C, Stauch M, Rengsberger M, Runnebaum IB, Pachmann U and
Pachmann K: Chemosensitivity testing of circulating epithelial
tumor cells (cetc) in vitro: Correlation to in vivo sensitivity and
clinical outcome. J Cancer Ther. 4:597–605. 2013. View Article : Google Scholar
|
|
19
|
Camara O, Rengsberger M, Egbe A, Koch A,
Gajda M, Hammer U, Jörke C, Rabenstein C, Untch M and Pachmann K:
The relevance of circulating epithelial tumor cells (CETC) for
therapy monitoring during neoadjuvant (primary systemic)
chemotherapy in breast cancer. Ann Oncol. 18:1484–1492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hekimian K, Meisezahl S, Trompelt K,
Rabenstein C and Pachmann K: Epithelial cell dissemination and
readhesion: Analysis of factors contributing to metastasis
formation in breast cancer. ISRN Oncol. 2012:6018102012.PubMed/NCBI
|
|
21
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munson JM and Shieh AC: Interstitial fluid
flow in cancer: Implications for disease progression and treatment.
Cancer Manag Res. 6:317–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu JQ and Cristofanilli M: Circulating
tumor cells and PET. J Nucl Med. 52:1501–1504. 2011. View Article : Google Scholar : PubMed/NCBI
|