Introduction
As an emerging and promising cancer therapy,
photodynamic therapy (PDT) activates and destroys
photosensitizer-incubated tumor cells using wavelength-matched
visible light irradiation. PDT initiates apoptotic and necrotic
cell death in vivo and in vitro (1); however, the factors involved in the
overall process and the contribution to either mechanism are not
completely elucidated.
PDT has previously demonstrated novel effects in the
treatment of oncologic diseases; however, the limitations of laser
penetration into normal tissues and long-lasting cutaneous
photosensitivity following irradiation are unavoidable, and may
affect the applicability of PDT to malignant tumor therapy
(2). As a novel chlorophyll derived
photosensitizer, 9-hydroxypheophorbide α (9-HPbD) has a relatively
longer absorption wavelength (664 nm) and a shorter half-life in
the body compared with other photosentisizers (3). In addition, 9-HPbD exhibited an
apoptosis-inducing effect and growth suppression in MCF-7 human
breast cancer cells (3). A previous
study concerning combination treatment with 9-HPbD-PDT and
carboplatin resulted in an enhanced photocytotoxicity and apoptosis
induction in laryngeal AMC-HN-3 (HN-3) cancer cells (4). Oxidative stress-directed cell death and
migration suppression of HN-3 cells using 9-HPbD-PDT is
additionally investigated in the present study.
The present study aimed to investigate the effect,
and elucidate the potential mechanisms, of 9-HPbD-PDT on apoptosis
and necrosis induction, as well as migration suppression, of HN-3
cells.
Materials and methods
Reagents and antibodies
All media and supplements for cell culture were
supplied by Hyclone™ (GE Healthcare Life Sciences, Logan, UT, USA).
Dimethyl sulfoxide (DMSO),
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
(MTT), RIPA buffer, protease [glutathione (GSH)] and phosphatase
(ascorbic acid) inhibitors, Hoechst 33342 dye and propidium iodide
(PI) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA;
D399) and rhodamine 123 were purchased from Molecular Probes
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Rabbit
anti-GAPDH polyclonal antibody (cat. no. ab9485; dilution 1:2,000)
was supplied by Abcam (Cambridge, UK); mouse anti-poly ADP-ribose
polymerase (PARP) polyclonal antibody (cat. no. AM30; dilution
1:200) was obtained from Merck Millipore (Darmstadt, Germany); and
goat anti-epidermal growth factor receptor (EGFR) polyclonal
antibody (cat. no. sc-03-G; dilution 1:200) was purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
The HN-3 cell line (5)
was developed from a 63-year-male patient with previously untreated
laryngeal squamous cell carcinoma, and was kindly provided by Asan
Medical Center (Seoul, Korea). The cell line was cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum,
penicillin (50 units/ml) and streptomycin (50 µg/ml) at 37°C in a
5% CO2 and 95% air atmosphere in a humidified
incubator.
PDT protocol
9-HPbD (3) was kindly
donated by Kumho Life and Environmental Science Laboratory
(Kwangju, Korea). 9-HPbD was stored in ethanol (1.5 mg/ml) and
aluminum foil at −20°C. HN-3 cells at 90% confluence were incubated
with 9-HPbD in the dark for 6 h at 37°C. Subsequent to changing the
culture medium, 9-HPbD-photosensitized cells were subsequently
exposed to a 664 nm diode laser (Hi-Tech Optoelectronics, Co.,
Ltd., Beijing, China) at 2.0 J/cm2 for 15 min. In order
to assess the antioxidant effect, HN-3 cells were incubated
simultaneously with either 5 mM GSH or 2.5 mM ascorbic acid and
0.59 µg/ml 9-HPbD. Subsequently, the incubated cells were
irradiated by laser, according to the aforementioned
conditions.
Cell viability assay
A MTT assay was used to measure cell viability. The
treated cells at 90% confluence were incubated with 50 µl MTT
solution (2 mg/ml) for 2 h. The MTT solution was exchanged with 100
µl DMSO and the absorbance at 540 nm was measured following 20 sec
of shaking. Cell viability was calculated according to the
following equation: Cell viability (%) = (mean absorbance in
treatment group / mean absorbance in control group) × 100.
Detection of reactive oxygen species
(ROS) and 9-HPbD fluorescence
Generation of ROS was detected by H2DCFDA
staining as previously described (6).
In brief, treated cells at 90% confluence were incubated with 2 µM
H2DCFDA at 37°C for 30 min, and then gently washed twice
with Dulbecco's phosphate-buffered saline (DPBS). Images of green
H2DCFDA were captured using an excitation light from a
488 nm argon laser, 560 nm dichroic mirror and 505–550 nm band pass
barrier filter. 9-HPbD was excited with a 633 nm helium/neon laser.
Band-pass emission filters of 530–600 and 420–480 nm were used to
identify ROS green signal (channel 1) and 9-HPbD red fluorescence
(channel 2). A 650 nm long pass emission filter was applied for
9-HPbD.
Hoechst 33342 and PI double
staining
The treated cells at 90% confluence were stained
with Hoechst 33342 for 30 min and PI for 10 min and observed using
confocal microscopy, as previously described (4). Hoechst 33342 is a fluorescent dye that
specifically stains nucleic acid. Hoechst 33342-stained normal
cells exhibit regular and round nuclei (blue), while the nuclei of
apoptotic cells are crimpy or condensed (bright blue). However, PI
penetrates the cytoplasmic membrane of oncotic cells (late
apoptotic and necrotic cells) and stains the nuclei pink. Due to
the intact cytoplasmic membrane, the nuclei of normal cells and
early apoptotic cells are not stained by PI.
Measurement of mitochondrial membrane
potential (MMP)
In brief, the treated cells at 60–70% confluence
were stained with 1 µM rhodamine 123 for 30 min, and subsequently
the cells were gently washed twice with DPBS. Images of green
fluorescence from rhodamine 123 stained mitochondria were captured
with confocal microscopy with an excitation wavelength of 488 nm,
560 nm dichroic mirror and 505–550 nm band pass barrier filter. MMP
was further detected using flow cytometry (BD Biosciences, San
Jose, CA, USA). Briefly, suspended cells were incubated with 1 µM
rhodamine 123 for 30 min and monitored by the FL1-H channel. Data
were analyzed using BD CellQuest™Pro software, version 2.0 (BD
Biosciences). A minimum of 20,000 events were counted.
Wound healing assay
The HN-3 cells were cultured in 10 cm petri dishes
until the monolayer was 80–90% confluent. Following PDT treatment,
the cell monolayer was lesioned using a 1.2 mm cell scraper without
damaging the dish surface. Images of the lesion areas were captured
at 0 and 24 h following PDT. The photos were analyzed using Adobe
Photoshop CS6 (Adobe Systems Inc., San Jose, CA, USA). The distance
of cell migration was calculated by subtracting the distance
between the lesion edges at 24 h from the distance measured at 0 h.
The values were expressed in mm.
Western blotting analysis
Following treatment with PDT, the cells at 90%
confluence were harvested and total proteins were extracted using
RIPA buffer. Protein concentrations were determined with Bradford
dye reagent. Equivalent amounts of protein (100 µg) were loaded
onto 10% polyacrylamide gels, subjected to electrophoresis, and
transferred to polyvinylidene difluoride membranes. Electrophoresis
and blotting were performed using the PowerPac™ Basic Power Supply
Electrophoresis System 200 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The membranes were blocked with 5% skimmed milk for 1 h,
followed by incubation with the primary antibodies against PARP,
EGFR and GAPDH overnight at 4°C. Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-mouse
IgG (cat. no. sc-2005; dilution 1:2,000; Santa Cruz Biotechnology,
Inc.), goat anti-rabbit IgG (cat. no. sc-2004; dilution 1:2000;
Santa Cruz Biotechnology, Inc.) or mouse anti-goat IgG (cat. no.
sc-2355; dilution 1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h.
The protein bands were detected by a Kodak in vivo Image
Analyzer (Kodak, Rochester, NY, USA).
Statistical analysis
Results are expressed as the mean ± standard
deviation. Significant differences were evaluated using one-way
analysis of variance followed by least significant difference
method. Statistical analyses were performed using SPSS 11.5
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
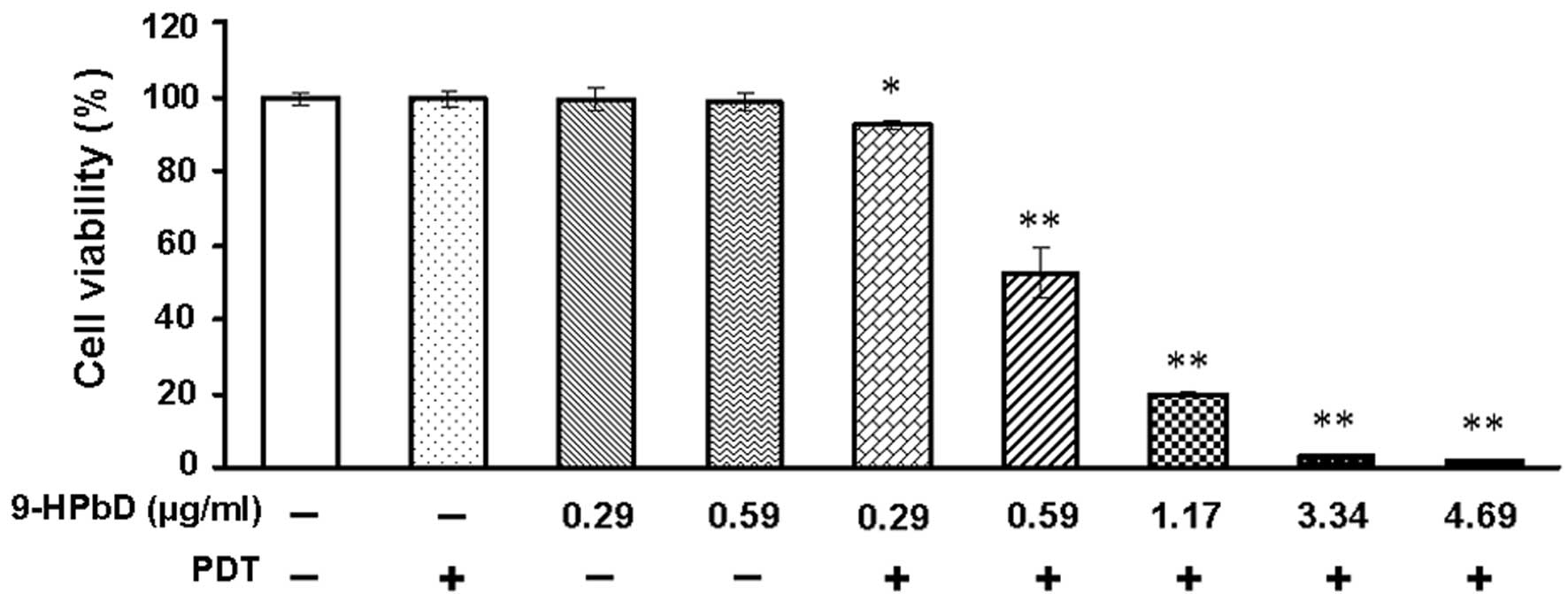
Photocytotoxic effect of 9-HPbD
The photocytotoxicity exhibited by 9-HPbD-PDT on
HN-3 cells was validated by a MTT assay. With diode laser
irradiation, the photocytotoxic effect of 9-HPbD on HN-3 cells
exhibited a photosensitizer dose-dependent pattern. Neither PDT nor
9-HPbD alone was photocytotoxic to HN-3 cells. As shown in Fig. 1, cell viability was significantly
suppressed at 0.29 µg/ml 9-HPbD-PDT compared with control cells
(P=0.021). At higher doses of 9-HPbD (≥0.59 µg/ml), cell viability
was also significantly suppressed compared with control cells
(P<0.01).
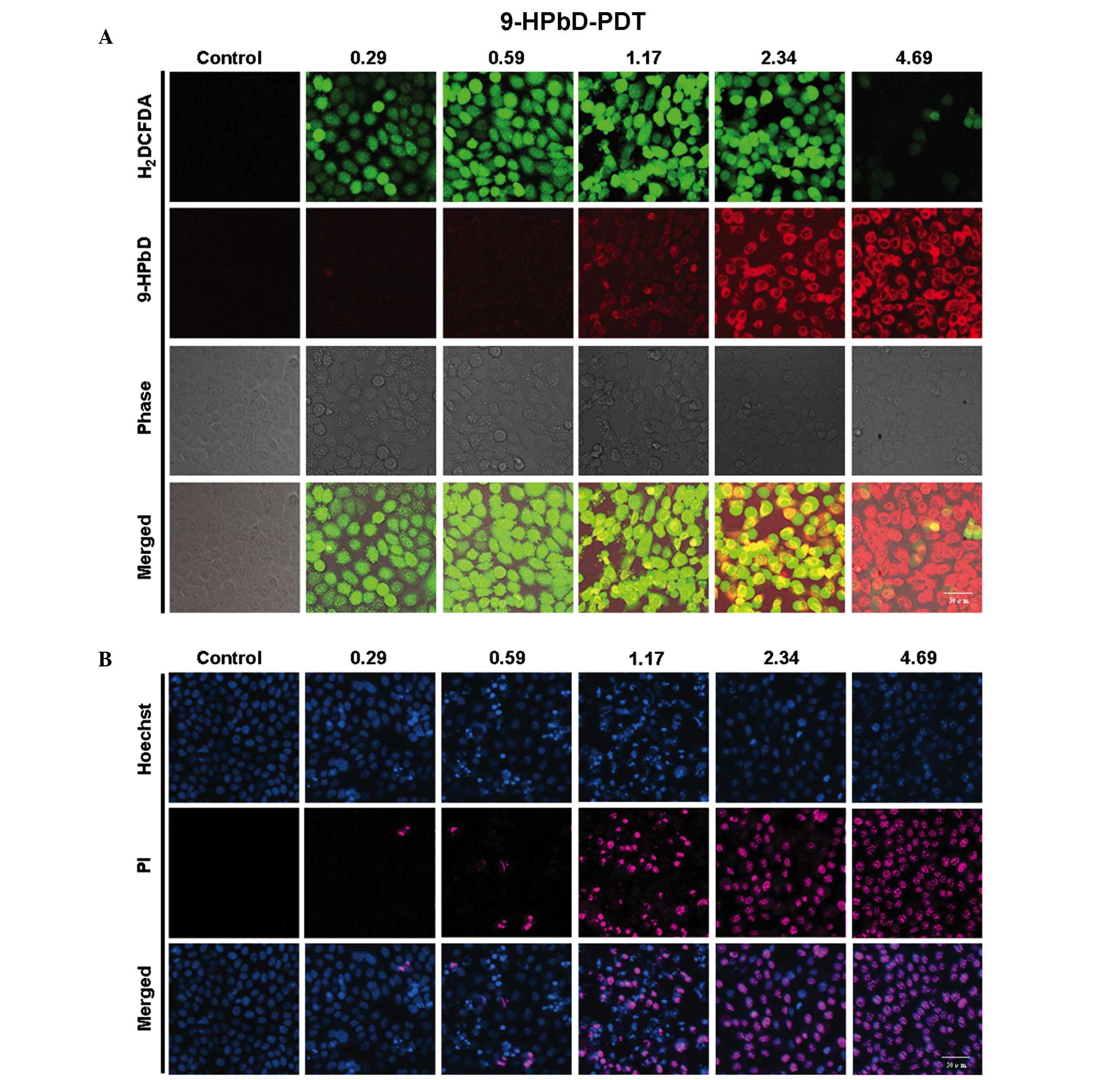
ROS generation, 9-HPbD fluorescence
and cell death induction by 9-HPbD-PDT
As shown in Fig. 2A,
9-HPbD fluorescence was increased with increasing doses of 9-HPbD.
Intracellular formation of ROS was detected by the conversion of
non-fluorescent H2DCFDA to fluorescent
2,7-dichlorofluorescein diacetate (DCFDA). As shown in Fig. 2A, DCFDA fluorescence increased between
0.29 and 1.17 µg/ml 9-HPbD-PDT. Subsequently, the intensity of
DCFDA fluorescence broke down gradually between 1.17 and 4.69 µg/ml
9-HPbD-PDT 1 h following laser irradiation.
A total of 24 h following PDT, the cells exhibited
blurred and shrinkable cellular contours. Apoptotic cells, marked
with condensed/fragmented blue or pink nuclei, were observed in a
dose-dependent manner following treatment with 0.29–1.17 µg/ml
9-HPbD-PDT. There was a gradual increase in necrotic cells, which
exhibited pink intact nuclei, in cells treated with higher doses of
9-HPbD (1.17–4.69 µg/ml). The cells in the control group exhibited
intact homogeneous blue and round nuclei (Fig. 2B). Apoptotic/necrotic cells were not
observed in cells treated with 9-HPbD or laser irradiation alone
(data not shown).
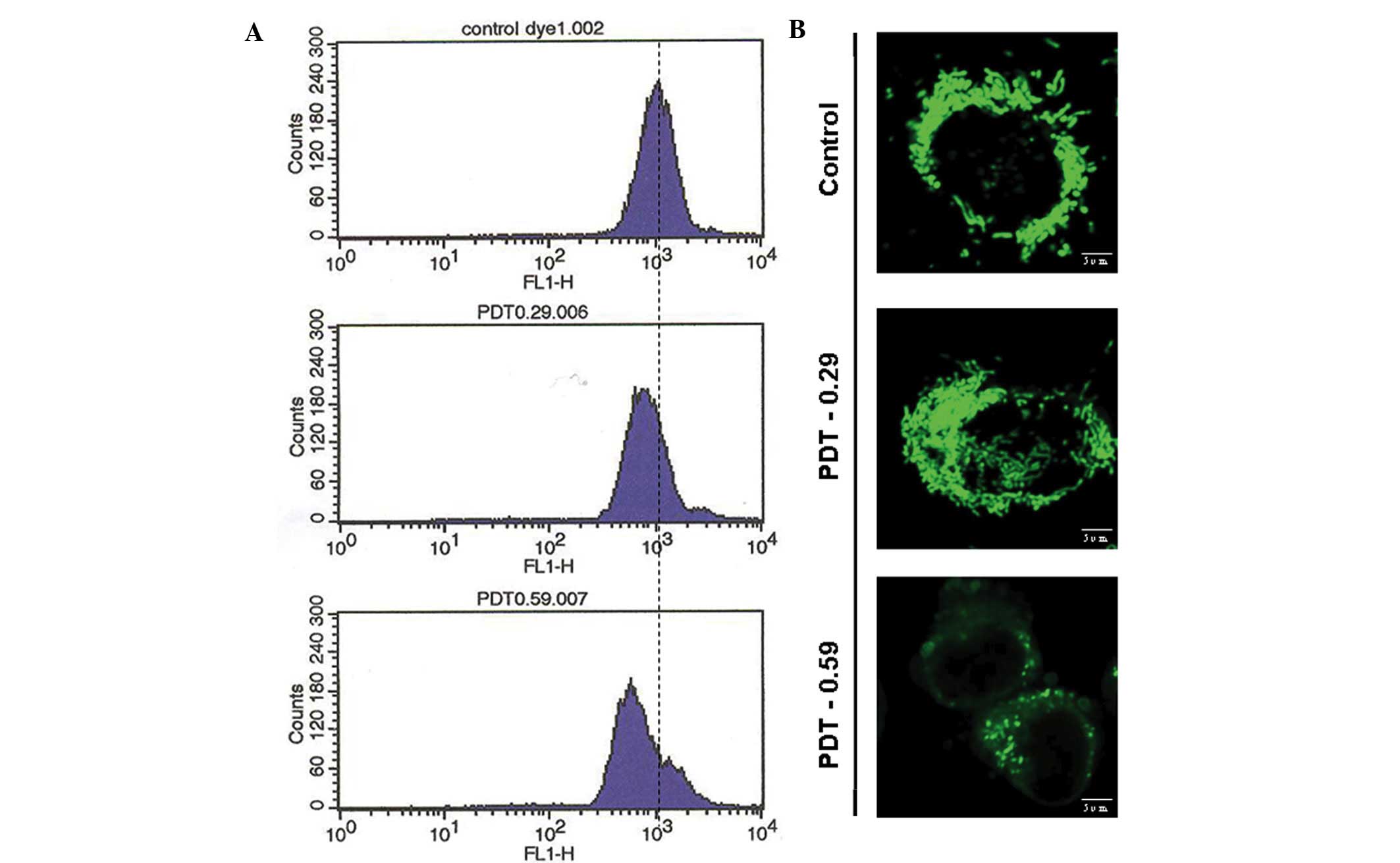
9-HPbD-PDT initiates mitochondrial
depolarization
Cells were stained with rhodamine 123, and MMP in
single cells was monitored using flow cytometry and confocal
microscopy. A collapsed MMP, shown as a leftward shift of the
fluorescence curve from flow cytometry, was observed in a 9-HPbD
dose-dependent manner (Fig. 3A).
Polarized mitochondria were demonstrated by the presence of bright
fluorescent spheres and tubes using confocal microscopy. A total of
2 h following PDT, the majority of the bright spheres became faint
and rhodamine 123 fluorescence intensity was caliginous, indicating
mitochondrial depolarization (Fig.
3B).
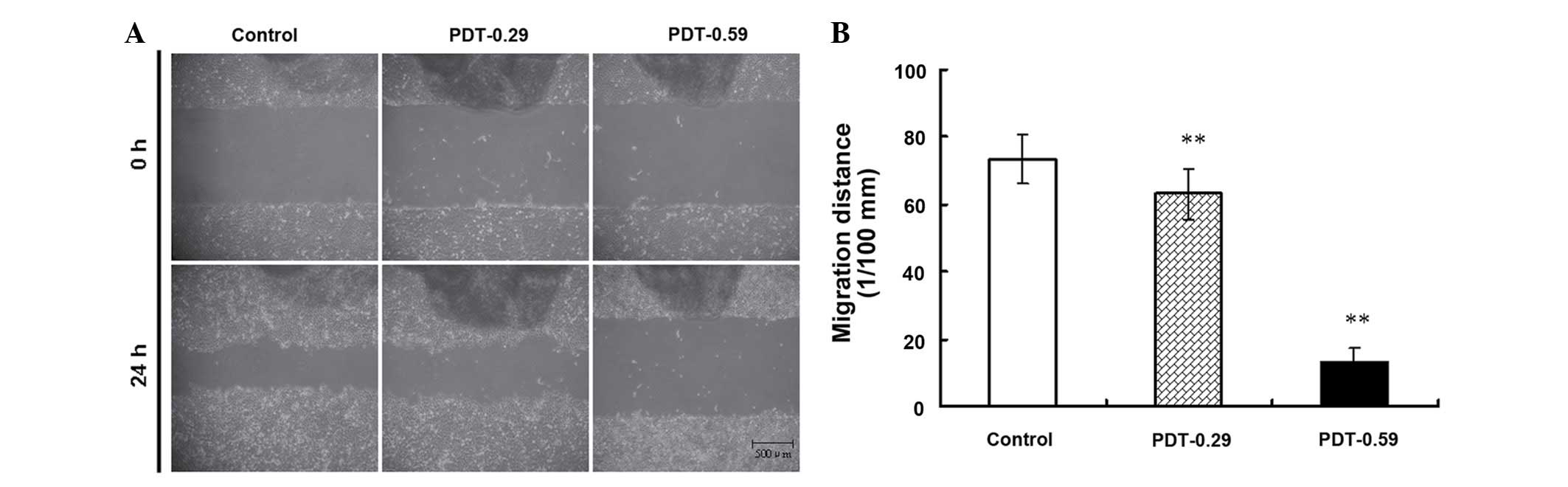
9-HPbD-PDT suppressed the migration
and EGFR expression in HN-3 cells
A wound healing assay was used to evaluate the
effect of 9-HPbD-PDT on the migration of HN-3 cells. As shown in
Fig. 4, PDT significantly suppressed
the migration of HN-3 cells in a sensitizer dose-dependent manner.
The migration distance of HN-3 cells decreased from 0.74±0.07
observed in the control group to 0.63±0.07 mm in the 0.29 µg/ml
9-HPbD-PDT group (P=0.007) and 0.13±0.05 mm in the 0.59 µg/ml
9-HPbD-PDT group (P=0.001) (Fig.
4).
In contrast to the untreated group, there was clear
inhibition of EGFR expression in PDT groups in a sensitizer
dose-dependent manner (Fig. 5A).
Downregulation in the expression of EGFR was partially inhibited
when the cells were pretreated with ascorbic acid (Fig. 5B). As the native substrate of
caspase-3, PARP was measured using western blotting. Similarly to
EGFR expression, there was an elevated expression of PARP in a
sensitizer dose-dependent manner (Fig.
5A). When cells were pretreated with GSH and ascorbic acid,
upregulation of PARP was significantly inhibited (Fig. 5B).
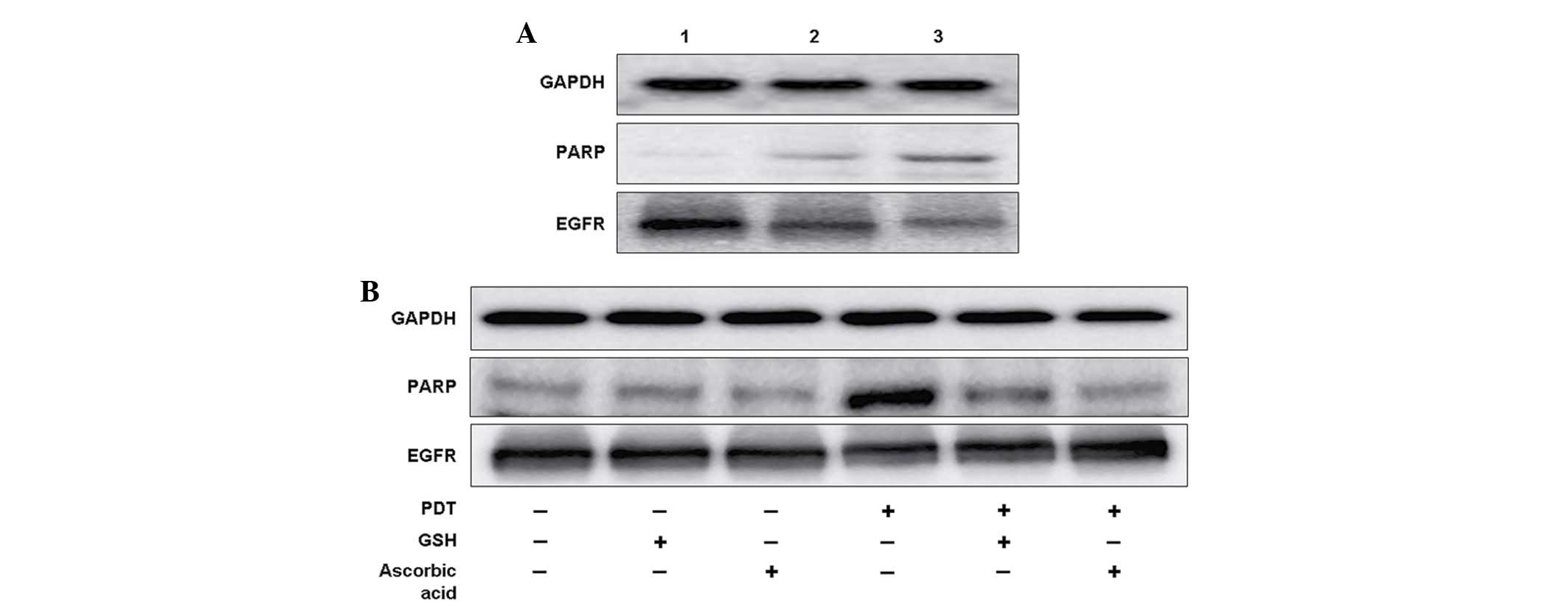 | Figure 5.9-HPbD-PDT induced the cleavage of
PARP (89 kDa), and inhibited the expression of EGFR (170 kDa).
Laryngeal cancer AMC-HN-3 cells were treated with a sublethal dose
of PDT, collected 24 h later, and subjected to western blot
analysis. GAPDH (37 kDa) was used as a control. (A) Lane 1, control
(no treatment); lane 2, 0.29 µg/ml 9-HPbD-PDT; lane 3, 0.59 µg/ml
9-HPbD-PDT. (B) Cells were treated with 0.59 µg/ml 9-HPbD-PDT alone
or pretreated with 5 mM GSH or 2.5 mM ascorbic acid. GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; PARP, poly ADP-ribose
polymerase; EGFR, epidermal growth factor receptor; 9-HpbD,
9-hydroxypheophorbide α; PDT, photodynamic therapy; GSH,
glutathione. |
Discussion
In the present study, phototoxicity was not observed
when HN-3 cells were treated with 9-HPbD or laser irradiation
alone. In the presence of a laser, 9-HPbD had significant
photocytotoxicity and an induction of apoptosis and necrosis was
observed, which was a photosensitizer dose-dependent response.
These results demonstrate the basic rule of PDT: There is a
synergistic effect between lasers and photosensitizers, which is
required since laser nor photosensitizer alone is photocytotoxic
(7).
The basic principle of PDT is that rapid ROS
generation, due to the photochemical activation of the
photosensitizer, initiates cell death (8). Consistent with increased ROS signals, in
the present study there was gradually induction of apoptosis in
HN-3 cells treated with 0.29–1.17 µg/ml 9-HPbD-PDT. In cells
treated with higher doses of 9-HPbD-PDT (1.17–4.69 µg/ml), there
was a gradual increase of necrotic cells with 9-HPbD concentration,
whereas ROS signals were remarkably attenuated. This may be
associated with nonspecific relocalization of photosensitizer
targets at higher doses, including to lysosomes or the plasma
membrane, which may either block or delay the apoptotic program,
thus predisposing the cells to necrosis (9). Apoptotic and necrotic cells were not
observed when cells were treated with 9-HPbD or a laser alone (data
not shown). These results suggest that 9-HPbD shares the same
characteristics as other photosensitizers; photosensitizers
minimize the undesirable effects of anti-tumor drugs towards normal
tissue during treatment (2). This is
one of the advantages of PDT over other conventional cancer
treatment modalities, including surgery, radiation and
chemotherapy. The advantage of selective tumor destruction with
normal tissue preservation is of particular importance for cancers
in the head and neck region, where excessive tissue loss results in
significant functional disabilities including effects on speech and
swallowing (2,10).
A positive regulatory function of PARP cleavage has
been observed in the onset of apoptosis (11). PARP, the native substrate of caspase-3
(12), was measured in the present
study by western blotting, and this revealed that PARP was
upregulated 24 h following PDT. The increased expression of cleaved
PARP, consistent with an enhanced cytotoxic and apoptotic effect of
PDT, was significantly inhibited by antioxidant pretreatment,
including GSH and ascorbic acid. The pattern of PARP expression
under oxidative stress further indicates the role of ROS in
9-HPbD-PDT-induced apoptosis.
Although the involvement of multiple pathways during
PDT-mediated cell death has been reported (1), the elucidation of the molecular
mechanisms underlying PDT-mediated apoptosis and cell cycle
deregulation is far from complete. An improved understanding of
these pathways may lead to development of strategies for improving
treatment protocols (4,13,14), and
therefore, increase the therapeutic efficacy of PDT.
Mitochondria are recognized as the most important
cellular organelle for apoptosis inducement (15,16).
Generally, prior to cell death, a collapse of MMP is initiated.
Collapsed MMP has been considered as an important factor that
initiates the release of cytochrome c (17). Following the release of mitochondrial
cytochrome c into the cytosol, activation of the caspase
cascade has been reported in PDT-induced apoptosis (18). In the present study, a clear leftward
shift of the fluorescence curve indicated a collapse of MMP in HN-3
cells treated with 9-HPbD-PDT, which occurred in a photosensitizer
dose-dependent pattern. Similarly with increasing doses of 9-HPbD,
mitochondrial morphology exhibited a gradual loss of
characteristically bright spheres/tubes and more diffuse and weaker
fluorescent signals were observed following PDT, due to leakage of
the dye from the mitochondria into the cytosol. The disruption of
MMP demonstrates the important role of mitochondria activation of
apoptosis in HN-3 cells treated with 9-HPbD-PDT.
An increased expression of EGFR has been reported in
90% of squamous cancer cells in the head and neck region (19). It has been indicated that EGFR is
important in tumor progression, in addition to its role in cellular
proliferation (20); therefore,
EGFR-inhibition has been recently considered as a candidate for the
treatment of head and neck carcinoma (21,22). In
the present study, there was a significant downregulation in the
expression of EGFR in PDT groups in a photosensitizer
dose-dependent manner compared with the high expression of EGFR
observed in the control group, which may subsequently lead to
migration suppression of HN-3 cancer cells, as observed in a
previous experiment. This indicates that EGFR may be the target of
9-HPbD-PDT in HN-3 cells. EGFR also has a role in the activation of
cell apoptosis and necrosis (23). A
downregulated expression of EGFR following PDT was partially
inhibited by pretreatment with ascorbic acid, indicating a
potential role of ROS in 9-HPbD-PDT-induced migration
suppression.
Overall, 9-HPbD-PDT exhibits a phototoxic effect in
HN-3 cells, as evidenced by an attenuated cell viability and
activation of apoptosis and necrosis observed by the present study.
Mitochondrial activation under oxidative stress is important in
9-HPbD-PDT-induced apoptosis of HN-3 cells. The subcellular
relocalization of 9-HPbD may lead to the distinguishing generation
of ROS and subsequently determine the type of cell death. Migration
suppression of HN-3 cells following PDT is partially due to the
ROS-mediated inhibition of EGFR expression and/or phosphorylation
(23). However, the potential
mechanisms involved in the photochemical effect of 9-HPbD-PDT on
HN-3 cancer cells require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81172557) and the Project Sponsored by the Scientific Research
Foundation for the Returned Overseas Chinese Scholars of State
Education Ministry (Beijing, China). In addition, the study was
partially funded by the Leading Foreign Research Institute
Recruitment Program through the National Research Foundation of
Korea funded by the Ministry of Education, Science and Technology
(grant no. 2012K1A4A3053142) and Beckman Laser Institute Korea,
Dankook University
References
|
1
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
2
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi SE, Sohn S, Cho JW, Shin EA, Song PS
and Kang Y: 9-Hydroxypheophorbide α-induced apoptotic death of
MCF-7 breast cancer cells is mediated by c-Jun N-terminal kinase
activation. J Photochem Photobiol B. 73:101–107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He P, Ahn JC, Shin JI, Hwang HJ, Kang JW,
Lee SJ and Chung PS: Enhanced apoptotic effect of combined modality
of 9-hydroxypheophorbide alpha-mediated photodynamic therapy and
carboplatin on AMC-HN-3 human head and neck cancer cells. Oncol
Rep. 21:329–334. 2009.PubMed/NCBI
|
|
5
|
Kim SY, Chu KC, Lee HR, Lee KS and Carey
TE: Establishment and characterization of nine new head and neck
cancer cell lines. Acta Otolaryngol. 117:775–784. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung PS, He P, Shin JI, Hwang HJ, Lee SJ
and Ahn JC: Photodynamic therapy with 9-hydroxypheophorbide alpha
on AMC-HN-3 human head and neck cancer cells: Induction of
apoptosis via photoactivation of mitochondria and endoplasmic
reticulum. Cancer Biol Ther. 8:1343–1351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moan J and Berg K: Photochemotherapy of
cancer: Experimental research. Photochem Photobiol. 55:931–948.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moan J and Berg K: The photodegradation of
porphyrins in cells can be used to estimate the lifetime of singlet
oxygen. Photochem Photobiol. 53:549–553. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oleinick NL, Morris RL and Belichenko I:
The role of apoptosis in response to photodynamic therapy: What,
where, why, and how. Photochem Photobiol Sci. 1:1–21. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biel MA: Photodynamic therapy treatment of
early oral and laryngeal cancers. Photochem Photobiol.
83:1063–1068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boulares AH, Yakovlev AG, Ivanova V,
Stoica BA, Wang G, Iyer S and Smulson M: Role of poly (ADP-ribose)
polymerase (PARP) cleavage in apoptosis: Caspase 3-resistant PARP
mutant increases rates of apoptosis in transfected cells. J Biol
Chem. 274:22932–22940. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tewari M, Quan LT, O'Rourke K, Desnoyers
S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verma S, Watt GM, Mai Z and Hasan T:
Strategies for enhanced photodynamic therapy effects. Photochem
Photobiol. 83:996–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang X, Wu P, Li J, Qi L, Tang Y, Jiang W
and Zhao S: Combination of apoptin with photodynamic therapy
induces nasopharyngeal carcinoma cell death in vitro and in vivo.
Oncol Rep. 28:2077–2082. 2012.PubMed/NCBI
|
|
15
|
Liu JX, Zhang JH, Li HH, Lai FJ, Chen KJ,
Chen H, Luo J, Guo HC, Wang ZH and Lin SZ: Emodin induces Panc-1
cell apoptosis via declining the mitochondrial membrane potential.
Oncol Rep. 28:1991–1996. 2012.PubMed/NCBI
|
|
16
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell
apoptosis through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013.PubMed/NCBI
|
|
17
|
Lai JC, Lo PC, Ng DK, Ko WH, Leung SC,
Fung KP and Fong WP: BAM-SiPc, a novel agent for photodynamic
therapy, induces apoptosis in human hepatocarcinoma HepG2 cells by
a direct mitochondrial action. Cancer Biol Ther. 5:413–418. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granville DJ, Shaw JR, Leong S, Carthy CM,
Margaron P, Hunt DW and McManus BM: Release of cytochrome c, Bax
migration, Bid cleavage, and activation of caspases 2, 3, 6, 7, 8
and 9 during endothelial cell apoptosis. Am J Pathol.
155:1021–1025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodburn JR: The epidermal growth factor
receptor and its inhibition in cancer therapy. Pharmocol Ther.
82:241–250. 1999. View Article : Google Scholar
|
|
21
|
Kim SG, Hong JW, Boo SH, Kim MG, Lee KD,
Ahn JC, Hwang HJ, Shin JI, Lee SJ, Oh JK and Chung PS: Combination
treatment of Cetuximab and photodynamic therapy in SNU-1041
squamous cancer cell line. Oncol Rep. 22:701–708. 2009.PubMed/NCBI
|
|
22
|
Koon HK, Chan PS, Wong RN, Wu ZG, Lung ML,
Chang CK and Mak NK: Targeted inhibition of the EGFR pathways
enhances Zn-BC-AM PDT-induced apoptosis in well-differentiated
nasopharyngeal carcinoma cells. J Cell Biochem. 108:1356–1363.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martínez-Carpio PA and Trelles MA: The
role of epidermal growth factor receptor in photodynamic therapy: A
review of the literature and proposal for future investigation.
Lasers Med Sci. 25:767–771. 2010. View Article : Google Scholar : PubMed/NCBI
|